Abstract
Oxidative stress and glucose affect the expression of various genes that contribute to both reactive oxygen species generation and antioxidant systems. However, systemic alteration of oxidative stress-related gene expression in normal brains and in brains with a high-glucose status after ischemic reperfusion has not been explored. Using a polymerase chain reaction array system, we demonstrate that thioredoxin-interacting protein (Txnip) is induced by both oxidative stress and glucose. We found that Txnip mRNA is induced by ischemic-reperfusion injury and that Txnip is located in the cytoplasm of neurons. Moreover, in vitro oxygen-glucose deprivation (OGD) and subsequent reoxygenation without glucose and in vivo administration of 3-nitropropionic acid also promoted an increase in Txnip in a time-dependent manner, indicating that oxidative stress without glucose can induce Txnip expression in the brain. However, calcium channel blockers inhibit induction of Txnip after OGD and reoxygenation. Using the polymerase chain reaction array with ischemic and hyperglycemic-ischemic samples, we confirmed that enhanced expression of Txnip was observed in hyperglycemic-ischemic brains after middle cerebral artery occlusion. Finally, transfection of Txnip small interfering RNA into primary neurons reduced lactate dehydrogenase release after OGD and reoxygenation. This is the first report showing that Txnip expression is induced in neurons after oxidative or glucose stress under either ischemic or hyperglycemic-ischemic conditions, and that Txnip is proapoptotic under these conditions.
Keywords: Middle cerebral artery occlusion, Oxygen-glucose deprivation, Polymerase chain reaction array, Reactive oxygen species, Thioredoxin, Thioredoxin-interacting protein
Introduction
Ischemic brain injury is associated with the production of reactive oxygen species (ROS) during reperfusion injury (Jung et al., 2010; Kim et al., 2009). ROS-generating systems, such as the electron transport chain in mitochondria and NADPH oxidase (NOX) in brains, are activated (Kim et al., 2009; Moro et al., 2005) and lead to oxidative stress. Excessive ROS production impairs various cellular components, including DNA, proteins, and lipids, and aggravates cell death after ischemic-reperfusion injury (Valko et al., 2007). Hence, reperfusion injury plays an important role in oxidative damage after ischemia/reperfusion. Hyperglycemia enhances ROS that are associated with exacerbated neuronal damage after ischemia/reperfusion (Kamada et al., 2007; Mehta et al., 2011). Thus, preventing ROS generation by inhibiting oxidases, or scavenging newly-formed ROS with antioxidant agents or endogenous antioxidants, such as thioredoxin (Trx), peroxiredoxin, and glutathione, is important as potential therapeutic strategies for treating stroke (Hattori et al., 2004; Hwang et al., 2010). The antioxidant system is tightly regulated to maintain the redox balance (Valko et al., 2007) and endogenous antioxidants can be rapidly used for defense against harmful ROS that are then degraded (Bulkley, 1987; Chan, 2001).
In the thiol-reduction system, Trx is ubiquitous and effectively works in cells to control redox (Powis and Montfort, 2001). With the help of peroxiredoxin, the antioxidant effects of Trx are enhanced and detoxify ROS (Rhee et al., 2005). This system consists of Trx, Trx reductase, and NADPH. Trx is 12 kDa (Koháryová and Kollárová, 2008) and was first isolated from Escherichia coli. The redox-active center of Trx interacts with oxidized proteins to reduce these proteins by forming a disulfide bond (Patwari et al., 2006). A study that used transgenic mice that overexpress Trx, showed that Trx is neuroprotective against ischemic brain damage (Takagi et al., 1999), suggesting that it has a potent antioxidant role and blocks the detrimental actions of ROS in the brain. Trx also participates in signal transduction by interaction with apoptosis signal-regulating kinase 1, which involves various biological processes in various cells (Saitoh et al., 1998; Watson, et al., 2004).
A novel intracellular endogenous inhibitor of Trx, Trx-interacting protein (Txnip), was identified by Schulze et al. (2004). Txnip was first known by the names of vitamin D3-up-regulated protein-1 and Trx binding protein-2 (Chen and DeLuca, 1994; Schulze et al., 2002). Using the yeast two-hybrid system, Yamanka and colleagues (2000) found that there was a possible interaction between Trx and vitamin D3-up-regulated protein-1 in HeLa cells. An emerging body of evidence suggests that Txnip inhibits Trx function in cells by directly binding with reduced Trx, forming stable, mixed sulfide (Patwari et al., 2006). This stable sulfide, with the catalytic motif of Trx, dramatically reduces Trx binding to other oxidized proteins. Txnip expression is affected by glucose stress, and carbohydrate response elements are located in the promoter of Txnip (Cha-Molstad et al., 2009). Txnip is believed to be strongly induced by glucose in diabetes mellitus (Shalev et al., 2002). Not only is Txnip induced by glucose, but it is associated with enhanced cell death because of accumulation of ROS mediated by Trx/Txnip interaction (Minn et al., 2005). Reports have shown that Txnip can be induced by the glucose status in many cell types, but it is still unknown if other signaling molecules, such as ROS or calcium, can induce Txnip expression after ischemic-reperfusion injury in the brain.
Using a polymerase chain reaction (PCR) array system focused on 98 genes related to oxidative stress in ischemic-reperfusion and hyperglycemic-ischemia mouse models, we show for the first time that Txnip is an inducible gene product of both oxidative stress and glucose stress. We observed that Txnip is markedly expressed in neurons after oxidative and glucose stress and that calcium is involved in the increase in Txnip expression. Our study provides evidence that down-regulation of Txnip can be effective in preventing neuronal cell death induced by ischemic reperfusion or hyperglycemic-ischemic reperfusion.
Material and methods
Transient focal cerebral ischemia
All experiments with mice were performed in accordance with National Institutes of Health guidelines, and the animal protocols were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. We used CD1 mice (2-month-old males, 30–35 g) that were purchased from Charles River Laboratories (Wilmington, MA). The mice were anesthetized with 1.5% isoflurane in 70% nitrous oxide and 30% oxygen and maintained with 1.5% isoflurane during surgery. Rectal temperature was controlled with a homeothermic blanket and kept at 37 °C. The left common carotid artery was exposed and a 6-0 suture, blunted at the tip with an electrocoagulator, was introduced into the internal carotid artery through the external carotid artery stump. After 30 or 45 min of occlusion, cerebral blood flow was resumed by the careful removal of the suture. Physiological parameters were monitored throughout the surgeries. The animals were allowed to recover until sampling at various time points for each experiment after reperfusion. Sham controls underwent the same procedure without insertion of the suture and occlusion of the vessels (Chen et al., 2011).
Streptozotocin-induced hyperglycemic model
Hyperglycemia was induced in mice 2 months of age by an injection of streptozotocin (STZ) (240 mg/kg) (Sigma-Aldrich, St. Louis, MO) dissolved in sodium citrate buffer. Three days after injection, the blood glucose level was measured with a OneTouch Ultra device (LifeScan, Inc., Milpitas, CA) using the blood from a tail snip (Kamada et al., 2007).
Oxygen-glucose deprivation and reoxygenation
The primary neuronal cell plates were subjected to oxygen-glucose deprivation (OGD) by replacing the medium with buffered salt solution without glucose and were placed in a gas-tight humidified anoxic chamber (Plas Labs, Inc., Lansing, MI) at 37 °C for 4 h. For calcium channel inhibitor experiments, verapamil was purchased from MP Biomedicals (Solon, OH) and diltiazem and 2-aminoethoxydiphenyl borate (2-APB) were purchased from Sigma-Aldrich. Cells were treated with the three calcium channel blockers right before OGD, after which the medium was changed to a glucose-free Neurobasal medium and the cells were reoxygenated for 24 h. A lactate dehydrogenase (LDH) assay (BioVision, Mountain View, CA) and a WST-1 assay (Roche Diagnostics, Indianapolis, IN) were performed according to the manufacturers’ instructions.
Primary cortical neuronal culture
Pregnant CD1 mice were used for the neuronal culture and were anesthetized by inhalation with isoflurane. At embryonic day 16, mouse cerebral cortices were isolated in ice-cold minimal essential medium (Invitrogen, Carlsbad, CA). Tissue was minced and digested with trypsin for 2 min. Cells were grown on Millicoat poly-D-lysine coated 6-well plates (Millipore, Billerica, MA) in minimal essential medium supplemented with 5% horse serum, GlutaMAX (2 mM), and D-glucose (25 mM). Cultures were maintained in a humidified 5% CO2 incubator at 37 °C. On day 2, the medium was changed to Neurobasal medium containing B27 supplement and glutamate. Experiments were performed on neurons grown in culture for 7 days.
Small interfering RNA transfection
Primary neuronal cells were grown on 6-well or 48-well plates, pre-coated with poly-D-lysine, in Neurobasal medium containing B27. The cells were then transfected for 48 h with 25 nM small interfering RNA (siRNA) using HiPerFect transfection reagent (QIAGEN, Valencia, CA). The transfected cells were subjected to OGD and allowed to reoxygenate in glucose-free medium. Negative control siRNA (46-2002; Invitrogen) that does not target any mRNA sequence was used as a control. Txnip siRNA (TXNIPMSS226056) (RNA- UCC UCC UUG CUA UAU GGA CAU CAU U) was purchased from Invitrogen.
Injection of 3-nitropropionic acid
The mice were anesthetized with 1.5% isoflurane in 70% nitrous oxide and 30% oxygen and were put onto a stereotactic apparatus. A hole was drilled in the skull. 3-Nitropropionic acid (3-NP) (71.4 mg/ml, 0.5 μl) (Sigma-Aldrich) was injected into the striatum (bregma: 2.0 mm lateral, 0.5 mm anterior, 3.3 mm deep) over 7 min. PBS was used as a vehicle.
Measurement of infarction volume
Twenty-four hours after reperfusion, the mice were killed and the brains were quickly isolated and chilled in ice-cold PBS. The brains were sliced coronally at 1-mm intervals using a mouse brain matrix. Individual slices were then incubated in 2% 2,3,5-triphenyltetrazolium chloride in 0.1 mol/L PBS (pH adjusted to 7.4) for 30 min followed by 3.7% formalin overnight. The total infarct volume was quantified using Adobe Photoshop (Adobe Systems, San Jose, CA).
Immunofluorescent staining
The animals were cardially perfused with heparinized (10 U/ml) saline and subsequently with 4% formaldehyde in PBS. The brains were sectioned at 50 μm using a vibratome and the slices were stored at −20 °C. The sections were washed three times with Tris-buffered saline (TBS) and were then blocked with TBS-blocking solution (1% bovine serum albumin, 0.2% skim milk, and 0.3% Triton X-100 in TBS) for 1 h and incubated in primary antibodies in TBS-blocking solution overnight on a shaker at 4 °C. These sections were washed three times with TBS and then incubated in secondary antibodies conjugated with Alexa dyes (1:250; Invitrogen) for 2 h. Sections were rinsed three times with TBS and were mounted on glass slides and covered with mounting medium containing 4′,6 diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA).
Western blot analysis
Brain tissue from the ipsilateral hemisphere was homogenized in ice-cold lysis buffer containing 10 mM HEPES-KOH pH 7.5, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 7.5 mM MgCl2, 2 mM EGTA, 1% Triton X-100, 0.7% protease inhibitor cocktail, and 1% phosphatase inhibitor cocktail 1 and 2 (Sigma-Aldrich). The tissue homogenates were centrifuged at 4 °C for 20 min at 10000 × g, and the supernatant was transferred to a fresh tube. Protein concentrations were determined by the bicinchoninic acid method using bovine serum albumin as a standard. Thirty micrograms of protein extracts were loaded and run on a NuPAGE Bis-Tris gel (4–12%) (Invitrogen). These proteins were then transferred to a polyvinylidene fluoride membrane that was incubated with blocking solution (5% skim milk in PBS/Tween 20) for 1 h, followed by incubation with a primary antibody overnight at 4 °C. After washing three times, the membrane was incubated with a secondary antibody that was conjugated with horseradish peroxidase for 1 h at room temperature. The signals were visualized using a chemiluminescence kit (GE Healthcare, Piscataway, NJ). Primary antibodies and titers used in this study are as follows: anti-Txnip antibody (Invitrogen), anti-3-nitrotyrosine antibody (Exalpha Biologicals, Shirley, MA), anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) antibody (Imgenex, San Diego, CA), and anti-β-tubulin antibody (Sigma-Aldrich). Horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Total RNA isolation
Total RNA isolation was performed using a Trizol reagent (Invitrogen) and purified with QIAGEN RNAeasy column following the manufacturer’s instructions. Brain tissues were homogenized with the Trizol reagent, and chloroform was added to brain homogenates. After centrifugation at 15000 × g for 10 min, the top layer of the supernatant was collected and applied to QIAGEN RNAeasy column to further purify the RNA. The amount and purity were quantified at absorbance ratios of 260 and 280 nm.
Profiling of gene expression
cDNA synthesis was performed using an RT2 First Strand Kit (QIAGEN). The Oxidative Stress and Antioxidant Defense PCR array (PAMM-065; QIAGEN) was performed according to instructions from the manufacturer, and quantitative real-time-PCR was performed with an Mx300P QPCR system (Stratagene, La Jolla, CA) using a cycling program with initial heating for 10 min at 95 °C and 40 cycles for 15 s at 95 °C and 1 min at 60 °C. The ΔΔCt method was used for data analysis, which was performed using the web-based tools provided by the manufacturer.
Statistical analysis
All data represent mean ± S.E.M. Statistical analysis was performed using Student’s t-test or analysis of variance (SigmaPlot; Systat Software, Chicago, IL) and a p-value of < 0.05 was accepted as statistically significant.
Results
Systemic changes in oxidative-related genes and up-regulation of the Txnip gene after ischemic-reperfusion injury
Oxidative stress can be generated and modulated by the actions of various gene products after ischemic injury, in turn altering their mRNA levels by the activity of transcriptional factors and their protein levels by degradation. Thus, the transcriptional changes in antioxidation and ROS-generating systems in oxidative stress-induced injury, especially in the ischemic brain, need to be explored. We used PCR array tools to elucidate the systemic changes in oxidative-related genes to isolate candidate genes responsible for ROS generation and antioxidant system maintenance. The mouse Oxidative Stress and Antioxidant Defense PCR array can simultaneously detect changes in 84 genes related to oxidative stress and defense mechanisms, such as glutathione peroxidases, peroxiredoxins, and superoxide dismutases. Using this PCR array, we compared the transcriptional changes in genes between sham controls and the damaged brains 6 h after ischemia and reperfusion. Among the 84 oxidative stress-related genes, 38 gene transcripts were up-regulated and 46 gene transcripts were down-regulated by ischemic-reperfusion injury (Fig. 1A). The group of down-regulated genes includes glutathione peroxidase 2, 3, and 4 and Trx reductase 1, 2, and 3. The NOX-related genes (NOX1, NOX4, NOX activator 1), prostaglandin-endoperoxide synthase 1 and 2, and glutathione peroxidase 5, 6, and 7 were up-regulated by ischemic damage. Seventeen gene mRNAs in the brains after ischemic injury were up-regulated more than 1.5-fold, compared with sham controls, including the mRNAs of myeloperoxidase (Mpo), NOX4, Ptgs2, Txnip, and extracellular superoxide-dismutase (SOD3) (Fig. 1B). Among them, the level of Txnip mRNA was markedly increased by 2.06-fold in brains that underwent middle cerebral artery occlusion (MCAO) and 6 h of reperfusion, compared with control brains, with a statistical significance of p = 0.026 (Fig. 1C, n = 3). These data indicate that ischemic and oxidative stressors induce changes in the mRNA of oxidative-related genes and specifically an increase in Txnip mRNA after brain damage.
Fig. 1.
Up-regulation of the Txnip gene after ischemic-reperfusion (I/R) injury. A real-time PCR-based array was performed to explore the overall changes in oxidative-related genes using samples of ischemic-damaged brains and sham brains (S). A) Among 84 genes, 38 were up-regulated and 46 gene transcripts were down-regulated 6 h after ischemic-reperfusion injury (n = 3). B) Among 46 genes, 17 were up-regulated over 1.5-fold compared with shams. These genes include Mpo, SOD3, NOX4, Txnip, and Ptgs2. C) Txnip mRNA was induced by 2.06-fold compared with sham controls (n = 3; *p = 0.026).
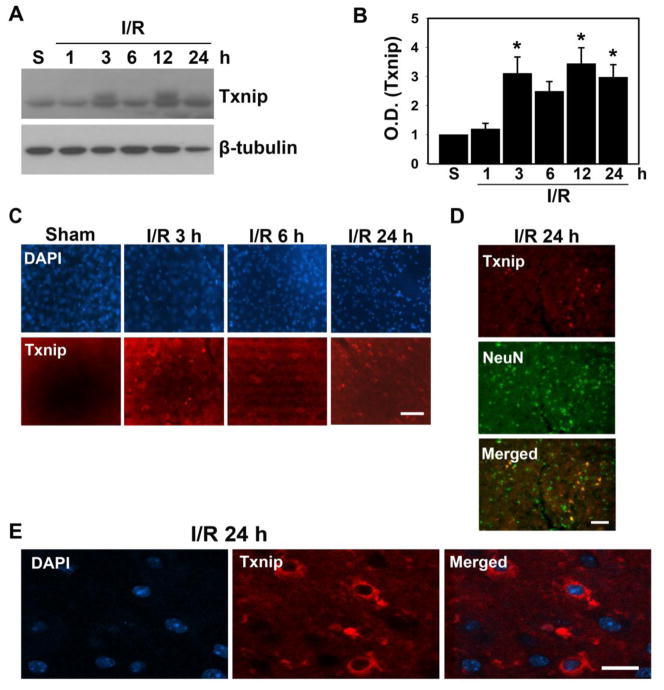
Txnip is induced by ischemic-reperfusion damage
We decided to focus on Txnip mRNA and protein levels after brain damage. An emerging body of evidence suggests that Txnip is induced by stimulation of glucose in various cells and tissue, but the level of Txnip expression in neurons and its role in the brain after ischemic-reperfusion injury are unknown. We also found that among many oxidative-related genes, Txnip mRNA was significantly changed by ischemic damage. After inducing transient focal cerebral ischemia in mouse brains using 45 min of MCAO, we examined changes in the levels of Txnip. Brain samples were collected at various time points after reperfusion. Western blotting and immunofluorescent staining were performed to detect changes in Txnip levels, which began to increase significantly at 3 h and persisted 12 and 24 h after reperfusion (Fig. 2A, B). In the damaged cortex, Txnip-positive cells increased 3, 6, and 24 h after ischemic reperfusion, as assessed by immunofluorescent staining (Fig. 2C). We found that most Txnip-positive cells were colocalized with a neuronal marker, NeuN, indicating that neurons in the damaged brains are responsible for inducing Txnip after ischemic reperfusion (Fig. 2D). Confocal microscopy showed that Txnip was increased 24 h after ischemic-reperfusion injury in the cytoplasm of neurons, but was not seen in nuclei (Fig. 2E). These data suggest that Txnip is expressed and active in the cytoplasm of neurons.
Fig. 2.
Txnip is induced by ischemic-reperfusion (I/R) injury in mice. Transient cerebral ischemia was induced by MCAO and the brains were isolated at various times after reperfusion. A) Western blot analysis was performed using sham (S) samples 1, 3, 6, 12, and 24 h after I/R with a Txnip antibody. β-tubulin was used as a loading control (n = 6). B) This graph shows that Txnip expression was enhanced 3, 6, 12, and 24 h after ischemic brain injury compared with the sham controls (n = 6; *p < 0.05). C) Immunofluorescence images show that Txnip-positive cells were increased by I/R injury (n = 4). Scale bar: 50 μm. D) Txnip-positive cells were colocalized with NeuN, a neuronal cell marker. Scale bar: 50 μm. E) Confocal images show that Txnip is located in the cytoplasm of neurons. Scale bar: 10 μm. O.D., optical density; DAPI, 4′,6 diamidino-2-phenylindole.
Oxidative stress can induce expression of Txnip
We showed that Txnip is expressed after ischemic-reperfusion injury. Many reports show that ischemic-reperfusion injury produces ROS when blood flow is restored, resulting in secondary damage to the brain. Glucose is regarded as fuel for ROS after ischemic brain injury (Suh et al., 2008) and participates in reperfusion injury. Txnip is an inducible protein that responds to intracellular concentrations of glucose in various cells (Minn et al., 2005). Upstream of the Txnip gene promoter, there is a putative glucose response element to which several transcription factors can be recruited when glucose is transported into cells (Yu and Luo, 2009). We examined whether oxidative stress can initiate Txnip expression without glucose. To exclude the effect of glucose in Txnip expression and to show the effect of ROS on Txnip expression, we utilized a modified OGD and reoxygenation experiment in vitro. We also addressed this in vivo by administering 3-NP, which is well-known for producing ROS without inducing glucose reperfusion (Beal et al., 1995; Kim et al., 2003). Primary cortical neurons underwent OGD for 4 h and were then allowed to reoxygenate in glucose-free medium. Txnip expression began to increase at 3 h and continued to markedly increase by 24 h after OGD and reoxygenation in a time-dependent manner (Fig. 3A, B). To demonstrate the role of ROS in Txnip expression, we treated primary cortical neurons with apocynin, an inhibitor of NADPH oxidase. Txnip expression was markedly decreased after 4 h of OGD and 10 h of reoxygenation (Supplemental Fig. 1). Similar to the in vitro data, there was significant Txnip expression 6 and 24 h after an injection of 3-NP into the striatum of the mice (Fig. 3C, D). These data show that without the concomitant effect of glucose, Txnip can be expressed by oxidative stress such as ROS.
Fig. 3.
Oxidative stress can induce Txnip expression. A) Primary neuronal cells were exposed to OGD and allowed to reoxygenate for 1, 3, 6, and 24 h using glucose-free medium. Cells were harvested and analyzed by Western blotting to verify Txnip expression. β-tubulin was used as a loading control (n = 4). B) OGD and reoxygenation without glucose significantly increased Txnip in a time-dependent manner (n = 4; *p < 0.05). C) 3-NP was injected into the striatum of brains using a stereotactic apparatus, and the damaged striatum was collected 3, 6, and 24 h after the injection. Western blot was preformed to detect Txnip. β-tubulin was used as a loading control (n = 4). D) 3-NP administration to the brains resulted in an increase in Txnip 6 and 24 h after injection (n = 4; *p < 0.05). O.D., optical density; Veh, vehicle.
Calcium influx is associated with induction of Txnip after OGD and reoxygenation in neurons
Cerebral ischemia accompanies the production of ROS and the influx of calcium into cells via activation of the N-methyl-D-aspartate receptor through calcium channels in the brain. Elevation of intracellular calcium leads to cell death by activation of calpain and microglia and inflammation of the ischemic brain. We hypothesized that the increase in intracellular calcium may affect Txnip expression after ischemic-reperfusion injury. To clarify the role of calcium influx in Txnip expression after OGD and reoxygenation, we used three types of calcium channel blockers. 2-APB blocks calcium channels related to calcium stores and inhibits inositol-1,4,5-triphosphate receptors. Diltiazem and verapamil are L-type calcium channel blockers. Treatment with 100 μM 2-APB in primary cortical neurons completely blocked Txnip expression 6 h after OGD and reoxygenation (Fig. 4A). Twenty-four hours after reoxygenation, 2-APB markedly reduced Txnip expression in a dose-dependent manner (Fig. 4A, B). Treatment with diltiazem in primary neurons subjected to OGD and reoxygenation significantly reduced expression of Txnip in a dose-dependent manner (Fig. 4C, E). Having similar effects as diltiazem, verapamil also completely prevented the induction of Txnip after OGD and reoxygenation in neurons (Fig. 4D, E). These data using the various calcium channel blockers strongly suggest that induction of Txnip after oxidative stress is mediated by an influx of calcium into the cytoplasm.
Fig. 4.
Calcium is important for induction of Txnip after oxidative stress. A) Primary neuronal cells treated with various concentrations of 2-APB were exposed to OGD and allowed to reoxygenate for 6 and 24 h using glucose-free medium. Western blot analysis was performed to confirm the level of Txnip. β-tubulin was used as a loading control (n = 4). B) This graph shows the dramatic reduction in Txnip by 2-APB after OGD and reoxygenation (n = 4; *p < 0.01). C) Primary neuronal cells treated with various concentrations of diltiazem were exposed to OGD and allowed to reoxygenate for 24 h using medium without glucose. Western blot analysis was performed to verify the level of Txnip. GAPDH was used as a loading control (n = 4). D) Primary neuronal cells were treated with 50 μM verapamil, exposed to OGD, and allowed to reoxygenate for 24 h using glucose-free medium. Western blotting was performed to confirm the level of Txnip. GAPDH was used as a loading control (n = 4). E) Treatment with diltiazem and verapamil in primary neurons subjected to OGD and reoxygenation showed a great reduction in Txnip in a dose-dependent manner (n = 4; p < 0.05). O.D., optical density.
Hyperglycemic-ischemic reperfusion injury significantly induces Txnip mRNA
To explore the changes in the expression profile of oxidative-related genes after oxidative and glucose stress, and to confirm that Txnip expression can be induced by both ischemic-reperfusion injury and glucose, we chose and established a hyperglycemic-ischemic model by injecting STZ into mice. STZ is widely used to mimic type I diabetes by killing insulin-producing beta cells in the pancreas. We confirmed the blood glucose level before and after MCAO. The level in mice injected with STZ was 514.7 ± 17.5 mg/dl, but the level in normal mice was 181.8 ± 6.2 before MCAO (Fig. 5A). The glucose level after MCAO was decreased compared with the level before MCAO in both the STZ-injected diabetic mice and normal mice. The glucose level in mice injected with STZ after the onset of ischemia was 409.4 ± 28.3.
Fig. 5.
Txnip mRNA induction is mediated by oxidative stress and glucose. A) A hyperglycemic-ischemia animal model was used, along with a single dose (240 mg/kg) of STZ injected intraperitoneally. Three days after the injection, the glucose level was confirmed with a OneTouch Ultra device using blood from a tail snip. Ischemic injury was then induced by 30 min of MCAO and reperfusion was induced by removal of the suture. Blood glucose levels were verified right before and after MCAO (n = 10). p < 0.01 compared with normoglycemia. B) Mice with normal blood glucose levels induced by citrate buffer or with high glucose levels induced by STZ were subjected to 30 min of MCAO by insertion of a suture. Brains were collected at 3, 6, and 24 h after reperfusion. Western blotting with an anti-3-nitrotyrosine (3-NT) and anti-β-tubulin antibody was performed to confirm ROS production after hyperglycemic ischemia (n = 4). C) Infarction volume was measured by 2,3,5-triphenyltetrazolium chloride staining using slices from the brains of normoglycemic-ischemic or hyperglycemic-ischemic brains 6 h after reperfusion (normoglycemic-ischemic, n = 5; hyperglycemic-ischemic, n = 6; *p < 0.05). D) Overall gene expression profiles induced by hyperglycemic stress and ischemic-reperfusion injury (n = 3). E) Genes up-regulated by glucose over 1.5-fold included Ccs, Txnip, Gpx1, and Ucp3 (hyperglycemic shams vs. normoglycemic shams). F) Genes up-regulated over 1.5-fold included Noxo1, Il19, Nos2, Txnip, and Zmynd17 (hyperglycemic-ischemic reperfusion for 6 h vs. normoglycemic-ischemic reperfusion for 6 h). G) Genes up-regulated over 1.5-fold included Mpo, Xirp1, Ptgs2, SOD3, Txnip, and Il19 (hyperglycemic-ischemic reperfusion for 6 h vs. hyperglycemic shams). H) Changes in Txnip mRNA after ischemic-reperfusion injury in hyperglycemic or normoglycemic mice (n = 3, *p < 0.05 compared with normoglycemic-ischemic reperfusion for 6 h and hyperglycemic shams). conc., concentration; NG, normoglycemic; I/R, ischemic-reperfusion; HG, hyperglycemic; S, sham.
We next asked if hyperglycemic ischemia and reperfusion injury results in more ROS than normoglycemic ischemia. Ischemic injury was induced by 30 min of MCAO using a suture. Ischemic injury in the hyperglycemic mice after 45 min of occlusion resulted in high lethality (data not shown). Western blotting data using a 3-nitrotyrosine antibody shows that hyperglycemic ischemia and reperfusion resulted in an increase in 3-nitrotyrosine compared with normoglycemic ischemia and reperfusion 6 and 24 h after reperfusion (Fig. 5B). Also, infarction volumes 6 h after reperfusion, induced by hyperglycemic ischemia and reperfusion, were greater than those after normoglycemic ischemia and reperfusion (5.0 ± 1.3 vs. 60.0 ± 8.2) (Fig. 5C). These data confirm the STZ-induced hyperglycemic mouse model and that STZ-induced hyperglycemic ischemia produced more ROS than normoglycemic ischemia, resulting in greater infarction.
To elucidate the changes in genes related to oxidative stress and the antioxidant defense system in hyperglycemic ischemia and normoglycemic ischemia and reperfusion, we utilized a real-time PCR array to detect the overall changes in gene transcripts. Using the samples of normoglycemic ischemia and hyperglycemic-ischemic reperfusion, we found changes caused by ischemic injury and glucose in 84 genes (shown in Fig. 5D). Expression of 17 gene transcripts was increased over 1.5-fold in the hyperglycemic sham mice compared with the normoglycemic sham controls. Six gene transcripts were decreased more than -1.5-fold. The genes highly up-regulated by glucose include Ccs, Txnip, Gpx1, and Ucp3 (hyperglycemic shams vs. normoglycemic shams) (Fig. 5E). We found that 14 genes were up-regulated over 1.5 times in brains that underwent hyperglycemic ischemia and 6 h of reperfusion compared with normoglycemic ischemia and 6 h of reperfusion. Up-regulated genes included Noxo1, Il19, Nos2, Txnip, and Zmynd17 (hyperglycemic-ischemic-reperfusion for 6 h vs. normoglycemic-ischemic-reperfusion for 6 h) (Fig. 5F). These data imply that glucose stress can affect the overall changes in oxidative stress-related genes after ischemia and reperfusion injury. Txnip was seen to be up-regulated in both comparisons, confirming that glucose is responsible for the induction of Txnip in the brain (Fig. 5E, F). We compared the expression profiles of oxidative-related genes in the brains of hyperglycemic shams and hyperglycemic mice that underwent MCAO and 6 h of reperfusion. Expression of 23 genes in the brains with hyperglycemic ischemia and reperfusion was increased over 1.5-fold compared with hyperglycemic sham controls. Among the 23 genes were Mpo, Xirp1, Ptgs2, SOD3, Il19 and Txnip, (hyperglycemic-ischemic-reperfusion for 6 h vs. hyperglycemic shams) (Fig. 5G). These data strongly suggest that oxidative stress induced by ischemia and reperfusion can facilitate mRNA expression in the Txnip gene. Txnip mRNA was markedly increased in brains that underwent MCAO and 6 h of reperfusion. Moreover, hyperglycemia strikingly increased Txnip mRNA compared with normoglycemic brains (Fig. 5H). These PCR array data employing the STZ-induced hyperglycemic model strongly show that expression of Txnip is closely associated with oxidative stress and glucose.
Down-regulation of Txnip is neuroprotective
We have shown that Txnip can be induced by oxidative stress and elevated glucose using models of 3-NP injection and ischemia or hyperglycemic ischemia in vivo and OGD and reoxygenation in vitro. Thus, we asked whether induced Txnip has a neuroprotective role or a proapoptotic role after brain injury. To elucidate this, we transfected Txnip siRNA into primary cortical neurons subjected to OGD and reoxygenation. Forty-eight hours after transfection, the primary cortical neurons were exposed to OGD and were allowed to reoxygenate for 20 h. A LDH assay was performed to detect the degree of neuronal cell death. We examined the effect of Txnip siRNA on Txnip expression. Txnip siRNA transfection for 48 h markedly decreased the basal level of Txnip expression (Fig. 6A). OGD and reoxygenation and scrambled siRNA transfection with OGD and reoxygenation resulted in a striking increase in LDH release compared with the controls. Interestingly, Txnip siRNA significantly reduced LDH release compared with OGD and reoxygenation and scrambled siRNA transfection plus OGD and reoxygenation (Fig. 6B). Down-regulation of Txnip increased neuronal cell viability assessed by the WST-1 assay (Fig. 6C). These data show that Txnip has a proapoptotic role in the oxidative-stressed brain. Thus, these data using Txnip siRNA indicate that down-regulation of Txnip can be a therapeutic approach to reducing brain damage induced by oxidative and hyperglycemic stress.
Fig. 6.
Knock-down of Txnip is neuroprotective after OGD in neurons. A) Txnip siRNA and scrambled siRNA were transfected with primary cortical neurons for 48 h. After transfection, the cells were harvested and the level of Txnip was analyzed by Western blotting. B) Forty-eight hours after siRNA transfection, the primary cortical neurons were subjected to OGD and allowed to reoxygenate for 20 h. The cell death rates were then measured by LDH assay (n = 6). *p < 0.05 compared with control without OGD and scrambled siRNA with OGD. C) Forty-eight hours after siRNA transfection, the primary cortical neurons were subjected to OGD and allowed to reoxygenate for 20 h. Cell viability was measured by WST assay (n = 6). *p < 0.05 compared with control without OGD and cells subjected to OGD. **p < 0.01 comparing Txnip siRNA#1-treated and untreated cells subjected to OGD. Scr, scrambled.
Discussion
Reperfusion brain injury after ischemia leads to an increase in neuronal damage caused by excessive ROS and excitotoxicity. Glucose is believed to be a critical fuel for producing ROS after reperfusion injury. Various pathways involved in ROS generation during ischemia/reperfusion have been examined to investigate possible strategies for the treatment of stroke (Girouard and Iadecola, 2006; Kirkland et al., 2002; Miller et al., 2006; Wang et al., 2006). In this report, we demonstrate that Txnip expression is mediated by oxidative stress and glucose stimuli using in vitro OGD and in vivo 3-NP-injection models with hyperglycemic ischemia and reperfusion. It is well known that Txnip expression is induced by glucose in many cells, especially in diabetic cells, but little is known about Txnip expression in ischemic brain injury in neurons under normal glucose conditions or in hyperglycemic neuronal cells after ischemic-reperfusion injury in mice. The level of Txnip expression was further confirmed with data obtained using an oxidative-related gene-focused PCR array. Among the 84 genes that we tested, changes in Txnip mRNA caused by oxidative stress and hyperglycemic-ischemic reperfusion were dramatic. To confirm the effect of ROS on Txnip expression, an in vitro model using a primary cortical neuronal culture with OGD and reoxygenation in glucose-free medium and an in vivo model using MCAO and injection of 3-NP were used. This data imply that an increase in ROS leads to an increase in Txnip expression. Since apocynin is a specific inhibitor of NADPH oxidase, our results indicate that NADPH oxidase may play a role in the increase in Txnip expression after OGD and reoxygenation. However, other ROS-generating processes may also be involved in oxidative stress associated with Txnip expression. Interestingly, we found that down-regulation of Txnip by siRNA transfection reduced LDH release after OGD and reoxygenation, suggesting that Txnip, when expressed, has a proapoptotic role in ischemic cell death. The WST-1 assay confirmed this finding. We have elucidated the role of oxidative stress in Txnip expression by comparing hyperglycemic mice that were subjected to ischemia/reperfusion with sham mice.
An earlier report showed two putative carbohydrate response elements in the Txnip promoter region where transcription factors bind and initiate Txnip mRNA expression (Minn et al., 2005). These carbohydrate binding elements could be where Txnip expression is inducible by glucose. Txnip induction by oxidative stress resulting from ischemia/reperfusion needs to be explored. Fang et al. (2011) have reported that Txnip expression is mediated by the ROS/MEK/MAPK pathway, supporting our view that oxidative stress is involved in Txnip expression. Another factor involved in Txnip induction by oxidative stress is Forkhead box transcription factor, class O (FOXO), known to regulate the oxidative stress response in many cells (Sengupta et al., 2011). FOXO transcription factors are closely involved in the induction of Txnip mRNA by oxidative stress. FOXO transcription factors act as repressors of Txnip induction. Thus, removal of FOXO transcription factors by oxidative stress could be responsible for Txnip expression (de Candia et al., 2008). Another report suggests that FOXO transcription factors can act as activators of Txnip mRNA in neurons (Al-Mubarak et al., 2009). This discrepancy may be due to the source or kind and duration of stress and the cell type. Further studies are needed to elucidate the detailed mechanism of FOXO family involvement in Txnip expression after ischemic brain injury.
In the present study we observed that Txnip was expressed in the cytoplasm after ischemic-reperfusion injury. A recent finding indicates that Txnip shuttles between the cytoplasm and the nucleus in pancreatic beta cells. In that report, Txnip was primarily located in the nucleus and translocated to the mitochondria after oxidative stress (Saxena et al., 2010). Our data suggest that Txnip in the cytoplasm acts as a mediator for dissociation of Trx from apoptosis signal-regulating kinase 1, leading to neuronal cell death. We also observed that down-regulation of Txnip by siRNA blocked neuronal cell death after OGD. Other than oxidative stress, calcium channel blockers inhibited Txnip induction after OGD and reoxygenation in primary cortical neurons in our study. Activation of the N-methyl-D-aspartate receptor facilitates elevation of intracellular calcium, leading to promotion of neuronal cell death after ischemic injury. Elevated calcium may contribute to induction of Txnip by control of proteins such as nuclear factor of activated T-cells and calmodulin and by pathways such as Ca2+-dependent protein kinase C and nuclear factor-κB (Mellström et al., 2008).
In conclusion, we have demonstrated that Txnip is induced by oxidative and glucose stress and calcium signaling, using well-known models to generate ROS (Supplemental Fig. 2). The Oxidative Stress and Antioxidant Defense PCR array confirms that transcription of Txnip mRNA is enhanced in hyperglycemic ischemia and reperfusion. Finally, we showed that down-regulation of Txnip is neuroprotective against ischemic brain damage. Our findings emphasize the important role of Txnip expression after hyperglycemic-ischemic injury and that modulation of Txnip expression can be an effective strategy for treating ischemic brain injuries in which ROS accumulation and neuronal apoptosis occur.
Supplementary Material
Apocynin blocked the increase in Txnip protein after OGD and reoxygenation. Primary neuronal cells treated with apocynin were subjected to OGD and allowed to reoxygenate for 10 h using glucose-free medium. Western blot analysis was performed showing changes in Txnip expression with apocynin treatment. The presence of apocynin prevented up-regulation of Txnip after OGD/reoxygenation, suggesting that ROS are important in Txnip expression. β-tubulin was used as a loading control (n = 4).
Schematic diagram of Txnip expression after brain injury. Txnip is induced by ROS, glucose, and intracellular calcium levels after normoglycemic and hyperglycemic-ischemic reperfusion injury. NG, normoglycemic; HG, hyperglycemic.
Highlights.
Ischemic reperfusion injury increases expression of Txnip protein in mice
Txnip can be induced in neurons by oxidative stress without the presence of glucose
Txnip and other gene profiles are altered by oxidative stress or glucose
Induction of Txnip is mediated by calcium in OGD and reoxygeneration model
Down-regulation of Txnip by siRNA is neuroprotective after OGD stimulus
Acknowledgments
This work was supported by grants PO1 NS014543, RO1 NS025372, and RO1 NS038653 from the National Institutes of Health, and by the James R. Doty Endowment (to P.H.C.).
Abbreviations
- ROS
reactive oxygen species
- NOX
NADPH oxidase
- Trx
thioredoxin
- Txnip
thioredoxin-interacting protein
- PCR
polymerase chain reaction
- STZ
streptozotocin
- OGD
oxygen-glucose deprivation
- 2-APB
2-aminoethoxydiphenyl borate
- LDH
lactate dehydrogenase
- siRNA
small interfering RNA
- 3-NP
3-nitropropionic acid
- TBS
Tris-buffered saline
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Mpo
myeloperoxidase
- SOD3
extracellular superoxide-dismutase
- MCAO
middle cerebral artery occlusion
- FOXO
Forkhead box transcription factor, class O
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site. FOXO1 is a FOXO target gene. Channels. 2009;3:233–239. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Henshaw R, Matthews RT, Chan PH, Kowall NW, Epstein CJ, Schulz JB. 3-Nitropropionic acid neurotoxicity is attenuated in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1995;65:919–922. doi: 10.1046/j.1471-4159.1995.65020919.x. [DOI] [PubMed] [Google Scholar]
- Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br J Cancer Suppl. 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42:341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- de Candia P, Blekham R, Chabot AE, Oshlack A, Gilad Y. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS One. 2008;3:e1670. doi: 10.1371/journal.pone.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Jin Y, Zheng H, Yan J, Cui Y, Bi H, Jia H, Zhang H, Wang Y, Na L, Gao X, Zhou H. High glucose condition upregulated Txnip expression level in rat mesangial cells through ROS/MEK/MAPK pathway. Mol Cell Biochem. 2011;347:175–182. doi: 10.1007/s11010-010-0626-z. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, Sugino T, Nishimura M, Hashimoto N, Yodoi J. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo K-Y, Kim DW, Lee CH, Choi JH, Kwon Y-G, Kim Y-M, Choi SY, Won M-H. Changes in the expression of mitochondrial peroxiredoxin and thioredoxin in neurons and glia and their protective effects in experimental cerebral ischemic damage. Free Radic Biol Med. 2010;48:1242–1251. doi: 10.1016/j.freeradbiomed.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats. Relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Gasche Y, Grzeschik S, Copin J-C, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood–brain barrier disruption? J Neurosci. 2003;23:8733–8742. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL. A Bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J Neurosci. 2002;22:6480–6490. doi: 10.1523/JNEUROSCI.22-15-06480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koháryová M, Kollárová M. Oxidative stress and thioredoxin system. Gen Physiol Biophys. 2008;27:71–84. [PubMed] [Google Scholar]
- Mehta SL, Lin Y, Chen W, Yu F, Cao L, He Q, Chan PH, Li PA. Manganese superoxide dismutase deficiency exacerbates ischemic brain damage under hyperglycemic conditions by altering autophagy. Transl Stroke Res. 2011;2:42–50. doi: 10.1007/s12975-010-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Miller AA, Dusting GJ, Roulston CL, Sobey CG. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006;1111:111–116. doi: 10.1016/j.brainres.2006.06.082. [DOI] [PubMed] [Google Scholar]
- Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces β-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- Moro MA, Almeida A, Bolaños JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39:1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin D3–hupregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Paik J-H, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFβ signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia–reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78:3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271:796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- Yu FX, Luo Y. Tandem ChoRE and CCAAT motifs and associated factors regulate Txnip expression in response to glucose or adenosine-containing molecules. PLoS One. 2009;4:e8397. doi: 10.1371/journal.pone.0008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apocynin blocked the increase in Txnip protein after OGD and reoxygenation. Primary neuronal cells treated with apocynin were subjected to OGD and allowed to reoxygenate for 10 h using glucose-free medium. Western blot analysis was performed showing changes in Txnip expression with apocynin treatment. The presence of apocynin prevented up-regulation of Txnip after OGD/reoxygenation, suggesting that ROS are important in Txnip expression. β-tubulin was used as a loading control (n = 4).
Schematic diagram of Txnip expression after brain injury. Txnip is induced by ROS, glucose, and intracellular calcium levels after normoglycemic and hyperglycemic-ischemic reperfusion injury. NG, normoglycemic; HG, hyperglycemic.