Abstract
Most research on decision making has focused on how human or animal decision makers choose between two or more options, posed in advance by the researchers. The mechanisms by which options are generated for most decisions, however, are not well understood. Models of sequential search have examined the trade-off between continued exploration and choosing one’s current best option, but still cannot explain the processes by which new options are generated. We argue that understanding the origins of options is a crucial but untapped area for decision making research. We explore a number of factors which influence the generation of options, which fall broadly into two categories: psycho-biological and socio-cultural. The former category includes factors such as perceptual biases and associative memory networks. The latter category relies on the incredible human capacity for culture and social learning, which doubtless shape not only our choices but the options available for choice. Our intention is to start a discussion that brings us closer toward understanding the origins of options.
Keywords: decision making, options, choice, goals, neuroeconomics, culture
Introduction
Neuroscientists and psychologists studying decision making generally follow a standard practice borrowed from economics, which is to assume a solitary decision maker who is presented with a set of options and asked to choose among them. The quintessential mathematical formulations of choice, decision theory and game theory, deal exclusively with actors with a finite and completely known set of action choices, and this framework has allowed for the development of coherent formal theories of economic, political, and evolutionary organization. This practice has also been fruitful for the experimental sciences: we have learned much about the psychological factors that influence decisions in ways contrary to the rational ideal of Homo economicus, and have uncovered neurophysiological mechanisms by which we process and assess those options. If we pull back from the domain of economic decision theory, however, we find that very few choices are made in this way. We are rarely given an explicit set of options from which to choose, or even an obvious goal toward which we can strive to optimize our choices. Rather, we make myriad decisions daily based on competing goals and options. Those options come not from a predetermined and ready-made basket, but are vaulted into the mind from sources that are not well understood. Uncovering those sources and classifying that order is therefore a task of vital importance to the sciences of decision making.
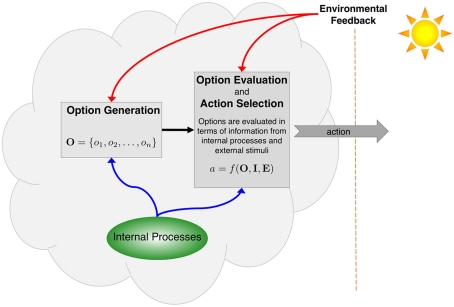
There is an important distinction between the act of choosing among options and the process by which those options are generated (Figure 1). The former is well studied in the fields of neuroscience, psychology, and behavioral economics. The latter has barely been studied at all. When an individual makes a choice, she evaluates a number of options in terms of her desired goal (or set of goals), using internal cognitive processes and perceptual information from the environment to select an action (Kahneman and Tversky, 2000; Cisek, 2007). Some researchers have also noted that organisms interact dynamically with the environment, and therefore the set of options is not static but rather shifts with the circumstances, with options competing for dominance based on available internal and external information (Cisek and Kalaska, 2010). This dynamic view of organism and environment is more realistic, but it still begs a question. Individuals must generate options for evaluation. Where do these options come from?
Figure 1.
Actions are selected through processes that evaluate options in terms of a given goal or set of goals (including subgoals). This evaluation utilizes information from internal processes, including memories and affective states, as well as perceptual feedback from the environment. These internal and external processes also contribute to the initial generation of options, but the mechanisms for doing so are much less well understood than are those for evaluation.
From a perspective of naïve epistemology, humans have a near infinite number of options available at any moment. Walking into a restaurant, for example, one usually thinks of the salient choice as being between which table to seat oneself, if such an act is permitted, or if it is not, of there being no choice at all but to go and see the host (or maître d’, depending on the fanciness of the establishment) to await seating. But there are countless other options. You could smack the headwaiter in the face. You could burst into song. Leap up on a table and tap dance. Try to walk through a wall. Take a nap on the floor. Drool. Check your watch. Scratch your leg. Stage a holdup. Turn around and leave. If there are limitless options, how are we ever to make any intelligent decisions?
The solution is that the operational set of options is not limitless. We are interested in the many processes that lead up to choice in the sense that it is usually modeled, the choice among a small set of options directly leading to action. Some of the near infinite number of theoretical options are not present at the point of decision because they have not been invented by the decision maker or communicated by some other individual. Holding up a restaurant is not an option unless you have learned how to use a pistol. Some options may be masked and others activated by many processes. For example, holding up a restaurant is masked for most people by a general commitment to being law abiding. Contrariwise, for some young males with poor job prospects and skills with a firearm, entering any prosperous business may activate an assessment of the prospects for a successful holdup. Many acts are not the result of choice at all. For example, when a behavior becomes habitual, the options are reduced to one; we enter our favorite restaurant for breakfast, sit at our usual table, and order our standard item without consulting the menu. Only a single option is salient even though the readily available menu lists a dozen or more. Throughout this paper, we will use “options” to denote those behaviors that are actually considered by an individual, consciously or unconsciously, rather than the infinite set of all possible actions.
Whether an option is considered has a lot to do with an individual’s goals. A person who had been awake for days and wasn’t concerned with social appearances might very well sit on the floor for a nap if he found himself in a restaurant (or anywhere else, for that matter). Goals influence choice in fundamental ways. An individual chooses from among actions in order to achieve a goal. Sometimes certain subgoals must be achieved en route to the superordinate goal, and actions will be selected to accomplish these (Brooks, 1991). Goals, in turn, may change dynamically in response to internal processes and external stimuli, and therefore understanding how goals interact with choice among a static set of options is a challenge in itself. Goals also play an important role in the generation of options, since goals help to define the cognitive and perceptual salience of potential behaviors (Minsky, 1985). That being said, goals influence the domain in which we search for options, but options are not fully defined by goals. Even if a goal is singular and extremely well-defined, which is rarely the case in natural settings, there are still a number of factors that will influence the available options. Some of these are provided by the environment itself – you cannot act upon what is not there, and what is there will be a source for ideas. Other factors are internal – options are influenced by an individual’s memories, motivational states, and personality. As social organisms, however, humans do not make decisions in a social void. Social and cultural factors influence the generation of options – we learn from each other, obey cultural norms, and respond to social influence. Thus a considerable number of processes interact with goals to lead to the options the decision maker comes to entertain.
The problem of options is related to a classic conundrum in cognitive science and artificial intelligence called the “frame” problem (Dennett, 1984; Shanahan, 2009). Given a task at hand, one needs to determine a set of options for evaluation, but this cannot be obtained simply by eliminating all the ineffective options, because the list of such options is effectively infinite, and an individual has limited time and computing power for decision making. Nor can the individual explicitly determine which options are irrelevant, because that still requires the discrete consideration of an infinite list. The frame problem is often formalized as a search for a set of generalized axioms that allow an individual to consider only relevant actions (Shanahan, 2009); however, a computational model that solved the frame problem for an actor of human-level complexity would effectively describe how options are generated.
It is worth noting that subjects in many decision making experiments evaluate choices that are not necessarily a priori “correct.” In addition to decisions concerning the optimization of an externally dictated reward, researchers have also considered actor-center choices evaluated on the basis of individual priorities. These two categories of decisions have been respectively referred to as veridical and adaptive decision making (Goldberg and Podell, 1999; Mograbi, 2011). While veridical decisions always have a best response, adaptive decision making experiments can shed light on how options are evaluated based on innate and learned preferences in such diverse domains as food (Arana et al., 2003; Paulus and Frank, 2003), leisure activities (Chaudry et al., 2009), esthetics (Goldberg and Podell, 1999), occupation (Nakao et al., 2009), altruistic behavior (Moll et al., 2006; Rilling et al., 2008), and moral decision making (Cikara et al., 2010; Kahane et al., 2011). Nevertheless, experiments in both veridical and adaptive decision making overwhelmingly tend to supply participants with predetermined options, and therefore still fail to shed light on the origins of options.
So, returning to the restaurant, why don’t we punch the waiter in the face? The rational response to this question is: why would we? To most people, this action has nothing to do with any salient goals, and therefore is not considered, even unconsciously. If, however, you are a jealous man, and the waiter has recently stolen your girlfriend, then voilà! Punching him becomes an option. That does not mean that you will choose this action – after all, you may be aware that this choice could land you in unwanted trouble – but it is considered where in the previous case it wasn’t. Continuing this line of thought, let’s now imagine that you have been looking for this man for the express purpose of punching him in the face. Now, even though it wasn’t your active goal a moment before you entered the restaurant, the sight of him makes you change gears and rush toward him, fists flailing. This new action plan, of course, entails a whole set of choices to be made, with the availability of specific options restricting the set of possible behaviors in the processing of those choices.
Whatever the situation, an individual’s course of action will depend on his evaluation of his available options, but those options are in turn influenced by a variety of factors – environmental, personal, and socio-cultural. These options are not necessarily available simultaneously for comparison. Decision makers may instead evaluate a sequential series of options, considering further solutions only until one is found that is satisfactory (Kahan et al., 1967). The process of considering options one at a time until a choice is made is known as sequential search, and can be characterized by a choice between selecting one’s best current option (“exploitation”) vs. continuing to search for a better solution (“exploration”). This is a classic problem in decision making, and has been extensively studied in neuroscience, economics, ecology, and computer science, but it is not the problem under consideration here. The complexities involved in the origins of options are fundamentally distinct from those of sequential search, recently framed (Cohen et al., 2007) in the immortal words of the Clash: should I stay or should I go? Once the decision to go has been made, the question becomes: where do I go, and how do I get there?
In this paper, we will consider how scientists might start thinking seriously about the origins of options. First, we will expand that discovering these origins cannot be achieved through solutions to sequential search problems, a traditional technique in decision making research. Following that, we will start fresh and discuss some of the factors involved in the generation of options, with the hope that a detailed enumeration of these factors will clarify the problem and inspire future work. First, we will briefly discuss the role of the environment on options. Next, we will explore the individual-level psycho-biological factors most familiar to neuroscientists and cognitive psychologists, which include things like memory and affect. We will then discuss the role socio-cultural factors on the origins of options in human decision making. While decisions are made by individuals, the intensely social nature of humankind necessitates the consideration of social and cultural forces. Finally, we will consider the implications and limitations of the ideas presented here.
Sequential Search
In choosing an example for the case of well-defined options, we used a situation in a restaurant. Why? It was likely chosen because the first draft of this paper was written in a café, and our mental models (Johnson-Laird, 1983) related to restaurants were primed. It is possible that other scenarios were evaluated, but more likely that we stuck with the first thing that came to mind. If “restaurant” was a satisfactory choice, then we likely deemed it “good enough,” and proceeded. If we had not been able to find a suitable example in the context of a restaurant, then we may have begun a sequential search for a more suitable choice. Most theoretical and experimental work on decision making under conditions where not all options are known to the decision maker have involved sequential search (Kahan et al., 1967; Hunt et al., 1989; Real, 1990; Hutchinson and Meyer, 1994; Daw et al., 2006; Cohen et al., 2007; Rendell et al., 2010), including so-called “naturalistic decision making” (Todd and Gigerenzer, 2001).
A sequential search is a two-stage process. An individual initiates search and finds a possible candidate solution for her problem. If the solution is not adequate, she searches again. In some cases, a decision to discontinue the search is made only when the perfect solution (if known) is found. In other cases, the search is discontinued in favor of the current “best” solution when the estimated cost of continuing the search outweighs the benefit of retaining the current solution. Optimal solutions for sequential search tasks have been discovered for various conditions in economics (Gittins, 1979; McKenna, 1979), artificial intelligence (Russell and Norvig, 2010), and behavioral ecology (Luttbeg, 2002; Stamps et al., 2005; Wiegmann et al., 2010), though the restriction of bounded rationality (Simon, 1990) makes it likely that evolved minds evaluate search decisions with fast and frugal heuristics (Gigerenzer et al., 1999), such as satisficing (i.e., choosing the first option to meet some evaluation threshold; Simon, 1956).
If options are evaluated one at a time (or even in parallel) with sequential search, then haven’t we reduced choice to two options: search or stay? This is a fundamental decision, analogous to the neuropsychological distinction between approach and withdrawal behaviors (Kinsbourne, 1993), and has received some well-deserved attention in the neuroscience literature under the computer science-inspired name of exploitation vs. exploration (Daw et al., 2006; Cohen et al., 2007). A problem endemic to all models of sequential search, however, is that the individual is assumed to know how to search. A mouse in search of a nest site can choose the best spot he has found so far or continue to search. This is a dichotomous choice, and one that may rely on a mental calculation of risk based on past experience. However, once the decision has been made to continue searching, where does the mouse look? While his options may not be technically infinite, in a complex environment such as those in which wild mice are found, the search space is nonetheless alarmingly vast. Yet somehow, a mouse searches for habitats without curling up in a fetal position and rocking back and forth while squeaking to itself, overwhelmed by an ocean of options. Similarly, a person entering a restaurant is not driven mad by an infinitude of possible behaviors. In fact, the ease with which we make choices is remarkable. Our philosophy departments are not littered with baffled epistemologists, too stunned by innumerable options to move.
The decision of whether to exploit or explore is a fundamental component of decision making, but it does not capture how the decision maker gathers the options for exploration. While much decision making theory assumes that the structure of the environment presents an individual with clear choices, this is rarely the case. Rather, our brains have evolved to detect salient features of the environment, or dimensions along which to search for those features. Those features and dimensions are then shaped and constrained by individual experiences and social factors, which in turn shape and constrain the perceived environment. The options available to an individual decision maker in natural contexts emerge organically from neural processes influenced by environmental, psycho-biological, and socio-cultural factors, and are not usually available a priori to an outside observer. We will now turn to explore in more detail the role these factors play in generating options.
Environmental Factors
The external environment shapes our options by providing structure to our behavior. This is so obvious that it will be given only cursory treatment here. The option to build a snowman only makes sense in a snowy environment; it is rarely ever considered by indigenous Hawaiians. Environments are also more than just rocks and trees and buildings and weather. Our environments also include other individuals. For example, while economists have noted the importance of market forces in constraining options, this also extends to what Noë and Hammerstein (1994) have called “biological markets” on the analogy of the markets that are so important in presenting options in the case of humans. The availability of and demand for interaction partners influences the pools from which we choose our friends, romantic partners, and business relations. One’s position in a social network also influences the spread of information to and from that individual, including cultural norms and expectations (Christakis and Fowler, 2009). How specific social factors influence perception and cognition will be discussed in greater detail in a subsequent section but we must first recognize that the individuals with whom we interact–-and how those individuals are themselves socially connected–-shape the types of decisions we will be in a position to make as well as the available options for those decisions (López-Pintado and Watts, 2008; Zerubavel and Smith, 2010).
Finally, a decision may be made to alter the environment (physical, social, or both) in order to provide the individual with new options. Gibson (1979) summed this up nicely when he posited that perception of an object is intrinsically related to the behaviors it affords the individual. Affordances are the passive natural analog of the selling points that salespersons use to convince us to buy their product. Options, then, are constrained by the potential behaviors afforded by the environment.
Psycho-Biological Factors
All aspects of psychology emerge from the interplay of neuronal, hormonal, and other biochemical processes. Psychology, then, is biology, but the nature of psychological phenomena demands that we abstract these phenomena in conceptual and linguistic terms (rather than in purely physiological terms) in order to discuss them coherently. In terms of decision making, it is often useful to articulate constraints in psychological rather than physiological terms. Here, we choose to use the designation “psycho-biological” to emphasize the connection between the two levels of abstraction. Whatever the articulation, there are a number of psycho-biological factors that constrain the options available for decision processes. The exploration of each of these in full would require much more space than we have here; what follows is by no means a complete list, but rather a broad survey of the mechanisms and processes that constrain our construction of options.
Perceptual biases
We cannot choose what we cannot perceive. The senses of each thinking organism have evolved to perceive the world in a way that reflects the salient cues that have been important for survival and reproduction throughout the species’ evolutionary history (von Uexküll, 1934/1957). An organism’s evolved perceptual biases therefore shape its options by dictating the relevant stimuli to which it reacts. Primates, for example, evolved in a niche where forward-facing eyes and good color vision were essential for navigation, foraging, and predator evasion. Swinging through trees and navigating quickly through dense, three-dimensionally complex forests requires good depth perception, and a dietary requirement of ripe fruits necessitates the ability to distinguish the color signals of fruits and leaves that are ready to eat. Grazing mammals such as deer or gazelles, on the other hand, have diets that are less dependent on color cues, and so have less precise color vision. They live in open plains, where they are vulnerable from predation from all sides, and so have eyes on each side of their head, with wide, oblong pupils for an almost completely panoramic visual field (Attenborough, 2002). Even closely related species have differences in organization of the sensory cortex related to different needs of their ecological niche, as demonstrated by recent work on rodents (Campi and Krubitzer, 2010; Krubitzer et al., 2011). Humans are famously unable to see the ultraviolet light, which renders invisible to us the often-beautiful UV-reflective patterns that guide many bird and insect species to find food, mates, and prey (Kevan et al., 2001).
These evolved biases have important effects on the ways organisms solve problems in a given environment. For example, the Norway Rat (Rattus norvegicus) is a semi-aquatic animal, and therefore is well-equipped to solve hidden-platform water maze, a common laboratory test of spatial learning. Mice, who in the wild spend much less time in water, have more difficulty solving the water maze, relying less on spatial cues than on random movement strategies (Frick et al., 2000). The Brazilian short-tailed opossum (Monodelphis domestica), a rat-sized arboreal marsupial, is generally unable to solve the hidden-platform task (Kimble and Whishaw, 1994). Each of these animals should be physically able to solve this task, but their evolved perceptual biases influence the strategic options available to them. These biases therefore influence the generation of options for decision making at a fundamental level.
There may also be differences in perceptual biases within species. These obviously include perceptual impairments such as blindness (and color blindness), deafness, etc. In addition, genetics and experience alter the salient options for decision making in many ways, which are explored in the subsequent sections.
Personality
Personality refers to individual differences in general behavioral tendencies, sometimes called behavioral syndromes when referring to non-humans (Sih et al., 2004). In humans, personalities are relatively stable throughout adulthood, though this stability largely depends on the constancy of the social environment and the individual’s role therein (Ardelt, 2000), and long-term changes can still be effected by certain life-changing events (MacLean et al., 2011). In the context of decision making, personalities refer to predictive behavioral regularities within individuals, which are influenced by complex interactions between genotype and developmental experience (Bouchard and Loehlin, 2001). Personality traits are useful descriptors that help us predict individual decision making. For example, riskier behavior for gains is correlated with increased Openness to Experience and decreased Neuroticism (Lauriola and Levin, 2001), and stable ambiguity-seeking tendencies have been shown to predict decision making behavior under both risk and ambiguity (Lauriola et al., 2007). The way in which reward is processed in the brain is also mediated by certain personality traits (Simon et al., 2010).
By defining behavioral and perceptual tendencies (Shrauger and Altrocchi, 1964; Perugini and Prestwich, 2007), personality can influence the options available to a decision maker. Imagine an individual going to a party where she does not know most of the guests. Many of her decisions, and the options thereof, will be dictated by personality-guided goals. If she is shy, she may try to associate only with people she already knows, and may stick to the edges of a room full of unfamiliar people. If she is thirsty, she may wait, or nervously ask the host for a glass. A socially bold person, on the other hand, might go directly to the refrigerator for a drink, and enthusiastically seek out conversations with strangers. Of course, it is possible that the shy person thought of going for the fridge, but rejected the action. However, the bold person assumes she will be liked (Sinclair and Lentz, 2010) and is unlikely to consider slinking along the walls or sneaking out to get a drink at the store around the block, while the shy person does. Importantly, personality traits influence more than just the way options are evaluated; they influence the determination of which options are available for evaluation.
A recent study by Gino and Ariely (2012) gives a simple example in a study of creativity, which can be characterized at least in part as a measure of the diversity of options an individual can generate. Subjects were given a difficult visual perception task of determining which of two adjacent triangles contained more circles, and could receive cash rewards. However, reward payoffs were not determined by accuracy but by absolute behavior: guessing the right triangle always paid off 10 times more than guessing the left. It was found that measures of creativity (a personality trait) correlated with the tendency to profit maximize rather than guess correctly. Though the authors characterize this behavior as dishonesty, a more parsimonious explanation of their results is that the possibility of “cheating” to maximize profits rather than perform as instructed simply did not occur to less creative individuals. As the authors note, “creativity may lead people to think of more and diverse ways they could benefit from the monetary gains from cheating, thus making cheating itself more tempting” (p. 11).
Affect
Affect is a broad term used to encompass moods, emotions, attitudes, evaluations, and preferences (Zeelenberg et al., 2008). Here we use the term to contrast with personality traits, which are more stable over the long-term; we define affective states as those situationally influenced brain states that alter the processing and prioritization of stimuli and behavioral choices. Though the variable nature of affect is often ignored by decision theorists, affective states are clearly a guiding factor in deciding among choices (Bechara et al., 2000; Zeelenberg et al., 2008). Zajonc (1980) has proposed, for example, that all perceptions contain some affect: we see not just a house but a nice house, an ugly house, etc. Building on this, Slovic et al. (2007) have proposed that many decisions are made using an affect heuristic. In these cases, the broad feelings associated with various options drive our choices more than a rational (profit-maximizing) evaluation of the associated payoffs. A similar idea has also been developed by Cunningham et al. (2007), with the additional proviso that evaluations are iteratively processed as relevant attitudes and associations are realized through spreading activation.
What is still overlooked, however, is that the options for many decisions are also guided by an individual’s affective state. Emotions, for example, may determine which goals are most salient, and therefore which options will come to the forefront (Zeelenberg et al., 2008). Damasio’s somatic marker hypothesis (Damasio, 1994; Bechara and Damasio, 2005) posits that the emotions experienced at the onset of and in response to a situation will bias the response options by activating in working memory those choices made in similar emotional states. Whether a person is angry, tired, hungry, manic, sad, or scared not only influences how she evaluates a set of options, but, given a minimal degree of agency, will influence what decisions are most important, and which options are available for consideration.
Memory and learning
Complex organisms are able to develop, adapt, and survive not only because they have been evolutionarily selected to do so, but also because the stimuli and experiences are internalized to guide future perceptions and decisions. This, of course, is learning, and the persistent effects of learning on cognition fall under the classification of memory. Memory obviously influences decision making in terms of the prior knowledge we can use to evaluate our decisions, whether in the Bayesian sense of prior probability distributions, or in terms of the relevant schemas and mental models used to evaluate situations. Memory is also related to affect, in the sense that one’s previous affective associations with a situation or option can guide choice (Damasio, 1994; Bechara and Damasio, 2005; Slovic et al., 2007). Memory can be an important factor in one’s motivational state, which we have already shown to influence the selection of options.
Since options must arise from the interplay of salient (external or internal) stimuli and preexisting cognitive structure, it is unsurprising that memory should be involved in influencing the origins of options. Perhaps the clearest influence of memory on the emergence of options is in the determination of the current goal or motivational state. As a simple example, consider a rodent exploring a dark arena. Research on Tristram’s jird (Meriones tristrami), a nocturnal rodent native in the Middle East, has shown that the animal has at least two distinct methods of exploration depending on its experience in the arena (Avni et al., 2006). At first, it “loops” around somewhat aimlessly, probably to gather enough spatial information to establish one or more “home bases.” Once a representation of the arena is internalized, the animal switches to “home-base behavior,” in which it makes short excursions from a preferred location, returning to the same location each time. Knowledge of the neural processes involved in this kind of spatial learning, at least in the hippocampal formation, is quite advanced (Moser et al., 2008). In this example, the animal must decide where to go (or whether to stay put), but the method for this decision process is determined by a mental schema dictated by the animal’s knowledge of the space.
Consider also the well-known influence of expertise in human decision making. A chess grandmaster can easily recall complex (but plausible) board positions and can make well-considered decisions with ease, which contrasts with the difficulty in both memorization and strategy found in chess novices (Simon, 1987). The grandmaster has not only memorized board positions, but has also internalized schemas and strategies, and can thus think many moves in advance, a difficulty for novices. Previous experience certainly influences the evaluation of choice options, but it also allows for the consideration of different options. Therefore, the difference between a master and a novice is not just the speed of search; through experience, the master has options unavailable to the beginner and conversely may not consider options that inexperienced players do. Even in chess, with a finite number of possible moves each turn, the expert may choose not only to make a particular move, but to embark on a planned series of moves, for which the choice of moves and the evaluation of the opponent’s moves are phenomenologically quite different than for the novice who chooses one move at a time. For more naturalistic decisions, the influence of experience on the generation of options can be even more severe and nuanced.
Individual learning is an error prone process. The information transferred in social learning processes is not always received without error either, nor are memories necessarily recalled without inaccuracies. We may misinterpret a communication not only because of imperfect perception, but also due to our own expectations and prior knowledge. Our memories are also imperfect, and we often fill in details of recalled events with conjectures and confabulations. Hirst et al. (2009) have shown this to be the case even when we are certain that our memories are accurate, as with so-called “flashbulb memories.” Moreover, conversations involving the recall of an important event can alter future recollections (Coman et al., 2009), introducing more errors. Errors introduce variation in our behavioral repertoires, and work as “mutations” for behavior selection. Acting on the basis of a previous choice, we may modify a behavior haphazardly to create a new option. If the new behavior is reinforced, it may become the dominant option around which further options are generated through haphazard modifications. Indeed, the operation of selective forces on errors may be a driving force in the production of creative thought (Campbell, 1960).
Other psycho-biological factors
At the individual-level, there are certainly other important factors that influence options. These include gender and biological sex, age, working memory (Bechara et al., 2000; Hinson et al., 2003), and cognitive biases such as framing and anchoring effects (Kahneman and Tversky, 2000). Evolution has supplied humans with useful decision making heuristics that work well under many conditions of limited information (Gigerenzer et al., 1999) and specific environmental structure (Bullock and Todd, 1999), the neural processes of which have begun to be uncovered (Volz et al., 2006). Additionally, individual differences related to both short- and long-term behavioral tendencies (i.e., affect and personality, respectively) are influenced by hormonal and genetic factors (Lee, 2008; Rilling et al., 2008). The nature of these influences may involve complex interplay between perception, cognition, and physiology (Wimsatt, 1972; Schank, 2001). Many facets of psychology and neurobiology are at work in the generation of choice options.
Socio-Cultural Factors
A decision is made by an individual and so, strictly speaking, all relevant factors shaping and constraining options reduce to those found within the individual, i.e., the psycho-biological factors discussed above1. However, social forces enter into the decision making processes of all social animals, and none more so than humankind. Humans are unique in the animal kingdom for the richness of their social ties and cultural phenomena, and for the ability of their cultures to rapidly evolve (Richerson and Boyd, 2005). Many other species engage in complex social behaviors of interest to decision scientists (de Waal and Tyack, 2003). The coordinated flocking behavior of birds in flight, for example, requires each individual to dynamically respond to its neighbors (Couzin, 2008), not to mention the intricate social dynamics found in non-human primates (de Waal and Tyack, 2003; Cheney and Seyfarth, 2007). Due to the unique role culture plays in human behavior (Chudek and Henrich, 2011), however, we will restrict this discussion to socio-cultural influences on human behavior, and the generation of options for human decision making.
Humans are social animals
Human cognition has been shaped by evolution to interpret and react to the behavior and intentions of others, and to collaborate and cooperate in shared goals in ways that differ fundamentally from our nearest primate relatives (Tomasello et al., 2005; Csibra and Gergely, 2009). There are many facets of humans as social animals that influence the options for decisions by interacting with many of the individual-level psycho-biological processes mentioned above, the diversity of which this section offers a mere taste.
The drive to be social
Humans are not content to act in solitude, a fact recognized long ago by Aristotle when he declared that “man is by nature a social animal.” We have a seemingly intrinsic drive to be for company and social acceptance, which will influence the options made in social or potentially social situations. Loneliness, for example, is a social emotion that influences perception and attention, which in turn influence available options. For example, Cacioppo et al. (2009) found that lonely individuals were less rewarded by pleasant social stimuli (e.g., a rollercoaster or a man and a dog running), and spent more time looking at images of social suffering than non-lonely individuals. Further, the desire for companionship and understanding is so strong that some individuals will even form relationships with anthropomorphized inanimate objects in an effort to stave off loneliness (Epley et al., 2008).
Social roles
Sociologists have long argued that one’s position within a society plays a large part in determining the roles that one can adopt and the actions that one can take (e.g., Goffman, 1974). These roles are often domain specific and dependent on the social landscape – a person behaves differently at work with her boss than at home with her friends. A woman may behave very differently in situations with her children, in which her role as “mother” is more salient, than in situations solely among her peers. On the other hand, tendencies developed in one sphere of life can influence behavior in other spheres. Kohn and Schoenbach (1983) found that individuals whose jobs were more “self-directed” were more likely to strive for autonomy in other domains, whereas those with more constrained job opportunities tended to favor conformity over autonomy. Importantly, these values of autonomy or conformity were transmitted both explicitly and implicitly to their children. Emphasizing one value system over another will influence an individual’s perceptions of situations as well as his goals within those situations.
Social roles also influence how we respond to various individuals. A generic social identity might drive behavior – we help an elderly woman carrying a heavy object, but not a strong young man. Our minds keep track of social relationships at the personal and interpersonal level that are quite complex, and the relevant schemas, motivations, and memories associated with those relationships influence the options and goals for decision making. Social roles and relationships influence who we trust, who we fear, and who we learn from. Humans’ amazing capacity for social learning in particular is a large part of what makes our species unique (Hermann et al., 2007), and who we target for social learning is important. In addition to our parents, we turn to people who are respected and venerated by others (Henrich and Gil-White, 2001) – indeed, this choice constitutes a sort of second-order social learning as we learn from whom to learn. Humans also preferentially reward and learn from individuals that are similar and punish, ostracize, or ignore those who are different (Aronson, 2004). This tendency appears very early – 12-month-olds preferentially copy the food selection choices of unfamiliar adults who speak their language compared with similar targets speaking a foreign language (Shutts et al., 2009).
Imitation, joint action, and emotion contagion
Our options for behaviors are influenced by what the people around us are doing. This refers to more than just environmental constraints like “I can’t walk there because Joe’s in the way.” Sociality is so deeply ingrained in humans that others’ behaviors can automatically trigger behavioral options in our brains. The mirror neuron system (Rizzolatti and Craighero, 2004) is the most famous example of this, but numerous brain networks in which action and observation comingle have been identified for sensations, emotions, and motor actions (Frith and Singer, 2008). This link between observational and behavioral pathways facilitates social learning, allows us to coordinate in complex joint tasks (Tomasello et al., 2005), and probably fosters social cohesion and the propagation of cultural norms and regional idiosyncrasies. When two people interact, they often unconsciously mimic each other’s postures, mannerisms, and facial expressions (Chartrand and Bargh, 1999). When this mimicry takes place, interactions occur more smoothly and the partners tend to like each other more (Lakin and Chartrand, 2003).
In addition to directly influencing options by activating behaviors, we can influence each others’ options by affecting their emotional states with our own. This may involve the simple spread of emotion, such as when we become fearful upon viewing another person expressing fear (Morris et al., 1996), or a reactive set of responses, such as exhibiting an expression of appeasement (e.g., embarrassment) in response to another’s anger (Keltner and Buswell, 1997).
Communication
We don’t get all our ideas from individual trial and error. While observational learning (Bandura, 1986) is an important source of information, we don’t socially learn solely by observation. The direct communication of ideas through gesture, symbol, and language represents a huge divide between humans and other species, and gives us immediate access to options generated by other minds. Indeed, seeking the advice or consultation of a friend or colleague can sometimes be an option in its own right. Whether solicited or not, advice is often most useful when it proposes options that were not previously considered, including the framing of a situation in a new light. Supporting this idea, work by Page (2007) has shown that groups are often best able to solve difficult problems when the constituent individuals are from diverse backgrounds, which increases the number and breadth of available options.
Humans are cultural animals
While all social animals are likely to be influenced by social learning, social contagion, and communication, these are hypertrophied in our species to create complex and diverse cultures (Tomasello, 1999; Jablonka and Lamb, 2005). The tremendous capacity for social learning coupled with an innate desire to learn the behaviors and customs of those around us leads to differentiations in groups, including customs, norms, and ethnic markers. It has become increasingly apparent that culture can fundamentally affect basic cognitive processes (Shore, 1996; Nisbett et al., 2001; Nisbett and Miyamoto, 2005), and that cognitive universality is largely mythical. Indeed, the fact that most psychological research is conducted on Western undergraduates should give us pause in considering how well we currently understand human cognitive and behavioral tendencies (Henrich et al., 2010). Culture guides social learning and shapes the schemas and associative networks of what is proper and what is possible in various circumstances – in other words, what behaviors are entertained as options. Indeed, cultural experience may even shape the way a given circumstance is perceived. A well-considered neural or psychological theory of decision making cannot ignore culture.
Culture influences cognition
Nisbett and colleagues (Nisbett et al., 2001; Nisbett and Miyamoto, 2005; Na et al., 2010; Varnum et al., 2010) have argued persuasively that many aspects of cognition and perception are fundamentally dependent on cultural influences. Their research emphasizes the differences between two general modes of thinking: the analytic style prevalent in the West, and the holistic style prevalent in East Asia. Analytic thinking involves the decontextualization of an object from its field, a focus on attributes of an object used to assign it into categories, and a preference for using rules about the categories to explain and predict behavior. In contrast, holistic thinking involves an orientation to the context or field as a whole, and a preference for explaining and predicting events based on relationships. Holistic thinking tends to rely on experience-based knowledge rather than abstract logic, and employs dialectic reasoning – emphasizing change, recognizing contradiction as an inherent property in the universe, and promoting a search for compromise in solutions.
These cultural differences in cognitive styles have been shown to influence both perception and memory. In a study by Masuda and Nisbett (2001), Japanese and American subjects were shown animated underwater scenes with a focal animal (a fish) and asked to describe what they had seen. The Japanese subjects were more likely to mention background information and relationships, whereas the Americans were more likely to concentrate on the focal animal. During a later recognition task, Japanese subjects had more difficulty remembering the focal animal if it was shown against a different background than the one originally seen; Americans did not show this effect. Cultural effects have also been shown in the perception of social events. Westerners are much more likely to explain another individual’s behavior in terms of inherent personality traits, while East Asians are more likely to consider explanations that take into account situational, contextual, and societal factors (Nisbett et al., 2001). If an event is perceived in a fundamentally different way, then it is probable that the options for decisions regarding that event will also differ.
Culture explicitly dictates options
Different cultures may be associated with differences in the physical environment, which alter decision making by providing different behavioral affordances (Miyamoto et al., 2006). In addition, cultural norms can influence options by suggesting or restricting choices, or by determining which behaviors will achieve specific social goals. We do not always cave to social pressures and cultural norms, but these factors still influence options even when we rebel. A secular teenager in an affluent US suburb may rebel by listening to hardcore punk music, while a rebellious teen in a fundamentalist religious community may get a thrill from sneaking a listen to a mainstream pop station.
Cultures may vary in terms of which behaviors are salient or even permitted. For example, cultures vary widely in the degree to which young people can make their own decisions concerning whom they marry (Buunk et al., 2010). A fascinating and somewhat horrific illustration of this type of cultural influence is the phenomenon of “bride abduction” in Central Asia (Werner, 2009). In Kazakhstan, a man wishing to marry a woman may forcibly abduct her, after which the woman is usually obligated to marry her abductor. The man’s friends and family are often complicit in the act, including actively assisting in the abduction and persuading or threatening the woman to accept the marriage. The bride is sometimes an accomplice in her own abduction (such as when she wishes to marry someone of whom her parents disapprove), but this is not always the case. Because female modesty plays an important role in a Kazakh family’s honor, “whether the abduction is consensual or not, it is the abduction itself that damages the family’s honour and the bride’s acceptance of the marriage serves to restore that honour” (Werner, 2009, p. 316). Werner further notes “Many of the same people who… believe it is wrong for a man to abduct a woman without her consent also believe that it is wrong for an abducted woman to reject the marriage” (p. 322). The option to forcibly abduct a woman he wishes to marry, let alone to recruit his friends and family to take part in the abduction, is not an option that occurs to most men in parts of the world like the United States, who are unaccustomed to the very concept of bride abduction. Again, this is not a matter of choice evaluation. Werner (2009) tells of a Kazakh man who was dissuaded from his original intent to abduct a bride by the power of persuasive rhetoric. That the origins of options are culturally influenced pertains to the fact that the option even occurred to him in the first place.
Conclusion
By focusing on choice behavior in the context of well-structured problems with pre-defined options, decision theorists limit the scope of their future understanding of decision processes. We cannot understand what we do not even try to study. Simon (1973) posited that it was not an overstatement to suggest that no real-world problems were well-structured in the way that experimental paradigms were – and are – generally presented. We propose that, to a large extend, problems become structured by the options that an individual considers.
Understanding how the brain generates options for decision making is a complex issue, and it is not clear that we are at all close to being able to produce a serious neural or cognitive theory. This is an open problem, and concerns neuroscientists, psychologists, economists, and anyone interested in fundamental decision making processes. Generally speaking, all behavior is decision making, and so a complete theory of behavior must account for the generation of options. We have not provided such a theory. We have merely stated the problem, and pointed out a wide array of factors for which a complete theory would need to account. Some insights into the origins of options may potentially be gleaned indirectly from previous decision making studies that look at different types of option sets (e.g., veridical vs. adaptive decision making), but these insights are limited because such studies have not considered the generation of options directly. We hope that the explicit recognition of this problem prompts future work toward a richer understanding of a fundamental component of decision processes. Given the scientific community’s accelerating knowledge of the organization and behavior of complex systems, progress toward such an understanding seems very plausible.
In some settings, an individual’s choices may be so constrained by social, cultural, and environmental factors (including legal and moral factors) that the set of options is in practice common across a wide range of individuals. In these cases, the available options may be so uniform that the paradigms of traditional decision making experiments seem applicable. This, however, still begs the question concerning the internal mechanisms that generate those admittedly common options. Moreover, we believe these situations are less common that often believed. Although broad behavioral patterns of individuals are statistically quite predictable in the aggregate (Ariely, 2008; Barabási, 2010), the precise, moment-to-moment behavior of individuals in naturalistic settings is inherently unpredictable. As we discussed in our Introduction, even the apparently simple and constrained act of ordering from a restaurant menu is rife with myriad factors that influence the available options for choice.
In contrast to the currently prevailing approach in the decision sciences of experiments with a priori options, we note that psychological experiments in which participants are allowed to respond in any way afforded by their environments are far from non-existent. Indeed, this type of experimental design has been common practice in social psychology since the 1960s. Such experiments, however, have thus far remained largely descriptive – e.g., people in larger groups wait longer to intervene in a social emergency (Darley and Latané, 1968); physical proximity, perceived power, and individual differences influence how individuals respond to counterintuitive orders from authority figures (Milgram, 1974); deeply entrenched cultural differences influence both behavioral and physiological responses to social insults (Cohen et al., 1996). The idea of integrating free response into a more rigorous neuroscience of human decision making is highly intriguing, though of course presents difficulties for experimental design. For example, implanted voltammetric microelectrodes have shed tremendous light on the role of dopamine in the reward-seeking behavior of free-moving rats (Phillips et al., 2003; Roitman et al., 2004), but similar experiments are obviously not feasible for human research. Bridging the gap between naturalistic behavior and rigorous scientific discovery of relevant decision mechanisms remains an important challenge.
One possible direction for future research might be in uncovering the neural bases for individual differences in option search strategies. For example, Schwarz et al. (2002) devised a scale which differentiated subjects’ tendencies either to seek more options in a choice task or to prefer a limited set of options as long as one met some threshold of worth, and called those at either end of the scale maximizers and satisficers, respectively. While we don’t know if satisficers and maximizers generate options in the same way, we do know they have different strategies for processing options, and that maximizers will evaluate more options when possible. These differences provide a potential starting point for understanding the neural bases for how the brain generates options. Another useful paradigm might be one that could determine whether an individual evaluated a given option (independent of final choice), or even whether two individuals consider the same options in a particular task.
We encourage researchers in the cognitive and behavioral sciences to start looking for neural mechanisms and cognitive models for the generation of options. We encourage all scientists interested in decision making to move beyond the assumptions that choices are (a) available a priori to the decision point, and (b) identical for all actors. We note that we have only presented a small number of options for future directions, but we are confident that creative decision scientists will generate many more.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper was greatly improved by careful readings by Bert Baumgaertner, Joshua Epstein, Kevin Hill, Emily Newton, and Jeffrey Schank. We also thank Mark Goldman for stimulating discussion, and Gabriel Mograbi and two anonymous reviewers for helpful comments.
Footnotes
References
- Arana F. S., Parkinson J. A., Hinton E., Holland A. J., Owen A. M., Roberts A. C. (2003). Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J. Neurosci. 23, 9632–9638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelt M. (2000). Still stable after all these years? Personality stability theory revisited. Soc. Psychol. Q. 63, 392–405 10.2307/2695848 [DOI] [Google Scholar]
- Ariely D. (2008). Predictably Irrational: The Hidden Forces That Shape Our Decisions. New York: HarperCollins [Google Scholar]
- Aronson E. (2004). The Social Animal, 9th Edn New York: Worth Publishers [Google Scholar]
- Attenborough D. (2002). The Life of Mammals. Princeton, NJ: Princeton University Press [Google Scholar]
- Avni R., Zadicario P., Eilam D. (2006). Exploration in a dark open field: a shift from directional progression and a proposed model of acquired spatial information. Behav. Brain Res. 171, 313–323 10.1016/j.bbr.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Bandura A. (1986). Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- Barabási A.-L. (2010). Bursts: The Hidden Patterns Behind Everything We Do. New York: Dutton [Google Scholar]
- Bechara A., Damasio A. R. (2005). The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 52, 336–372 10.1016/j.geb.2004.06.010 [DOI] [Google Scholar]
- Bechara A., Damasio H., Damasio A. R. (2000). Emotion, decision making, and the orbitofrontal cortex. Cereb. Cortex 10, 295–307 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- Bouchard T. J., Loehlin J. C. (2001). Genes, evolution, and personality. Behav. Genet. 31, 243–273 10.1023/A:1012294324713 [DOI] [PubMed] [Google Scholar]
- Brooks R. A. (1991). Intelligence without representation. Artif. Intell. 47, 139–159 10.1016/0004-3702(91)90053-M [DOI] [Google Scholar]
- Bullock S., Todd P. M. (1999). Made to measure: ecological rationality in structured environments. Mind Mach. 9, 497–541 10.1023/A:1008352717581 [DOI] [Google Scholar]
- Buunk A. P., Park J. H., Duncan L. A. (2010). Cultural variation in parental influence in mate choice. Cross Cultur. Res. 44, 23–40 10.1177/1069397109337711 [DOI] [Google Scholar]
- Cacioppo J. T., Norris C. J., Decety J., Monteleone G., Nusbaum H. (2009). In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J. Cogn. Neurosci. 21, 83–92 10.1162/jocn.2009.21007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. (1960). Blind variation and selection retention in creative thought as in other knowledge processes. Psychol. Rev. 67, 380–400 10.1037/h0040373 [DOI] [PubMed] [Google Scholar]
- Campi K. L., Krubitzer L. (2010). Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J. Comp. Neurol. 518, 4491–4512 10.1002/cne.22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand T. L., Bargh J. A. (1999). The chameleon effect: the perception-behavior link and social interaction. J. Pers. Soc. Psychol. 76, 893–910 10.1037/0022-3514.76.6.893 [DOI] [PubMed] [Google Scholar]
- Chaudry A. M., Parkinson J. A., Hinton E. C., Owen A. M., Robert A. C. (2009). Preference judgments involve a network of structures within frontal, cingulate and insula cortices. Eur. J. Neurosci. 29, 1047–1055 10.1111/j.1460-9568.2009.06646.x [DOI] [PubMed] [Google Scholar]
- Cheney D. L., Seyfarth R. M. (2007). Baboon Metaphysics: The Evolution of a Social Mind. Chicago: University of Chicago Press [Google Scholar]
- Christakis N. A., Fowler J. H. (2009). Connected: How YOUR Friends’ Friends’ Friends Affect Everything you Feel, Think, and Do. New York: Little, Brown and Co [Google Scholar]
- Chudek M., Henrich J. (2011). Culture-gene coevolution, norm-psychology and the emergence of human prosociality. Trends Cogn. Sci. (Regul. Ed.) 15, 218–226 10.1016/j.tics.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Cikara M., Farnsworth R. A., Harris L. T., Fiske S. T. (2010). On the wrong side of the trolley track: neural correlates of relative social valuation. Soc. Cogn. Affect. Neurosci. 5, 404–413 10.1093/scan/nsq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. (2007). Cortical mechanisms of action selection: the affordance competition hypothesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1585–1599 10.1098/rstb.2007.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P., Kalaska J. F. (2010). Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 33, 269–298 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]
- Cohen D., Nisbett R. E., Bowdle B. F., Schwarz N. (1996). Insult, aggression, and the Southern culture of honor: an “experimental ethnography.” J. Pers. Soc. Psychol. 70, 945–960 10.1037/0022-3514.70.5.945 [DOI] [PubMed] [Google Scholar]
- Cohen J. D., McClure S. M., Yu A. J. (2007). Should i stay or should i go? How the human brain manages the trade-off between exploitation and exploration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 933–942 10.1098/rstb.2007.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman A., Manier D., Hirst W. (2009). Forgetting the unforgettable through conversation: socially shared retrieval-induced forgetting of September 11 memories. Psychol. Sci. 20, 627–633 10.1111/j.1467-9280.2009.02343.x [DOI] [PubMed] [Google Scholar]
- Couzin I. D. (2008). Collective cognition in animal groups. Trends Cogn. Sci. (Regul. Ed.) 13, 36–43 10.1016/j.tics.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Csibra G., Gergely G. (2009). Natural pedagogy. Trends Cogn. Sci. (Regul. Ed.) 13, 148–153 10.1016/j.tics.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Cunningham W. A., Zelazo P. D., Packer D. J., Van Bavel J. J. (2007). The iterative reprocessing model: a multilevel framework for attitudes and evaluation. Soc. Cogn. 25, 736–760 10.1521/soco.2007.25.5.736 [DOI] [Google Scholar]
- Damasio A. R. (1994). Descartes’ Error. New York: G. P. Putnam’s Sons [Google Scholar]
- Darley J. M., Latané B. (1968). Bystander intervention in emergencies. J. Pers. Soc. Psychol. 8, 377–383 10.1037/h0025589 [DOI] [PubMed] [Google Scholar]
- Daw N. D., O’Doherty J. P., Seymour B., Dayan P., Dolan R. J. (2006). Cortical substrates for exploratory decisions in humans. Nature 441, 876–879 10.1038/nature04766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F. B. M., Tyack P. L. (2003). Animal Social Complexity: Intelligence, Culture, and Individualized Societies. Cambridge, MA: Harvard University Press [Google Scholar]
- Dennett D. C. (1984). “The frame problem of AI,” in Minds, Machines and Evolution, ed. Hookway C. (New York: Cambridge University Press; ), 129–151 [Google Scholar]
- Epley N., Akalis S., Waytz A., Cacioppo J. T. (2008). Creating social connections through inferential reproduction: loneliness and perceived agency in gadgets, gods, and greyhounds. Psychol. Sci. 19, 114–120 10.1111/j.1467-9280.2008.02056.x [DOI] [PubMed] [Google Scholar]
- Frick K. M., Stillner E. T., Berger-Sweeney J. (2000). Mice are not little rats: species differences in a one-day water maze task. Neuroreport 11, 3461–3465 10.1097/00001756-200011090-00013 [DOI] [PubMed] [Google Scholar]
- Frith C. D., Singer T. (2008). The role of social cognition in decision making. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3875–3886 10.1098/rstb.2008.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. J. (1979). The Ecological Approach to Visual Perception. Boston, MA: Houghton Mifflin [Google Scholar]
- Gigerenzer G., Todd P. M., the ABC Research Group (1999). Simple Heuristics that Make Us Smart. New York: Oxford University Press [Google Scholar]
- Gino F., Ariely D. (2012). The dark side of creativity: original thinkers can be more dishonest. J. Pers. Soc. Psychol. 102, 445–459 10.1037/a0026406 [DOI] [PubMed] [Google Scholar]
- Gittins J. C. (1979). Bandit processes and dynamic allocation indices. J. R. Stat. Soc. Series B Stat. Methodol. 41, 148–177 [Google Scholar]
- Goffman E. (1974). Frame Analysis: An Essay on the Organization of Experience. Cambridge, MA: Harvard University Press [Google Scholar]
- Goldberg E., Podell K. (1999). Adaptive versus veridical decision-making and the frontal lobes. Conscious. Cogn. 8, 364–377 10.1006/ccog.1999.0395 [DOI] [PubMed] [Google Scholar]
- Henrich J., Gil-White F. J. (2001). The evolution of prestige: freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol. Hum. Behav. 22, 165–196 10.1016/S1090-5138(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Henrich J., Heine S. J., Norenzayan A. (2010). The weirdest people in the world? Behav. Brain Sci. 33, 61–135 10.1017/S0140525X10000725 [DOI] [PubMed] [Google Scholar]
- Hermann E., Call J., Hernandez-Lloreda M. V., Hare B., Tomasello M. (2007). Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366 10.1126/science.1146282 [DOI] [PubMed] [Google Scholar]
- Hinson J. M., Jameson T. L., Whitney P. (2003). Impulsive decision making and working memory. J. Exp. Psychol. Learn. Mem. Cogn. 29, 298–306 10.1037/0278-7393.29.2.298 [DOI] [PubMed] [Google Scholar]
- Hirst W., Phelps E. A., Buckner R. L., Budson A. E., Cuc A., Gabrieli J. D., Johnson M. K., Lustig C., Lyle K. B., Mather M., Meksin R., Mitchell K. J., Ochsner K. N., Schacter D. L., Simons J. S., Vaidya C. J. (2009). Long-term memory for the terrorist attach of September 11: flashbulb memories, event memories, and the factors that influence their retention. J. Exp. Psychol. Gen. 138, 161–176 10.1037/a0015527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. G., Krzysofiak F. J., Meindl J. R., Yousry A. M. (1989). Cognitive style and decision making. Organ. Behav. Hum. Decis. Process. 44, 436–453 10.1016/0749-5978(89)90018-6 [DOI] [Google Scholar]
- Hutchinson J. W., Meyer R. J. (1994). Dynamic decision making: optimal policies and actual behavior in sequential choice problems. Mark. Lett. 5, 369–382 10.1007/BF00999211 [DOI] [Google Scholar]
- Jablonka E., Lamb M. J. (2005). Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. Cambridge, MA: MIT Press [Google Scholar]
- Johnson-Laird P. N. (1983). Mental Models: Towards a Cognitive Science of Language, Inference, and Consciousness. Cambridge, MA: Harvard University Press [Google Scholar]
- Kahan J. P., Rapoport A., Jones L. V. (1967). Decision making in a sequential search task. Percept. Psychophys. 2, 374–376 10.3758/BF03210074 [DOI] [Google Scholar]
- Kahane G., Wiech K., Shackel N., Farias M., Savulescu J., Tracey I. (2011). The neural basis of intuitive and counterintuitive moral judgment. Soc. Cogn. Affect. Neurosci. [Epub ahead of print]. 10.1093/scan/nsr005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D., Tversky A. (2000). Choices, Values, and Frames. New York: Cambridge University Press [Google Scholar]
- Keltner D., Buswell B. N. (1997). Embarassment: its distinct form and appeasement functions. Psychol. Bull. 112, 250–270 10.1037/0033-2909.122.3.250 [DOI] [PubMed] [Google Scholar]
- Kerr N. L., Tindale R. S. (2004). Group performance and decision making. Annu. Rev. Psychol. 55, 623–655 10.1146/annurev.psych.55.090902.142009 [DOI] [PubMed] [Google Scholar]
- Kevan P. G., Chittka L., Dyer A. G. (2001). Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. J. Exp. Biol. 204, 2571–2580 [DOI] [PubMed] [Google Scholar]
- Kimble D., Whishaw I. Q. (1994). Spatial behavior in the Brazilian short- tailed opossum (Monodelphis domestica): comparison with the Norway rat (Rattus norvegicus) in the Morris water maze and radial arm maze. J. Comp. Psychol. 108, 148–155 10.1037/0735-7036.108.2.148 [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. (1993). Integrated cortical field model of consciousness. Ciba Found. Symp. 174, 43–60 [DOI] [PubMed] [Google Scholar]
- Kohn M., Schoenbach C. (1983). “Class, stratification, and psychological functioning,” in Work and Personality: An Inquiry Into the Impact of Social Stratification, eds Kohn M., Schooler C. (Norwood, NJ: Able; ), 154–189 [Google Scholar]
- Krubitzer L., Campi K. L., Cooke D. F. (2011). All rodents are not the same: a modern synthesis of cortical organization. Brain Behav. Evol. 78, 51–93 10.1159/000327320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin J. L., Chartrand T. L. (2003). Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol. Sci. 14, 334–339 10.1111/1467-9280.14481 [DOI] [PubMed] [Google Scholar]
- Lauriola M., Levin I. P. (2001). Personality traits and risky decision-making in a controlled experimental task: an exploratory study. Pers. Individ. Dif. 31, 215–226 10.1016/S0191-8869(00)00130-6 [DOI] [Google Scholar]
- Lauriola M., Levin I. P., Hart S. S. (2007). Common and distinct factors in decision making under ambiguity and risk: a psychometric study of individual differences. Organ. Behav. Hum. Decis. Process. 104, 130–149 10.1016/j.obhdp.2007.04.001 [DOI] [Google Scholar]
- Lee D. (2008). Game theory and neural basis of social decision making. Nature Neurosci. 11, 404–409 10.1038/nn.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pintado D., Watts D. J. (2008). Social influence, binary decisions and collective dynamics. Rationality Soc. 20, 399–443 10.1177/1043463108096787 [DOI] [Google Scholar]
- Luttbeg B. (2002). Assessing the robustness and optimality of alternative decision rules with varying assumptions. Anim. Behav. 63, 805–814 10.1006/anbe.2001.1979 [DOI] [Google Scholar]
- MacLean K. A., Johnson M. W., Griffiths R. R. (2011). Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J. Psychopharmacol. (Oxford) 25, 1453–1461 10.1177/0269881111420188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Nisbett R. E. (2001). Attending holistically versus analytically: comparing the context sensitivity of Japanese and Americans. J. Pers. Soc. Psychol. 81, 922–934 10.1037/0022-3514.81.5.922 [DOI] [PubMed] [Google Scholar]
- McKenna C. J. (1979). A solution to a class of sequential decisions problems. Econ. Lett. 3, 115–118 10.1016/0165-1765(79)90102-2 [DOI] [Google Scholar]
- Milgram S. (1974). Obedience to Authority. New York: HarperCollins [Google Scholar]
- Minsky M. (1985). The Society of Mind. New York: Simon and Schuster [Google Scholar]
- Miyamoto Y., Nisbett R. E., Masuda T. (2006). Culture and the physical environment. Psychol. Sci. 17, 113–119 10.1111/j.1467-9280.2006.01673.x [DOI] [PubMed] [Google Scholar]
- Mograbi G. J. C. (2011). Neural basis of decision-making and assessment: issues on testability and philosophical relevance. Mens Sana Monogr. 9, 251–259 10.4103/0973-1229.77441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. (2006). Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. U.S.A. 103, 15623–15628 10.1073/pnas.0604475103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. S., Frith C. D., Perrett D. I., Rowland D., Young A. W. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383, 812–815 10.1038/383389a0 [DOI] [PubMed] [Google Scholar]
- Moser E. I., Kropff E., Moser M.-B. (2008). Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 31, 69–89 10.1146/annurev.neuro.31.061307.090723 [DOI] [PubMed] [Google Scholar]
- Na J., Grossman I., Varnum M. E. W., Kitayama S., Gonzalez R., Nisbett R. E. (2010). Cultural differences are not always reducible to individual differences. Proc. Natl. Acad. Sci. U.S.A. 107, 6192–6197 10.1073/pnas.1001911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T., Osumi T., Ohira H., Kasuya Y., Shinoda J., Yamada J. (2009). Neural bases of behavior selection without an objective correct answer. Neurosci. Lett. 459, 30–34 10.1016/j.neulet.2009.04.056 [DOI] [PubMed] [Google Scholar]
- Nisbett R. E., Miyamoto Y. (2005). The influence of culture: holistic versus analytic perception. Trends Cogn. Sci. (Regul. Ed.) 9, 467–473 10.1016/j.tics.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Nisbett R. E., Peng K., Choi I., Norenzayan A. (2001). Culture and systems of thought: holistic versus analytic cognition. Psychol. Rev. 108, 291–310 10.1037/0033-295X.108.2.291 [DOI] [PubMed] [Google Scholar]
- Noë R., Hammerstein P. (1994). Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. (Print) 35, 1–11 [Google Scholar]
- Page S. E. (2007). The Difference: How the Power of Diversity Creates Better Groups, Firms, Schools, and Societies. Princeton, NJ: Princeton University Press [Google Scholar]
- Paulus M. P., Frank L. R. (2003). Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport 14, 1311–1315 10.1097/00001756-200307180-00005 [DOI] [PubMed] [Google Scholar]
- Perugini M., Prestwich A. (2007). The gatekeeper: individual differences are key in the chain from perception to behaviour. Eur. J. Pers. 21, 303–317 10.1002/per.633 [DOI] [Google Scholar]
- Phillips P. E. M., Stuber G. D., Heien M. L. A. V., Wightman R. M., Carelli R. M. (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 10.1038/nature01476 [DOI] [PubMed] [Google Scholar]
- Real L. (1990). Search theory and mate choice: models of single-sex discrimination. Am. Nat. 136, 376–405 10.1086/285103 [DOI] [Google Scholar]
- Rendell L., Boyd R., Cownden D., Enquist M., Eriksson K., Feldman M. W., Fogarty L., Ghirlanda S., Lillicrap T., Laland K. N. (2010). Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213 10.1126/science.1184719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson P. J., Boyd R. (2005). Not By Genes Alone: How Culture Transformed Human Evolution. Chicago: University of Chicago Press [Google Scholar]
- Rilling J. K., King-Casas B., Sanfey A. G. (2008). The neurobiology of social decision-making. Curr. Opin. Neurobiol. 18, 159–165 10.1016/j.conb.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. (2004). The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- Roitman M. F., Stuber G. D., Phillips P. E. M., Wightman R. M., Carelli R. M. (2004). Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271 10.1523/JNEUROSCI.3823-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S., Norvig P. (2010). Artificial Intelligence: A Modern Approach, 3rd Edn Upper Saddle River, NJ: Pearson [Google Scholar]
- Schank J. C. (2001). Beyond reductionism: refocusing on the individual with individual-based modeling. Complexity 6, 33–40 10.1002/cplx.1040 [DOI] [Google Scholar]
- Shrauger S., Altrocchi J. (1964). The personality of the perceiver as a factor in person perception. Psychol. Bull. 62, 289–308 10.1037/h0044083 [DOI] [PubMed] [Google Scholar]
- Schwarz B., Ward A., Monteross J., Lyubomirsky S., White K., Lehman D. R. (2002). Maximizing versus satisficing: happiness is a matter of choice. J. Pers. Soc. Psychol. 83, 1178–1197 10.1037/0022-3514.83.5.1178 [DOI] [PubMed] [Google Scholar]
- Shanahan M. (2009). “The frame problem,” in The Stanford Encyclopedia of Philosophy (Winter 2009 Ed.), ed. Zalta E. N. Available at: http://plato.stanford.edu/archives/win2009/entries/frame-problem/ [Google Scholar]
- Shore B. (1996). Culture in Mind: Cognition, Culture, and the Problem of Meaning. New York: Oxford University Press [Google Scholar]
- Shutts K., Kinzler K. D., McKee C., Spelke E. S. (2009). Social information guides infants’ selection of foods. J. Cogn. Dev. 10, 1–17 10.1080/15248370902966636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A., Johnson J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. (Amst.) 19, 372–378 10.1016/j.tree.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Simon H. A. (1956). Rational choice and the structure of the environment. Psychol. Rev. 63, 129–138 10.1037/h0042769 [DOI] [PubMed] [Google Scholar]
- Simon H. A. (1973). The structure of ill structured problems. Artif. Intell. 4, 181–201 10.1016/0004-3702(73)90011-8 [DOI] [Google Scholar]
- Simon H. A. (1987). Making management decisions: the role of intuition and emotion. Acad. Manag. Exec. 1, 57–64 10.5465/AME.1987.4275905 [DOI] [Google Scholar]
- Simon H. A. (1990). Invariants of human behavior. Annu. Rev. Psychol. 41, 1–19 10.1146/annurev.ps.41.020190.000245 [DOI] [PubMed] [Google Scholar]
- Simon J. J., Walther S., Fiebach C. J., Friederich H.-C., Stippich C., Weisbrod M., Kaiser S. (2010). Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage 49, 1868–1874 10.1016/j.neuroimage.2009.09.016 [DOI] [PubMed] [Google Scholar]
- Sinclair L., Lentz T. (2010). Self-esteem, social inclusionary status, and inhibition of rejection. Self Identity 9, 434–443 10.1080/15298860903395826 [DOI] [Google Scholar]
- Slovic P., Finucane M. L., Peters E., MacGregor D. G. (2007). The affect heuristic. Eur. J. Oper. Res. 177, 1333–1352 10.1016/j.ejor.2005.04.006 [DOI] [Google Scholar]
- Stamps J. A., Krishnan V. V., Reid M. L. (2005). Search costs and habitat selection by dispersers. Ecology 86, 510–518 10.1890/05-0290 [DOI] [Google Scholar]
- Sumpter D. J. T. (2006). The principles of collective animal behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 5–22 10.1098/rstb.2005.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. M., Gigerenzer G. (2001). Putting naturalistic decision making into the adaptive toolbox. J. Behav. Decis. Mak. 14, 353–384 10.1002/bdm.382 [DOI] [Google Scholar]
- Tomasello M. (1999). The Cultural Origins of Human Cognition. Cambridge, MA: Harvard University Press [Google Scholar]
- Tomasello M., Carpenter M., Call J., Behne T., Moll H. (2005). Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–735 10.1017/S0140525X05540123 [DOI] [PubMed] [Google Scholar]
- Varnum M. E. W., Grossman I., Kitayama S., Nisbett R. E. (2010). The origin of cultural differences in cognition: the social orientation hypothesis. Curr. Dir. Psychol. Sci. 19, 9–13 10.1177/0963721409359301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K. G., Schooler L. J., Schubotz R. I., Raab M., Gigerenzer G., von Cramon D. Y. (2006). Why you think Milan is larger than Modena: neural correlates of the recognition heuristic. J. Cogn. Neurosci. 18, 1924–1936 10.1162/jocn.2006.18.11.1924 [DOI] [PubMed] [Google Scholar]
- von Uexküll J. (1934/1957). “A stroll through the worlds of animals and men: a picture book of invisible worlds,” in Instinctive Behavior: The Development of a Modern Concept, ed. Schiller C. (New York: International Universities Press; ), 5–80 [Google Scholar]
- Werner C. (2009). Bride abduction in post-Soviet Central Asia: marking a shift towards patriarchy through local discourses of shame and tradition. J. R. Anthropol. Inst. 15, 314–331 10.1111/j.1467-9655.2009.01555.x [DOI] [Google Scholar]
- Wiegmann D. D., Weinersmith K. L., Seubert S. M. (2010). Multi-attribute mate choice decisions and uncertainty in the decision process: a generalized sequential search strategy. J. Math. Biol. 60, 543–572 10.1007/s00285-009-0274-7 [DOI] [PubMed] [Google Scholar]
- Wimsatt W. C. (1972). Complexity and organization. Philos. Sci. Assoc. 1, 67–86 [Google Scholar]
- Zajonc R. B. (1980). Feeling and thinking: preferences need no inferences. Am. Psychol. 35, 151–175 10.1037/0003-066X.35.7.662 [DOI] [Google Scholar]
- Zeelenberg M., Nelissen R. M. A., Breugelmans S. M., Pieters R. (2008). On emotion specificity in decision making: why feeling is for doing. Judg. Decis. Mak. 3, 18–27 [Google Scholar]
- Zerubavel E., Smith E. R. (2010). Transcending cognitive individualism. Soc. Psychol. Q. 73, 321–325 10.1177/0190272510388998 [DOI] [Google Scholar]