Background: For unknown reasons, p21 expression induces different effects in cells, including arrest, death, and growth/prosurvival signals.
Results: Cancer cell lines respond with arrest or apoptosis to p21 expression depending on mitochondria sensitivity to oxidants.
Conclusion: Cell-specific sensitivity to oxidative stress determines p21-induced cell death.
Significance: This provides a rationale to find therapies that up-regulate p21 in cells that are more sensitive to oxidants to favor a death response.
Keywords: Apoptosis, Cancer Therapy, Oxidative Stress, p53, Reactive Oxygen Species (ROS), Senescence, p21
Abstract
p21Waf1/Cip1/Sdi1 is a cyclin-dependent kinase inhibitor that mediates cell cycle arrest. Prolonged p21 up-regulation induces a senescent phenotype in normal and cancer cells, accompanied by an increase in intracellular reactive oxygen species (ROS). However, it has been shown recently that p21 expression can also lead to cell death in certain models. The mechanisms involved in this process are not fully understood. Here, we describe an induction of apoptosis by p21 in sarcoma cell lines that is p53-independent and can be ameliorated with antioxidants. Similar levels of p21 and ROS caused senescence in the absence of significant death in other cancer cell lines, suggesting a cell-specific response. We also found that cells undergoing p21-dependent cell death had higher sensitivity to oxidants and a specific pattern of mitochondrial polarization changes. Consistent with this, apoptosis could be blocked with targeted expression of catalase in the mitochondria of these cells. We propose that the balance between cancer cell death and arrest after p21 up-regulation depends on the specific effects of p21-induced ROS on the mitochondria. This suggests that selective up-regulation of p21 in cancer cells could be a successful therapeutic intervention for sarcomas and tumors with lower resistance to mitochondrial oxidative damage, regardless of p53 status.
Introduction
p21Waf1/Cip1/Sdi1 is a member of a family of inhibitors of the cyclin-dependent kinases, together with p27Kip1 and p57Kip2 (1). p21 arrests cells by affecting the activity of cyclin D-, E-, and A-dependent kinases, which regulate progression through the G1 phase of the cell cycle and initiation of DNA synthesis (1). It is a target gene of the tumor suppressor p53 (2) and a key mediator of p53-induced G1 arrest in response to DNA damage (3, 4). It can also be induced independently of p53 in response to stimuli such as TGFβ (5), histone deacetylase inhibitors (6), or Ras (7).
Increased p21 protein levels have been detected in cultured human fibroblasts undergoing replicative senescence (8), a terminal differentiation state triggered by shortening and/or dysfunction of telomeres (9). This phenotype is characterized by an irreversible growth arrest, as well as distinctive morphological changes and markers (10, 11). Senescence is also a tumor suppressor mechanism that prevents emergence of transformed cells (9). It has been shown that lack of p21 delays or abolishes the onset of senescence (12) and that continuous p21 expression induces a senescent arrest in normal and cancer cells in a p53-independent manner (13–15).
Reactive oxygen species (ROS)2 are generated by cellular oxidative processes, and are normally buffered by antioxidant mechanisms (16). Elevation of ROS above basal levels trigger different cellular responses such as cell cycle arrest, senescence, apoptosis, or necrosis, depending on the intensity of the oxidative damage (17). We have shown that ROS are important in determining cell fate after p53 up-regulation (18). Moreover, we have reported that p21 can increase ROS levels independently of p53 and that this is required for the permanent arrest observed in senescence (15). Recent studies confirmed that p21 is necessary for the induction of ROS and mitochondrial dysfunction observed in senescence and showed that this maintains a constant DNA damage response responsible for prolonged cell cycle arrest (19).
p21 prevents cancer cell growth due to its ability to transiently or permanently stop proliferation, thus being an important component of tumor suppressor mechanisms. Indeed, it has recently been shown that p21 can be down-regulated by several microRNA that are expressed in cancer cells (20, 21). However, p21 levels are often elevated in cancers without signs of growth inhibition (22). Moreover, it has been proposed that p21 can actually favor transformation by inhibiting apoptosis and inducing growth and prosurvival signals, genomic destabilization, and expression of secreted mitogenic factors (23). It is not well understood how p21 exerts these radically different functions or even if they reside in separate domains of the protein (23). Due to the difficulty of selectively activating its tumor suppressor properties without also inducing its potentially oncogenic features, the design of antineoplastic therapies involving p21 regulation has so far been controversial (22, 23).
Further adding to its complex pleiotropic functions, it has been recently shown that p21 can also trigger cell death (24, 25), although the mechanisms involved in these processes have not been fully elucidated. Depending on the context, p21 can induce proapoptotic effectors such as Bax or members of the TNF family (24), as well as p53 (25). Also, p21-mediated depletion of proteins that control cell division can lead to abnormal mitosis and genetic destabilization when arrested cells attempt to re-enter cell cycle after p21 down-regulation, causing death by mitotic catastrophe independently of p53 or the apoptotic pathway (26). Here, we characterize the mechanisms involved in the induction of death by p21 and find that a cell-specific sensitivity to p21-mediated ROS, likely determined by mitochondrial responses, plays a role in defining apoptosis after p21 up-regulation.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments
HT1080p21–9, HOS, A431, HA847, and MDA-MB-175 cells were maintained in DMEM supplemented with 10% FBS and penicillin-streptomycin (50 units/ml). U2OSp53−/− (U2OS stably transfected with a pLKO-p53-shRNA-941 vector to suppress p53 expression using methods described previously (27)) were maintained in DMEM supplemented with 10% FBS, penicillin-streptomycin (50 units/ml), and puromycin (2 μg/ml). U2OSp53−/− were generously provided by Dr. Nickolai Barlev (University of Leicester, UK). EJ and PC3 cells with a tetracycline (Tet)-regulated expression system (EJp21 and PC3p21, respectively) (14, 15) were maintained in DMEM supplemented with 10% FBS, penicillin-streptomycin (50 units/ml), hygromycin (100 μg/ml), and geneticin (750 μg/ml), plus 1 μg/ml Tet to repress expression of p21. Fresh medium with Tet was added every 3 days. EJp21 and PC3p21 were provided generously by Dr. Stuart Aaronson (Mount Sinai School of Medicine, New York, NY). Cells were treated by adding to the medium 10 mm N-acetyl-l-cysteine (NAC, Sigma-Aldrich), 10–20 μm pifithrin (Sigma-Aldrich), or different concentrations of tert-butyl-hydroperoxide (tBH, Sigma-Aldrich) for the specified time.
Modulation of Gene Expression
To induce p21 expression in HT1080p21–9, 25 μm IPTG was added to the medium, except otherwise noted. To induce p21 expression in EJp21 and PC3p21, cells were washed three times with PBS and seeded in medium in the absence of Tet. In all other cell lines, p21 was induced by infection with a retrovirus containing p21 (generously provided by Dr. Stuart Aaronson, Mount Sinai School of Medicine) in the presence of 2 μg/ml polybrene (Sigma) as described (15). For adenoviral studies, cells were infected with 2.5 × 107 adenovirus containing LacZ (AdLacZ, BD Biosciences), a catalase that expresses specifically in the mitochondria (28) (AdCat, generous gift of Dr. Arthur I. Cederbaum, Mount Sinai School of Medicine) or p53 (Adp53, a generous gift of B. Vogelstein, The Johns Hopkins University, Baltimore, MD). Generation and titration of p53 shRNA-containing lentivirus was performed as described previously (29). Catalase expression was suppressed using specific predesigned ChimeraRNAi (Abnova). shRNA against luciferase was used as control. All oligonucleotides were transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions.
Senescence-associated β-Galactosidase Staining
Cells were stained following the standard protocols as described (11).
FACS Analysis
Fluorescently stained cells were transferred to Polystyrene tubes and subjected to FACS (FACScan, BD Biosciences) using Cell Quest software (version 3.2, BD Biosciences) for acquisition and analysis.
Cell Cycle Analysis
Cells were stained with propidium iodide (PI) using the CycleTEST Plus DNA reagent kit (BD Biosciences), following the instructions provided. Fluorescent stained cells were then subjected to FACS analysis.
Measurement of Apoptosis
Cells were stained with annexin and PI using the annexin V-Fluos staining kit (Boehringer, Mannheim, Germany) as reported (30) followed by FACS analysis.
Measurement of Intracellular ROS and Glutathione
Cells were incubated with 5 μg/ml of 2′,7′-difluorodihydrofluorescein diacetate (DCF, Invitrogen) for 30 min at 37 °C, and then washed with PBS, trypsinized, and collected in 1 ml of PBS, followed by FACS analysis. Values of mean fluorescence intensity were reported. An assay kit that determines intracellular concentrations of total glutathione (Sigma-Aldrich) was used, following the manufacturer's instructions.
Measurement of Oxidative Damage to DNA
A comet assay was performed as described previously (31) with the following modifications. After lysis, the slides were washed once with distilled water and immersed in three changes of enzyme digestion buffer (40 mm HEPES, 0.1 m KCl, 0.5 mm EDTA and 0.2 mg/ml bovine serum albumin (pH 8.0)), for 5 min each time, at room temperature. Fpg (Sigma-Aldrich) was added to the gel (50 μl/gel) at 1/500 or 1/1000 dilutions; gels were covered with a coverslip and incubated in a humidified chamber at 37 °C for 30 min. The coverslips were removed, and the slides were placed in a horizontal electrophoresis tank. DNA damage was expressed as the percentage of DNA in the comet tails.
Mitochondrial Studies
Measurements of mitochondrial membrane potential were made using tetramethylrhodamine, ethyl ester, perchlorate (TMRE, Invitrogen). Cells were incubated with 0.1 μm TMRE for 30 min at 37 °C before being collected for FACS analysis. Total mitochondria mass was assessed by staining with 10 μm nonyl acrydil orange (Invitrogen) for 30 min at 37 °C, followed by FACS analysis. For immunofluorescence, cells were seeded onto glass coverslips and incubated with 180 nm MitoTracker Red CMXRos (Invitrogen) for 25 min at 37 °C. Coverslips were rinsed with PBS, fixed with 4% paraformaldehyde in PBS for 15 min, washed three times with PBS, and incubated with 0.1% Triton X-100 for 5 min. They were then washed three times with PBS and blocked with 1% BSA for 30 min. Cells were stained for p21 using rabbit polyclonal antibody (Santa Cruz Biotechnology) for 1 h. Following staining, cell were washed three times with PBS and incubated for 1 h with Alexa Fluor® 488 chicken anti-rabbit IgG (H+L) secondary antibody (Invitrogen). Coverslips were washed three times with PBS, mounted upside down onto slides using ProLong® Gold antifade reagent, and images were taken with an Olympus FV1000 confocal laser scanning microscope.
Immunobolot Analysis
Immunoblots were performed as described (32). 15 μg of total cell protein per sample was subjected to 10% SDS-PAGE and transferred to Immobilon polyvinvylidene difluoride filter (Millipore, Billerica, MA). The antibodies used were as follows: p21 (polyclonal, Santa Cruz Biotechnology), p53 (1801 monoclonal, Abcam, Cambridge, UK), HDM2 (monoclonal, Abcam), PUMA (polyclonal, Cell Signaling), vimentin (monoclonal, BD Biosciences), ZEB1 (polyclonal, Santa Cruz Biotechnology), e-cadherin (monoclonal, BD Biosciences), SOD1 (polyclonal, Abcam), SOD2 (polyclonal, Abcam), catalase (polyclonal, Abcam), actin (monoclonal, Abcam), and tubulin (monoclonal, Abcam).
RESULTS
p21 Expression Can Induce Both Cell Cycle Arrest and Death in Same Cell Line
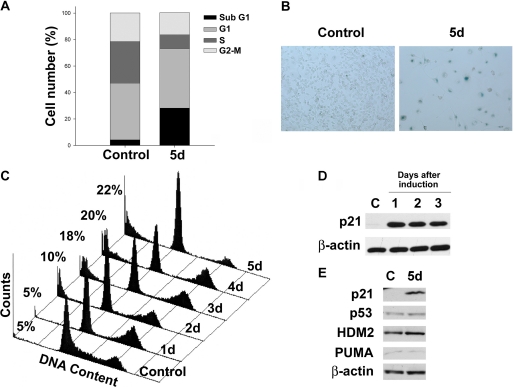
To better understand cell fate decisions after p21 induction, we studied HT1080p21–9, a WT p53-containing human fibrosarcoma cell line with an IPTG-regulatable p21 expression system. These cells undergo senescent-like changes when p21 is up-regulated for more than 3 days at levels similar to those observed in physiological responses (13, 33). FACS analysis of HT1080p21–9 after 5 days of p21 expression confirmed that cells entered cell cycle arrest, as shown by an important reduction in S phase (Fig. 1A). As expected, p21 induced morphological changes consistent with the establishment of senescence, accompanied by positive staining with the senescence-specific marker senescence-associated β-gal (Fig. 1B). However, we also observed a concomitant increase in the sub-G1 fraction of the cell cycle (Fig. 1, A and C), indicating a significant percentage of dead cells. Induction of cell cycle arrest and death occurred progressively after p21 up-regulation (Fig. 1C), although maximum levels of p21 expression were already achieved 1 day after exposure to IPTG and remained constant (Fig. 1D).
FIGURE 1.
p21 induces arrest and cell death in HT1080p21–9. A, FACS analysis of PI-stained HT1080p21–9 cells uninduced (control) or 5 days after p21 induction with 25 μm IPTG (5d). Bars show percentages of cells in each phase of the cell cycle. All plots shown in this and other figures represent mean values of at least three independent experiments, and error bars indicate S.D. B, HT1080p21 cells uninduced (control) or induced for 5 days with 100 μm IPTG (5d) stained for senescence-associated β-gal. Three independent experiments were performed in duplicate. Magnification, 20×. C, FACS analysis of PI-stained HT1080p21–9 1 to 5 days after p21 induction, compared with untreated cells (Control). Percentages indicate amount of events in sub-G1 phase of the cell cycle. D, immunoblot analysis of protein levels in lysates of the cells used in C. For this and other figures, immunoblot results were confirmed with at least two independent experiments. E, immunoblot analysis of HT1080p21–9 uninduced or induced for 5 days.
p53 induction has been observed after p21 has been highly overexpressed (25). On the other hand, previous reports have shown that p21 can negatively regulate p53 stability in different cell types in the context of a normal DNA damage response (34). In HT1080p21–9, neither p53 nor its targets genes, HDM2 and PUMA, were induced in response to p21 expression (Fig. 1E), suggesting that the cell death observed was not mediated by changes in p53 expression or activity. This was confirmed by inhibiting p53 up-regulation in HT1080p21–9 with a specific shRNA (supplemental Fig. 1A). p21-mediated cell death did not vary when p53 levels were reduced (supplemental Fig. 1B). These results were also reproduced using pifithrin, a reversible inhibitor of p53-dependent apoptosis and transcriptional activity (supplemental Fig. 1, C and D).
p21-dependent Increases in ROS Determine Induction of Cell Death
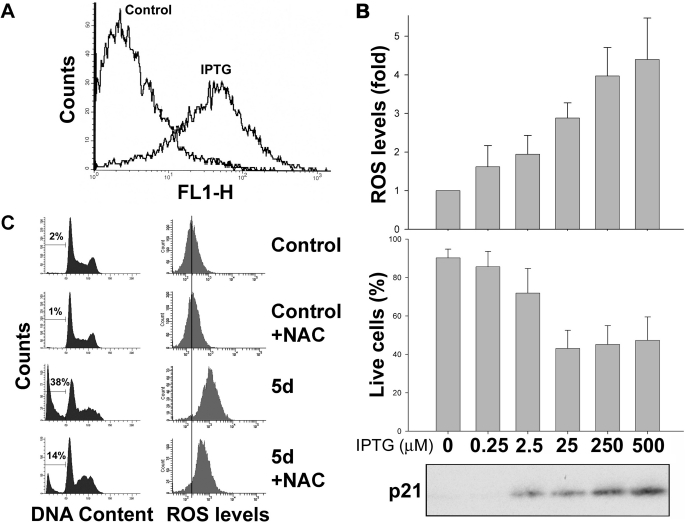
Intracellular ROS are important mediators of apoptosis (35), and it has been proposed that p21-induced ROS increases could determine cell fate decisions (25). To test whether ROS play a role in p21-induced cell death in HT1080p21–9, we first analyzed whether p21 could elevate ROS levels in these cells using DCF, a probe that fluoresces when oxidized. We found that intracellular ROS were substantially increased 5 days after p21 up-regulation (Fig. 2A). When the concentration of IPTG was titrated to control the amount of p21 expressed, levels of ROS varied accordingly (Fig. 2B, top panel). This confirms that ROS is induced in these cells proportionally to p21 protein levels, consistent with previous results obtained in bladder (15), colorectal, cervical, and ovarian cancer cell lines (25). Of note, no cell death or ROS changes were detected in the parental cell line HT1080 treated with any of the IPTG concentrations tested (data not shown), proving that IPTG itself was not responsible for the effect. Although senescent cells usually exhibit a degree of autofluorescence due to the accumulation of lipofuscin (36), this was not sufficient to influence the measurement of ROS (supplemental Fig. 2A). Moreover, bigger cells did not show higher DCF staining, indicating that cell size had no effect on dye accumulation (supplemental Fig. 2B).
FIGURE 2.
Increase in intracellular ROS correlates with p21 induction of apoptosis in HT1080p21–9. A, FACS analysis of DCF-stained HT1080p21–9, 5 days after exposure to 25 μm IPTG, compared with untreated cells. B, HT1080p21–9 exposed to from 0 to 500 μm IPTG for 5 days. Top panel, ROS levels compared with untreated cells, measured by DCF staining. Middle panel, percentage of live cells as measured by annexin/PI staining. Cells that were both annexin-negative and PI-negative (lower left quadrant) were considered alive. Bottom panel, immunoblot of p21 protein expression. C, left panels, FACS analysis of PI-stained HT1080p21–9, uninduced or 5 days after p21 up-regulation, in the presence or absence of 10 mm NAC. Cells were cultured in the presence of NAC for the duration of the experiment, and NAC was added every time the medium was changed. Right panels, FACS analysis of the same cells stained with DCF. The gray vertical line indicates mean fluorescence intensity of control cells.
By using the apoptosis-specific annexin/PI staining (30), we determined that a significant fraction of the p21-induced cell death in HT1080p21–9 was of apoptotic origin (Fig. 2B, bottom panel, and supplemental Fig. 2C ). This correlated with the cleavage of PARP, a hallmark of apoptosis (supplemental Fig. 2D). The induction of apoptosis also correlated with p21 protein and ROS levels (Fig. 2B), which suggests a causal link. We explored this possibility by inducing p21 in HT1080p21–9 for 5 days, in the presence of the antioxidant NAC, a reduced glutathione provider and direct ROS scavenger. As shown in Fig. 2C (left column), NAC ameliorated cell death after p21 expression in HT1080p21–9. This was confirmed with an annexin/PI staining (supplemental Fig. 2E). Non-toxic concentrations of NAC were capable of substantially reducing intracellular ROS, although not entirely (Fig. 2C, right column), which could explain why its protection against p21-dependent cell death was not complete. Also, p21 depletion causes mitotic crisis in HT1080p21–9, which could be responsible for ROS-independent death in a small percentage of cells that can lose p21 expression in culture spontaneously (26). Of note, NAC had no effect on p21 expression (supplemental Fig. 2F). These results show that p21-mediated increases in intracellular ROS are determinant in the induction of cell death in HT1080p21–9 after p21 expression and that this is, at least in part, due to the onset of apoptosis.
In contrast to these results, most studies of p21 overexpression in cancer cell lines have shown an induction of cell cycle arrest in the absence of cell death (15, 37). To explore whether this could be a cell type-specific response, we induced a prolonged p21 up-regulation in an U2OS osteosarcoma cell line in which p53 expression had been previously suppressed (U2OSp53−/−) (supplemental Fig. 3A). Similar to our observations in HT1080p21–9, p21 elevated intracellular ROS levels in these cells and induced a substantial increase in cell death (supplemental Fig. 3, B–D), which could also be ameliorated with antioxidants (supplemental Fig. 3E). This suggests that the induction of ROS-dependent cell death in response to p21 could be a common feature of sarcomas.
Comparison of Arrest and Cell Death Responses to p21 Expression
Our data indicate that cell-specific factors could be important to favor different cell fates after p21 up-regulation. To further explore this possibility, we studied the differences between arrest an apoptotic responses to p21 in cancer cells. Our previous reports using EJp21, a p53 mutant EJ human bladder cancer cell line with a Tet-regulatable p21 expression, have shown that p21 induces arrest, senescence, and intracellular ROS elevation in the absence of significant apoptosis (supplemental Fig. 4, A and B) (14, 15). This is also the case in PC3p21, a p53-null prostate cancer cell line in which a Tet-regulatable p21 expression system was introduced. Prolonged expression of p21 in these cells induced cell cycle arrest in the absence of significant death (supplemental Fig. 4C) (15).
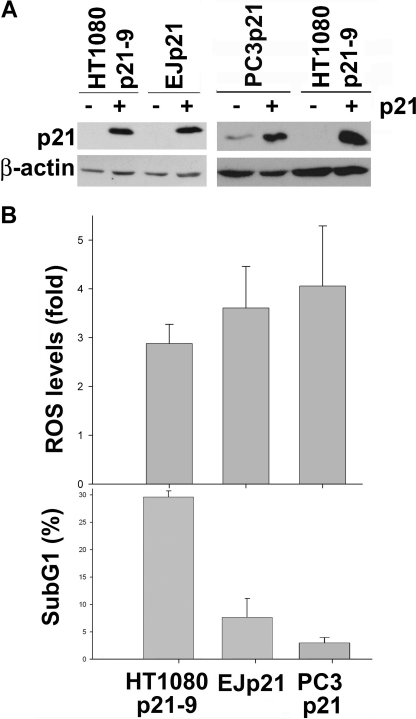
We first investigated whether the different responses to p21 of HT1080p21–9 and EJp21/PC3p21 could be due to variations in protein levels expressed and/or the intracellular ROS generated as a result. Fig. 3A shows a similar p21 up-regulation in these cells, which proves that protein levels did not determine the selective induction of cell death. These levels of p21 expression have been shown previously to be comparable with those induced after DNA damage (14), underscoring the physiological relevance of our results. ROS generated in HT1080p21–9 were slightly lower than those in induced EJp21 or PC3p21 (Fig. 3B, top panel). This indicates that the increased cell death observed in HT1080p21–9 (Fig. 3B, bottom panel) was not due to higher p21-mediated induction of ROS in these cells. These results support the hypothesis of cell-specific factors determining the sensitivity to p21 expression.
FIGURE 3.
Cell fate decisions after p21 up-regulation do not depend on differences in p21 expression or ROS levels. A, immunoblot analysis of HT1080p21–9, EJp21, and PC3p21 uninduced (−) and induced to express p21 for 5 days (+). B, top panel: intracellular ROS levels as measured by DCF staining of HT1080p21–9, EJp21, and PC3p21 cells induced for 5 days. Bars represent fold changes in mean fluorescence intensity from control to induced cells. Bottom panel, percentage of events in the sub-G1 phase, as measured by PI staining of the same cells.
Because HT1080/U2OS and EJ/PC3 are derived from mesenchymal and epithelial tumors, respectively, it is possible that cell origin could determine their general sensitivity to p21. As shown in supplemental Fig. 5A, EJp21 expressed mesenchymal markers such as vimentin and ZEB1 but not the epithelial marker E-cadherin. This suggests that they had undergone an epithelial-mesenchymal transition and that their characteristics are closer to those of mesenchymal cells. Thus, cellular responses to p21 are unlikely to be due only to the epithelial/mesenchymal differences of HT1080 and EJ. This was confirmed by the fact that p21 was also able to trigger cell death in ovarian (HA847) and breast (MDA-MB-175) cancer cell lines (supplemental Fig. 5B). Consistent with previous data (24, 25), this shows that p21-induced cell death is not limited to tumors of mesenchymal origin.
Involvement of Mitochondria in p21-induced Cell Death
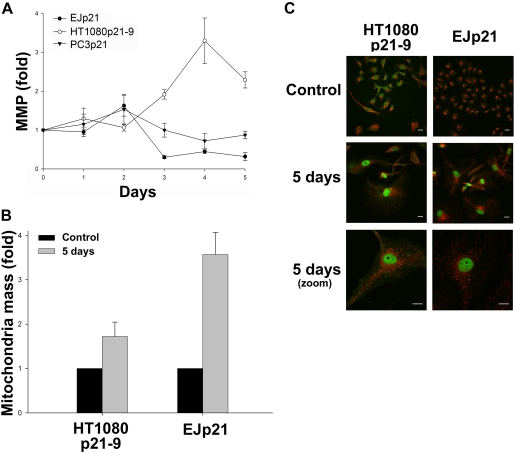
ROS-mediated changes in mitochondrial membrane potential have been implicated in apoptosis (38). Moreover, the fact that p21 triggers the expression of the mRNA of certain mitochondrial genes (33) suggests that the effects of p21 on mitochondria could play a role in the induction of cell death. We hypothesized that divergences in mitochondria responses after p21 up-regulation could explain a selective induction of apoptosis. To test this, we measured changes in mitochondrial membrane potential using TMRE, a cell-permeant fluorescent dye that accumulates in mitochondria depending on their membrane potential. As shown in Fig. 4A, a mild mitochondrial membrane hyperpolarization was evident in EJp21 and PC3p21 after 2 days of p21 expression, followed by a strong depolarization. This is consistent with previous reports showing mitochondrial membrane depolarization associated with senescence (19, 39, 40). On the other hand, HT1080p21–9 responded with a delayed and higher hyperpolarization that peaked at day 4 (Fig. 4A). Of note, mitochondria hyperpolarization has been previously linked to apoptosis in some models (41, 42). This shows that the mitochondrial response to p21 expression is substantially different in these two cell lines and suggests that this could determine the cellular sensitivity to p21-induced ROS.
FIGURE 4.
Mitochondrial differences between HT1080p21–9 and EJp21. A, mitochondrial membrane potential (MMP) as measured by fold changes in mean fluorescence intensity of TMRE-stained HT1080p21–9, EJp21, and PC3p21 with p21 expression induced for 1 to 5 days, normalized to values in uninduced cells. B, total mitochondrial mass in nonyl acrydil orange-stained HT1080p21–9 and EJp21 after 5 days of p21 induction, normalized to values in uninduced cells. C, confocal microscopy images (40×, bottom row magnified 2×) of HT1080p21–9 and EJp21 cells stained with MitoTracker Red CMXRos for mitochondria (red) and p21 expression (green). Scale bar, 20 μm.
An increase in mitochondrial mass has been described in cells undergoing senescence (39). Using the mitochondria-specific dye nonyl acrydil orange, we observed an augmented mitochondrial mass in induced EJp21 that was 2-fold higher than that in HT1080p21–9 (Fig. 4B). This was confirmed with another mitochondria-specific dye, MitoTracker Red, which also showed that EJp21 with induced p21 have a dense mitochondrial network, whereas mitochondria in HT1080p21–9 exhibit a more discrete punctuated pattern (Fig. 4C). It has been suggested that presence of cytoplasmic p21 may interfere with apoptosis (43), but induced p21 was only nuclear in both cell lines (Fig. 4C), indicating that differences in p21 localization did not contribute to the induction of apoptosis. These results show that the ratio of depolarized mitochondria is elevated in cells preferentially undergoing senescence after p21 expression, confirming that increased mitochondrial mass and depolarization are hallmarks of this process (39). Moreover, because elongated mitochondria have been recently associated with resistance to oxidative stress (44), our results suggest that HT1080p21–9 could be more sensitive to ROS due to the punctuated morphology of their mitochondria.
Higher Sensitivity of HT1080p21–9 Mitochondria to ROS Explains Preferential Induction of Apoptosis by p21
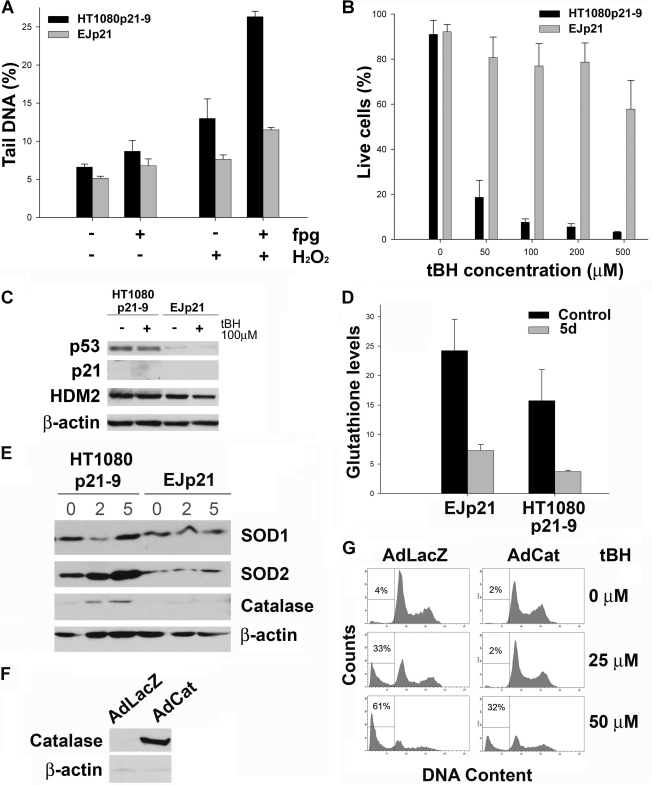
Our data shows that HT1080/U2OS and EJ/PC3 cells react differently to similar levels of p21 and ROS and suggest that these cells may have different mitochondrial resistances to oxidative stress, which could determine a preference for arrest or apoptosis after p21 up-regulation. To investigate this hypothesis, we measured the amount of oxidative damage to DNA in response to oxidants. As shown in Fig. 5A, the same concentration of peroxide induced a significantly higher oxidative DNA damage in HT1080p21–9 than in EJp21. We confirmed this result by treating HT1080p21–9 and EJp21 with increasing concentrations of the oxidant tBH, which is capable of triggering apoptosis. Annexin V/PI staining showed that EJp21 were significantly more resistant than HT1080p21–9 to similar concentrations of tBH (Fig. 5B). This was also the case in PC3p21 (supplemental Fig. 5C). The difference in cell viability was not due to oxidative stress-induced p53 in HT1080p21–9 because p53 or its target genes were not up-regulated in response to tBH (Fig. 5C).
FIGURE 5.
Increased sensitivity to ROS in HT1080p21–9. A, comet assay of HT1080p21–9 and EJp21 cells treated or not with 100 μm H2O2 for 10 min on ice right before analysis. Experiments were performed in the absence or presence of fpg to determine specific damage due to oxidation (the difference in tail DNA percentage between the two conditions). Controls show results using fpg buffer. Mean percentage of tail DNA from three experiments is plotted. Error bars represent S.D. Experiments were performed twice in triplicate. B, annexin/PI-stained uninduced HT1080p21–9 (black bars) and EJp21 cells (gray bars), 24 h after exposure to different concentrations of tBH. Percentages of live cells are plotted. C, immunoblot analysis of uninduced HT10180p21–9 and EJp21 untreated (−) or 24 h after being treated with 100 μm tBH for 2 h and washed thereafter with PBS (+). D, intracellular levels of total glutathione in EJp21 and HT1080p21–9 cells, uninduced (Control) and after 5 days of p21 expression (5d). E, immunoblot analysis of HT10180p21–9 and EJp21 uninduced or after 2 or 5 days of p21 expression. F, immunoblot analysis of uninduced HT10180p21–9 cells infected for 24 h with adenovirus expressing LacZ (AdLacZ, control) or catalase (AdCat). G, representative plots of FACS analysis of PI-stained uninduced HT1080p21–9 infected for 24 h with AdLacZ or AdCat after being treated with 25 or 50 μm tBH as described. Percentages indicate amount of events in sub-G1 phase of the cell cycle.
We further explored the different sensitivities of these cells to ROS increases by upregulating p53 expression using an adenovirus containing p53. It is known that p53 expression induces ROS and that this is a crucial component of its induction of apoptosis (45). As expected, EJp21 were more resistant to p53 than HT1080p21–9 (supplemental Fig. 6A). Of note, the levels of p53 protein achieved by adenoviral infection in the two cell lines were comparable (supplemental Fig. 6B).
These results together suggest that HT1080p21–9 have reduced tolerance to oxidative stress, which could explain its higher sensitivity to p21-induced ROS. This could be determined by a reduction in the levels of intracellular antioxidants in these cells. To explore this possibility, we measured the concentration of total glutathione, the main cellular ROS buffer. As shown in Fig. 5D, glutathione levels decreased in both EJp21 and HT1080p21–9 when p21 was induced, consistent with an increase in ROS. Importantly, both the basal and post-induction concentrations of intracellular glutathione were lower in HT1080p21–9, suggesting a reduced capacity to neutralize ROS of these cells when compared with EJp21. These data were supported by a p21-dependent increase in HT1080p21–9 of the expression of enzymes involved in the clearance of ROS, such as superoxide dismutases (SOD1 and SOD2) and catalase (Fig. 5E), which our results show that is still insufficient to block ROS and prevent cell death. Of note, these enzymes were not up-regulated in response to p21 in EJp21, suggesting that these cells probably rely on their higher glutathione levels as a primary mechanism to buffer ROS. When we used an adenorival system to increase the levels of a catalase specifically engineered to be expressed in mitochondria (Fig. 5F) (28), induction of cell death in HT1080p21–9 in response to oxidants was reduced importantly (Fig. 5G). Expression of a cytoplasmic catalase did not change the levels of cell death (data not shown). These results indicate that the levels of intracellular antioxidant mechanisms could determine the sensitivity to p21 expression and that protecting mitochondria against ROS can enhance the resistance to cell death.
We also observed that catalase expression in EJp21 did not change the response of these cells to p21 induction (supplemental Fig. 6. C and D). However, inhibition of catalase expression in EJp21 increased cell death in response to p21 (supplemental Fig. 6, E and F). This confirms that sensitivity to oxidative stress is determinant in induction of p21-mediated cell death. In summary, our data establish that HT1080p21–9 and EJp21 differ in the sensitivity of their mitochondria to oxidative stress, which provides a mechanistic explanation for the different responses to p21-mediated increases in intracellular ROS.
DISCUSSION
Despite its promising potential, there are no antineoplastic therapies specifically directed at up-regulating p21 expression in cancer cells. This is in part due to the fact that the pleiotropic effects of p21 are not understood completely. Indeed, although p21 can induce a permanent cell cycle arrest in cancer cells and thus inhibit tumor growth, there is also the possibility of pro-oncogenic side effects (23, 33). Moreover, arresting cells can protect them against DNA-damage induced apoptosis and thus promote transformation (22). The antitumoural functions of p21 could be enhanced if its recently discovered abilities to cause cell death were favored over induction of arrest. Thus, strategies that upregulate p21 and promote its apoptotic functions in cancer cells could be an effective therapeutic approach.
In our attempt to characterize the mechanisms that define cell fate decisions after p21 expression, we uncovered that p21 can trigger cell death in the sarcoma cell lines HT1080 and U2OS. We found this to be p53-independent, cell type-specific, and, at least in part, ROS-dependent and of apoptotic nature. Although it has been shown before that HT1080p21–9 undergo death by mitotic crises after p21 withdrawal (26), this is the first report of p21-dependent apoptosis in these cells. Apoptosis upon p21 induction in HT1080 p21–9 cells was not seen in earlier studies (26), and cell death upon p21 induction varies depending on the stock of the cell line and cell culture conditions.3 In the current experiments, this response was consistent, with the percentage of cell death ranging from 22 to 38% (see Figs. 1C, 2C, and 3B). We did not observe the presence of mitotic cells in HT1080p21–9 once p21 was induced (data not shown), consistent with previous studies (26).
Our results provide evidence that the elevation of intracellular ROS levels is an important part of the mechanism by which p21 can induce apoptosis, and that this is likely due to their effects on mitochondria. How p21 expression affects the oxidative balance of the cell has been investigated for years, but there are still no conclusive observations. In our experiments, induction of cell death was not immediate and required prolonged expression of p21. This could reflect the necessity to accumulate sufficient intracellular ROS to trigger a certain amount of mitochondrial damage. According to this hypothesis, short term expression of p21 at physiologically relevant levels would induce cell cycle arrest, whereas apoptosis would only be achieved at a later time point if the stimulus is maintained, and a threshold of oxidative stress is surpassed. Also, our results indicate that this would be more likely to occur in cells that have increased mitochondrial sensitivity to ROS; otherwise, cells would undergo a less drastic response in the form of senescence. Our results are also consistent with the idea that p21 levels could have a dose-dependent effect in cell fate decisions (25) because apoptosis increased proportionally to p21 and the induction of ROS (see Fig. 2B). However, we showed that protein levels are not necessary determinant because similar p21 induction caused different effects depending on cell context.
Identifying cells that undergo apoptosis after prolonged p21 expression could help selecting tumor types that would be more susceptible to p21-based treatments. The intrinsic features of mesenchymal cells could make them more sensitive to p21-induced ROS, although this would probably be only one of the defining characteristics (see supplemental Fig. 5, A and B). Our results suggest that the apoptotic functions of p21 could be preferentially observed in those cancer cells that have accumulated higher mitochondrial damage or defects in the intracellular/mitochondrial ROS buffers. This is consistent with recent data showing that cancer cells with primed mitochondria respond better to cell death stimuli (46). Because normal cells usually have intact antioxidant and DNA repair mechanisms, therapies that up-regulate p21 could have the potential to be selectively toxic for sensitive cancer cells.
Recent studies show that cancer cells that die after p21 up-regulation are not a rare event (24, 25), which supports the hypothesis that inducing p21-mediated apoptosis could be a relevant form of therapy. Moreover, within the physiological levels of p21 expression tested in this study, we found that there was no increase in p53 expression or activity to account for the induction of apoptosis, indicating that p53-null cancers would benefit from these treatments. This suggests that compounds that induce p21 independently of p53, such as MLN4924 (47), could trigger cell death in certain cancer types. Our data support the concept that chemical up-regulation of p21 could be a useful therapeutic approach in selected tumors and that the mitochondrial response to ROS could be a good predictive marker of cancer cell sensitivity to p21.
Supplementary Material
Acknowledgments
We thank S. A. Aaronson for help in this project. We thank S. Cowley and D. Critchley for useful discussions and critical reading of the manuscript. We also thank K. Straatman and the Advanced Image Facilities for assistance with immunofluorescence experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AG028687 (to I. B. R.). This work was also supported by New Blood Lectureship Research Support funding from the Medical Research Council (to S. M.), Consejo Nacional de Ciencia y Tecnologia (to S. C.), and the Swedish Cancer Society Grant Cancerfonden 2007/649 (to P. J. d. V.).

This article contains supplemental Figs. 1–6.
I. B. Roninson, unpublished results.
- ROS
- reactive oxygen species
- NAC
- N-acetyl-l-cysteine
- tBH
- tert-butyl-hydroperoxide
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- PI
- propidium iodide
- DCF
- 2′,7′-difluorodihydrofluorescein diacetate
- TMRE
- tetramethylrhodamine, ethyl ester, perchlorate
- Tet
- tetracycline
- DCF
- 2′,7′-difluorodihydrofluorescein.
REFERENCES
- 1. Sherr C. J., Roberts J. M. (1999) CDK inhibitors: Positive and negative regulators of G1 phase progression. Genes Dev. 13, 1501–1512 [DOI] [PubMed] [Google Scholar]
- 2. el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 3. Brugarolas J., Chandrasekaran C., Gordon J. I., Beach D., Jacks T., Hannon G. J. (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377, 552–557 [DOI] [PubMed] [Google Scholar]
- 4. Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. (1995) Mice lacking p21CIP1/WAF1 undergo normal development but are defective in G1 checkpoint control. Cell 82, 675–684 [DOI] [PubMed] [Google Scholar]
- 5. Gartel A. L., Tyner A. L. (1999) Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp. Cell Res. 246, 280–289 [DOI] [PubMed] [Google Scholar]
- 6. Richon V. M., Sandhoff T. W., Rifkind R. A., Marks P. A. (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U.S.A. 97, 10014–10019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gartel A. L., Najmabadi F., Goufman E., Tyner A. L. (2000) A role for E2F1 in Ras activation of p21(WAF1/CIP1) transcription. Oncogene 19, 961–964 [DOI] [PubMed] [Google Scholar]
- 8. Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211, 90–98 [DOI] [PubMed] [Google Scholar]
- 9. Campisi J., Kim S. H., Lim C. S., Rubio M. (2001) Cellular senescence, cancer, and aging: The telomere connection. Exp. Gerontol. 36, 1619–1637 [DOI] [PubMed] [Google Scholar]
- 10. Hayflick L., Moorhead P. (1961) Exp. Cell Res. 25, 585–621 [DOI] [PubMed] [Google Scholar]
- 11. Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dulic V., Beney G. E., Frebourg G., Drullinger L. F., Stein G. H. (2000) Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Molecular and cellular biology 20, 6741–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang B. D., Xuan Y., Broude E. V., Zhu H., Schott B., Fang J., Roninson I. B. (1999) Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18, 4808–4818 [DOI] [PubMed] [Google Scholar]
- 14. Fang L., Igarashi M., Leung J., Sugrue M. M., Lee S. W., Aaronson S. A. (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 18, 2789–2797 [DOI] [PubMed] [Google Scholar]
- 15. Macip S., Igarashi M., Fang L., Chen A., Pan Z. Q., Lee S. W., Aaronson S. A. (2002) Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 21, 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bond J. A., Wyllie F. S., Wynford-Thomas D. (1994) Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9, 1885–1889 [PubMed] [Google Scholar]
- 17. Barzilai A., Yamamoto K. (2004) DNA damage responses to oxidative stress. DNA Repair 3, 1109–1115 [DOI] [PubMed] [Google Scholar]
- 18. Macip S., Igarashi M., Berggren P., Yu J., Lee S. W., Aaronson S. A. (2003) Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol. Cell. Biol. 23, 8576–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Passos J. F., Nelson G., Wang C., Richter T., Simillion C., Proctor C. J., Miwa S., Olijslagers S., Hallinan J., Wipat A., Saretzki G., Rudolph K. L., Kirkwood T. B., von Zglinicki T. (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borgdorff V., Lleonart M. E., Bishop C. L., Fessart D., Bergin A. H., Overhoff M. G., Beach D. H. (2010) Multiple microRNAs rescue from Ras-induced senescence by inhibiting p21(Waf1/Cip1). Oncogene 29, 2262–2271 [DOI] [PubMed] [Google Scholar]
- 21. Wu S., Huang S., Ding J., Zhao Y., Liang L., Liu T., Zhan R., He X. (2010) Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′-untranslated region. Oncogene 29, 2302–2308 [DOI] [PubMed] [Google Scholar]
- 22. Abbas T., Dutta A. (2009) p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roninson I. B. (2002) Oncogenic functions of tumor suppressor p21(Waf1/Cip1/Sdi1): Association with cell senescence and tumor-promoting activities of stromal fibroblasts. Cancer Lett. 179, 1–14 [DOI] [PubMed] [Google Scholar]
- 24. Gartel A. L. (2005) The conflicting roles of the CDK inhibitor p21(CIP1/WAF1) in apoptosis. Leuk. Res. 29, 1237–1238 [DOI] [PubMed] [Google Scholar]
- 25. Inoue T., Kato K., Kato H., Asanoma K., Kuboyama A., Ueoka Y., Yamaguchi S. I., Ohgami T., Wake N. (2009) Level of reactive oxygen species induced by p21Waf1/CIP1 is critical for the determination of cell fate. Cancer Sci. 100, 1275–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang B. D., Broude E. V., Fang J., Kalinichenko T. V., Abdryashitov R., Poole J. C., Roninson I. B. (2000) p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene 19, 2165–2170 [DOI] [PubMed] [Google Scholar]
- 27. Ivanov G. S., Ivanova T., Kurash J., Ivanov A., Chuikov S., Gizatullin F., Herrera-Medina E. M., Rauscher F., 3rd, Reinberg D., Barlev N. A. (2007) Methylation-acetylation interplay activates p53 in response to DNA damage. Mol. Cell. Biol. 27, 6756–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai J., Cederbaum A. I. (2001) Adenovirus-mediated overexpression of catalase in the cytosolic or mitochondrial compartment protects against cytochrome P450 2E1-dependent toxicity in HepG2 cells. J. Biol. Chem. 276, 4315–4321 [DOI] [PubMed] [Google Scholar]
- 29. Pearce L., Morgan L., Lin T. T., Hewamana S., Matthews R. J., Deaglio S., Rowntree C., Fegan C., Pepper C., Brennan P. (2010) Genetic modification of primary chronic lymphocytic leukemia cells with a lentivirus expressing CD38. Haematologica 95, 514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aubry J. P., Blaecke A., Lecoanet-Henchoz S., Jeannin P., Herbault N., Caron G., Moine V., Bonnefoy J. Y. (1999) Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry 37, 197–204 [DOI] [PubMed] [Google Scholar]
- 31. Duarte T. L., Cooke M. S., Jones G. D. (2009) Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic. Biol. Med. 46, 78–87 [DOI] [PubMed] [Google Scholar]
- 32. Carrera S., de Verdier P. J., Khan Z., Zhao B., Mahale A., Bowman K. J., Zainol M., Jones G. D., Lee S. W., Aaronson S. A., Macip S. (2010) Protection of cells in physiological oxygen tensions against DNA damage-induced apoptosis. J. Biol. Chem. 285, 13658–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang B. D., Watanabe K., Broude E. V., Fang J., Poole J. C., Kalinichenko T. V., Roninson I. B. (2000) Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc. Natl. Acad. Sci. U.S.A. 97, 4291–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broude E. V., Demidenko Z. N., Vivo C., Swift M. E., Davis B. M., Blagosklonny M. V., Roninson I. B. (2007) p21 (CDKN1A) is a negative regulator of p53 stability. Cell Cycle 6, 1468–1471 [PubMed] [Google Scholar]
- 35. Johnson T. M., Yu Z. X., Ferrans V. J., Lowenstein R. A., Finkel T. (1996) Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. U.S.A. 93, 11848–11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jongkind J. F., Verkerk A., Visser W. J., Van Dongen J. M. (1982) Isolation of autofluorescent “aged” human fibroblasts by flow sorting. Morphology, enzyme activity, and proliferative capacity. Exp. Cell Res. 138, 409–417 [DOI] [PubMed] [Google Scholar]
- 37. Chen X., Ko L. J., Jayaraman L., Prives C. (1996) p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10, 2438–2451 [DOI] [PubMed] [Google Scholar]
- 38. Li P. F., Dietz R., von Harsdorf R. (1999) p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 18, 6027–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ksiazek K., Passos J. F., Olijslagers S., von Zglinicki T. (2008) Mitochondrial dysfunction is a possible cause of accelerated senescence of mesothelial cells exposed to high glucose. Biochem. Biophys. Res. Commun. 366, 793–799 [DOI] [PubMed] [Google Scholar]
- 40. Sugrue M. M., Wang Y., Rideout H. J., Chalmers-Redman R. M., Tatton W. G. (1999) Reduced mitochondrial membrane potential and altered responsiveness of a mitochondrial membrane megachannel in p53-induced senescence. Biochem. Biophys. Res. Commun. 261, 123–130 [DOI] [PubMed] [Google Scholar]
- 41. Giovannini C., Matarrese P., Scazzocchio B., Sanchez M., Masella R., Malorni W. (2002) Mitochondria hyperpolarization is an early event in oxidized low density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS Lett. 523, 200–206 [DOI] [PubMed] [Google Scholar]
- 42. Paglin S., Lee N. Y., Nakar C., Fitzgerald M., Plotkin J., Deuel B., Hackett N., McMahill M., Sphicas E., Lampen N., Yahalom J. (2005) Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 65, 11061–11070 [DOI] [PubMed] [Google Scholar]
- 43. Blagosklonny M. V. (2002) Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle 1, 391–393 [DOI] [PubMed] [Google Scholar]
- 44. Mai S., Klinkenberg M., Auburger G., Bereiter-Hahn J., Jendrach M. (2010) Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J. Cell Sci. 123, 917–926 [DOI] [PubMed] [Google Scholar]
- 45. Polyak K., Xia Y., Zweier J. L., Kinzler K. W., Vogelstein B. (1997) A model for p53-induced apoptosis. Nature 389, 300–305 [DOI] [PubMed] [Google Scholar]
- 46. Ni Chonghaile T., Sarosiek K. A., Vo T. T., Ryan J. A., Tammareddi A., Moore Vdel G., Deng J., Anderson K. C., Richardson P., Tai Y. T., Mitsiades C. S., Matulonis U. A., Drapkin R., Stone R., Deangelo D. J., McConkey D. J., Sallan S. E., Silverman L., Hirsch M. S., Carrasco D. R., Letai A. (2011) Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jia L., Li H., Sun Y. (2011) Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia 13, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.