Abstract
A number of small-molecule poly (ADP-ribose) polymerase (PARP) inhibitors are currently undergoing advanced clinical trials. Determining the distribution and target inhibitory activity of these drugs in individual subjects, however, has proven problematic. Here, we used a PARP agent for positron emission tomography-computed tomography (PET-CT) imaging (18F-BO), which we developed based on the Olaparib scaffold using rapid bioorthogonal conjugation chemistries. We show that the bioorthogonal 18F modification of the parent molecule is simple, highly efficient, and well tolerated, resulting in a half maximal inhibitory concentration (IC50) of 17.9 ± 1.1 nM. Intravital imaging showed ubiquitous distribution of the drug and uptake into cancer cells, with ultimate localization within the nucleus, all of which were inhibitable. Whole-body PET-CT imaging showed tumoral uptake of the drug, which decreased significantly, after a daily dose of Olaparib. Standard 18F-fludeoxyglucose imaging, however, failed to detect such therapy-induced changes. This research represents a step toward developing a more generic approach for the rapid codevelopment of companion imaging agents based on small-molecule therapeutic inhibitors.
Introduction
DNA breaks occur naturally during the course of each cell cycle. In order for a cell to survive, however, these defects must be repaired. Poly (ADP-ribose) polymerase (PARP) and breast cancer susceptibility proteins (BRCA) are two proteins that have specific roles in repairing such breaks, namely PARP repairs single-strand breaks and BRCA repairs double-strand breaks. In cases where BRCA is mutated (i.e., not functional), PARP is able to repair both types of DNA breaks, and thus, these cells depend solely on the PARP repair mechanism. Small-molecule PARP inhibitors have received significant attention when combined with DNA-damaging agents in BRCA-negative tumors (synthetic lethality) [1]. Yet, despite the compelling scientific rationale for the use of these agents as well as encouragement from early clinical trials [1–3], more recent trials in triple-negative breast cancers [4] and in ovarian cancer [5] have resulted in disappointment. One issue in these studies has been the lack of clearly defined molecular biomarkers of efficacy. Indeed, recent trials have been heavily reliant on indirect measures of cancer regression derived from RECIST (Response Evaluation Criteria In Solid Tumors), tumor volumetrics or plasma biomarker methods (CA125, circulating cancer cells). These approaches could be considerably enhanced by the performance of tandem measurements of drug distribution (pharmacokinetics) as well as direct measurements of drug effects (pharmacodynamics) on their intended target.
Using newer bioorthogonal approaches, a number of platform technologies have been recently introduced, which facilitate rapid labeling of small-molecule drugs with 18F for rapid proof-of-principle preclinical imaging [6]. Although it is synthetically feasible to produce 18F-labeled small-molecule PARP inhibitors, how these agents might behave in different cancers, particularly ovarian and pancreatic cancers, has remained unclear. In the current study, we thus performed a more systematic preclinical approach involving biologic validation studies in murine models and using a prototype 18F radioactive and a fluorescent PARP inhibitor platform based on the Olaparib (AZD2281) scaffold. The 18F bioorthogonally (BO)-labeled Olaparib (18F-BO) [6] and the fluorescent analog (FL-BO) were then tested in mouse models of ovarian and pancreatic cancer. We hypothesized that use of these BO agents would allow quantitation of PARP expression levels in vivo. To test this hypothesis, we designed a series of experiments that address the following questions: 1) What is the imaging profile of 18F-BO in cancer models? 2) Do imaging signals from these agents correlate with PARP expression levels in vivo? 3) Do the imaging agents colocalize with PARP inside cancer cells? 4) How does 18F-BO imaging compare to 18F-FDG positron emission tomography (PET) imaging? Here, we show that 18F-BO represents a highly selective agent for imaging PARP in vivo. Furthermore, 18F-BO allows accurate prediction of target inhibition (superior to PET FDG imaging), and it has been found to accumulate inside the nucleus of tumor cells. We anticipate that this class of agent will be beneficial to current clinical trials not only by enabling direct measures of target occupancy but also for establishing drug doses based on biologic rather than toxicity grounds and for identifying treatment failures.
Materials and Methods
Unless otherwise noted, all reagents were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. BODIPY FL succinimidyl ester was purchased from Invitrogen (Carlsbad, CA). Olaparib (AZD2281) was purchased from Selleck Chemicals (Houston, TX). 4-[[4-Fluoro-3-(piperazine-1-carbonyl)phenyl]methyl]-2H-phthalazin-1-one, AZD2281-Tz, 18F-TCO, and 18F-BO were synthesized as described elsewhere [6–8]. [18F]-Fluoride (n.c.a.) and 18F-fludeoxyglucose (18F-FDG) were purchased from PETNET Solutions (Woburn, MA). High performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS) and high-performance liquid chromatography (HPLC) purifications were performed on a Waters (Milford, MA) LC-MS system. For LC-ESI-MS analyses, a Waters XTerra C18 5 µm column was used. For preparative runs, an Atlantis Prep T3 OBD 5-µm column was used. High-resolution ESI mass spectra were obtained on a Bruker Daltonics APEXIV 4.7-T Fourier transform mass spectrometer (FT-ICR-MS) in the Department of Chemistry Instrumentation Facility at the Massachusetts Institute of Technology. Proton nuclear magnetic resonance (1H nuclear magnetic resonance [NMR]) spectra were recorded on a Varian AS-400 (400 MHz) spectrometer. Chemical shifts for protons are reported in parts per million (ppm) and are referenced against the dimethylsulfoxide lock signal (1H, 2.50 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), coupling constants (Hz), and integration.
Synthesis of FL-BO
A solution of BODIPY FL succinimidyl ester (5.0 mg, 12.8 µmol) in acetonitrile (250 µl) was added to a solution of 4-[[4-fluoro-3(piperazine-1-carbonyl)phenyl]methyl]-2H-phthalazin-1-one (4.7 mg, 12.8 µmol) and triethylamine (4.6 µl, 64.2 µmol) in acetonitrile (250 µl). The reaction mixture was stirred for 4 hours at room temperature and purified through HPLC to yield the title compound as an orange solid (5.7 mg, 8.9 µmol, 70%). 1HNMR (400 MHz, DMSO-d6) δ = 12.59 (s, 1H), 8.26 (d, 3JHH = 7.6, 1H), 7.96 (d, 3JHH = 7.8, 1H), 7.92-7.80 (m, 2H), 7.69 (s, 1H), 7.47-7.41 (m, 1H), 7.39-7.34 (m, 1H), 7.23 (t, 3JHH = 9.0, 1H), 7.09 (s, 1H), 6.45-6.38 (m, 1H), 6.30 (s, 1H), 4.33 (s, 2H), 3.66-3.05 (m, 10H), 2.80-2.68 (m, 2H), 2.48-2.44 (m, 3H), 2.26 (s, 3H); 19F NMR (376 MHz, DMSO-d6) δ = -119.4 (s, 1F), -142.8 (q, JBF = 33 Hz, 2F); LC-ESI-MS(+) m/z = 621.4 [M - F]+, 641.4 [M + H]+, 663.4 [M + H]+; LC-ESI-MS(-) m/z = 619.3 [M - 2H - F]-, 639.3 [M - H]-; HRMS-ESI [M - H]+ m/z calculated for [C32H33BF3N6O3]+ 641.2671, found 641.2688 [M + H]+.
PARP-1 IC50 Determination
A commercially available colorimetric assay (Trevigen, Gaithersburg, MD) was used to measure PARP-1 activity in vitro in the presence of varying concentrations of FL-BO. Three-fold dilutions of FL-BO (final concentrations ranging from 3.3 µM to 0.1 nM) were incubated with 0.5 U of PARP high specific activity (HSA) enzyme for 10 minutes in histone-coated 96-well plates. All experiments were carried out in triplicate. Positive control samples did not contain inhibitor, and background measurement samples did not contain PARP-1. All reaction mixtures were adjusted to a final volume of 50 µl and a final concentration of 2% dimethyl sulfoxide (DMSO) in assay buffer. The remainder of the assay was performed according to the manufacturer's instructions. PARP-1 activity was measured by absorbance at 450 nm in each well using a Tecan Safire2 microplate reader (Tecan Group, Mannedorf, Switzerland).
Cancer Cell Lines
We chose a number of ovarian and pancreatic cancer cells lines with variable PARP expression levels to correlate imaging findings and because these primaries are the focus of ongoing clinical trials. UCI 101, UCI 107, OVCA429, and A2780 cell lines were generously provided by Dr Michael Birrer (Massachusetts General Hospital, Boston, MA). All other cell lines were obtained from the ATCC (Manassas, VA). MDA-MB-231, MDA-MB-436, MIA PaCa-2, A2780, OVCAR429, UCI 101, and UCI 107 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, l-glutamine, 100 IU penicillin, and 100 µg/ml streptomycin. RAW 264.7, PANC-1, CaOV3, and HT1080 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 2% sodium bicarbonate, l-glutamine, 100 IU penicillin, and 100 µg/ml streptomycin. SKOV-3 cells were cultured in McCoy 5A medium supplemented with 10% fetal bovine serum, L-glutamine, 100 IU penicillin, and 100 µg/ml streptomycin. OVCAR-3 cells were cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum, 1% bovine insulin, l-glutamine, 100 IU penicillin, and 100 µg/ml streptomycin. OV-90 cells were cultured in 50% MCDB 105 medium, 50% medium 199 supplemented with 15% fetal bovine serum, and 2% sodium bicarbonate. All cell lines were cultured at 37°C and 5% CO2.
HT1080 H2B-Apple
pmApple-N1 (Myo1E-pmApple-C1; Addgene [Cambridge, MA], Prof. Christien Merrifield [9]) was cloned by ligating mApple into pmCherry-N1 (Clontech, Mountain View, CA) using AfeI and BsrG1 restriction enzymes, followed by ligation. The pTag-H2B-Apple construct was produced by subcloning mApple from pmApple-N1 into pTag-H2B-BFP (Evrogen, Moscow, Russia) using the AgeI and NotI restriction enzyme sites. Correct insertion of mApple was confirmed by sequencing the insert in its entirety. pTag-H2B-Apple was transfected into HT1080 cells using the X-tremeGENE HP transfection reagent (Roche, Basel, Switzerland), followed by selection in 500 µg/ml G418. Single clones were screened for H2B-Apple expression by fluorescence microscopy. Cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum, 100 IU penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, nonessential amino acids, and 100 µg/ml G418.
In Vitro Cell Assays
RAW 264.7, PANC-1, MIA PaCa-2, A2780, OVCAR429, UCI 101, UCI 107, SKOV-3, OVCAR-3, and OV-90 cells (200 µl, 35,000 cells/ml) were each seeded in their respective growth medium on 96-well plates and allowed to attach for 48 hours. After incubation with FL-BO (2 µl, 100 µM, 20 minutes, 37°C), the medium was removed, and the cells were subsequently washed (1x medium, 200 µl, and 2x 1x phosphate-buffered saline [PBS]), fixed (4% paraformaldehyde in PBS), and permeabilized (ice-cold PBS/0.1% Triton X-100/0.5% bovine serum albumin [3x 200 µl, 2x 5-minute and 1x 30-minute incubations>]). Cells were then incubated with anti-PARP-1/2 Pab (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight, washed with PBS/ 0.1% Triton X-100 (3x 200 µl), and stained with secondary IgG-Cy5 Pab (1:100; Millipore, Billerica, MA) for 3 hours at 4°C. Before imaging, cells were washed with PBS (1x, 200 µl), stained with both Hoechst 33342 (Invitrogen) and Cellomics blue whole cell stain (Thermo Scientific, Waltham, MA) for 30 minutes at room temperature, and washed again with PBS (3x, 200 µl). Imaging was performed on a DeltaVision microscope (Applied Precision Instruments, Issaquah, WA) at 20x. For each cell line, nine different areas were measured per well. Each cell line was measured in biologic triplicate. FL-BO fluorescence for each cell line was determined by quantifying the total fluorescence and by subtracting the cells' respective green autofluorescence. Relative PARP-1/PARP-2 expression levels were determined by quantifying the fluorescence signal for each cell line and then subtracting unspecific secondary IgG-Cy5 Pab staining.
Mice
Experiments were performed in either C57BL/6 (B6) mice obtained from the Jackson Laboratory (biodistribution, pharmacokinetics; n = 9) or in nu/nu mice obtained from Massachusetts General Hospital (tumor implantations, imaging; n = 18). For all surgical procedures and imaging experiments, mice were anesthetized with 2.0% isoflurane in oxygen at 2.0 L/min. For imaging experiments lasting longer than 1 hour, the isoflurane flow rate was reduced to ∼1 L/min. Surgeries were conducted under sterile conditions with a zoom stereo microscope (Olympus SZ61; Olympus America, Center Valley, PA). All procedures and animal protocols were approved by the institutional subcommittee on research animal care.
For window chamber imaging experiments, HT1080 cells (2 x 106 cells, 50 µl 1x PBS) were implanted into dorsal skin chambers (DSCs; APJ Trading Co, Inc, Ventura, CA) in the dorsal skinfold of nu/nu mice as described before [10–14]. To allow neovascularization, HT1080 tumors were allowed to grow for 8 days. Spacers between both halves of the DSCs frame prevented excess compression of the tissue and vessels.
To obtain standardized uptake values (SUVs) for 18F-BO, nu/nu mice received four subcutaneous injections, each containing SKOV-3, MIA PaCa-2, A2780, or PANC-1 cells, into their flanks and shoulders (2.5 x 106 cells in 100 µl of 70:30 PBS/BD Matrigel [BD Biosciences, Bedford, MA] per injection). Tumors were then allowed to grow for 2 weeks before imaging. For dose-response experiments, nu/nu mice each received two subcutaneous injections containing A2780 cells into the flanks (2.5 x 106 cells in 100 µl of 70:30 PBS/BD Matrigel [BD Biosciences] per injection). Tumors were then allowed to grow for 10 to 15 days before the start of imaging experiments.
Intravital Window Chamber Imaging
Mice with HT1080 tumors in their DSCs were injected with 75 nmol of FL-BO (7.5 µl of DMSO, 67.5 µl of N,N-dimethylacetamide/Solutol (1:1), 150 µl 1x PBS). Accumulation of the probe in HT1080 tumor tissue was imaged in vivo in nu/nu mice as described previously [11]. A custom-made dorsal skinfold chamber holder was used to stabilize the sample and allow intravital imaging of probe accumulation at single-cell resolution for hours. Static and time series images were collected using a customized Olympus FV1000 based on a BX61-WI confocal microscope (Olympus America). A XLUMPLFLN 20x water immersion objective (NA 1.0) and a 60x LUMFLN (NA 1.10) water immersion objective were used for data collection (both Olympus America). FL-BO and H2B-Apple were scanned and excited sequentially using a 473-nm and a 559-nm diode laser, respectively, in combination with a DM405/488/559/635-nm dichroic beam splitter. Emitted light was then separated and collected using an SDM560 beam splitter and BA490-540 and BA575-675 band-pass filters (all Olympus America). Control tumors were used to optimize imaging conditions by ensuring that no photobleaching or phototoxicity occurred with the imaging settings used.
18F-BO Biodistribution Studies
B6 mice were used for blood half-life determinations. Mice were administered 34 ± 5 µCi of 18F-BO by intravenous tail vein injection. Blood sampling was performed by retro-orbital puncture using tared, heparinized capillary tubes. Samples were subsequently weighed, and activity was measured using an automatic gamma counter (Wallac Wizard 3″ 1480 Automatic Gamma Counter; PerkinElmer, Waltham, MA). Blood half-life data were fitted to a biexponential model (Graph-Pad Prism 4.0c; GraphPad Software, Inc, San Diego, CA), and results are reported as the weighted average of the distribution and clearance phases. For biodistributions, B6 mice were intravenously injected through the tail vein with either 43 ± 5 µCi (for the 2-hour time point) or 410 ± 22 µCi (for the 18-hour time point) 18F-BO. Animals were then sacrificed at 2 or 18 hours after injection, respectively. Tissues were subsequently harvested and weighed, and their radioactivity was counted using the PerkinElmer Wallac Wizard 3″ 1480 Automatic Gamma Counter. Statistical analysis was performed using GraphPad Prism 4.0c.
PET-Computed Tomography Imaging
Mice were imaged with PET-computed tomography (CT) using an Inveon small animal scanner (Siemens, Munich, Germany). Each PET acquisition took approximately 25 minutes. A high-resolution Fourier rebinning algorithm was used to rebin sinograms, followed by a filtered back-projection algorithm to reconstruct three-dimensional images without attenuation correction. Isotropic image voxel size was 0.796 x 0.861 x 0.861 mm, for a total of 128 x 128 x 159 voxels. Peak sensitivity of the Inveon accounts for 11.1% of positron emission, with a mean resolution of 1.65 mm. More than 100 counts were acquired per pixel, and the mean signal-to-noise ratio was greater than 20. Calibration of the PET signal with a cylindrical phantom containing 18F isotope was performed before all scans. CT images were reconstructed from 360 cone-beam x-ray projections with a power of 80 keV and 500 µA. The isotropic resolution of the CT images was 60 µm. Reconstruction of data sets, PET-CT fusion, and image analysis were done using IRW software (Siemens). Three-dimensional visualizations were produced using the DICOM viewer OsiriX (The OsiriX Foundation, Geneva, Switzerland).
In Vivo Correlation Experiments
nu/nu mice bearing A2780 (left shoulder), PANC-1 (right shoulder), MIA PaCa-2 (left flank), and SKOV3 (right flank) tumors were injected with 480 ± 20 µCi of 18F-BO and subjected to PET-CT imaging 2 hours after injection. After imaging, the mice were sacrificed. The tissues were then harvested and weighed, and their radioactivity was counted. After PET and CT image fusion, tumor SUV margins were drawn to span the entire tumor. Drawings were guided with the aid of the CT image using the Siemens Research Workplace v3.0 analysis application.
Western Blot of PARP-1/PARP-2 Expression
After PET imaging and gamma counting, tumors were homogenized in 400 µl of 1x radioimmunoprecipitation assay buffer and 2x Mini Complete Protease Inhibitor Cocktail tablet (Roche, Indianapolis, IN). The samples were spun at 4°C at 10,000 rpm for 10 minutes, and the supernatant was collected. Protein concentration was measured using a bicinchoninic acid protein assay according to the manufacturer's instructions (Thermo Scientific). Tumor lysate (10 µg) was loaded onto NuPAGE Novex 4% to 12% Bis-Tris 1.0-mm gels, and electrophoresis was performed with the XCell SureLock Mini-Electrophoresis system (both Invitrogen). Protein was transferred using the iBlot Dry Blotting System to a nitrocellulose membrane (Invitrogen). The blot was then blocked in 5% nonfat milk for 1 hour, washed with 1x TBS-Tween 20 (Boston BioProducts, Ashland, MA), and incubated overnight at 4°C with 1:1000 anti-PARP-1/2 in 5% nonfat milk (Santa Cruz Biotechnology). After three 5-minute washes and three 30-minute washes with 1x TBS-Tween 20, blots were incubated with 1:5000 goat antirabbit horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) at room temperature for 1 hour. After three 5-minute washes with 1x TBS-Tween 20, blots were incubated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), exposed for 20 minutes, and then processed with the Kodak (Rochester, NY) X-OMAT 2000A processor. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Western blots (after blocking and after three 5-minute washes with 1x TBS-Tween 20), blots were incubated for 1 hour at room temperature with 1:5000 anti-glyceraldehyde-3-phosphate dehydrogenase in 5% nonfat milk (R&D Systems, Minneapolis, MN). After three 5-minute washes with 1x TBS-Tween 20, blots were incubated with donkey antigoat horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) at room temperature for 1 hour. After three 5-minute washes with 1x TBS-Tween 20, blots were incubated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), exposed for 10 seconds, and then processed with the Kodak X-OMAT 2000A processor.
PET Imaging of Therapeutic Response
nu/nu mice bearing A2780 tumors were injected with 440 ± 40 µCi of 18F-BO through the tail vein and subjected to PET-CT imaging 2 hours after injection. Thereafter, the same mice were reinjected with 18F-FDG (523 ± 47 µCi) and reimaged. After 18F-FDG imaging, the mice were subjected to intraperitoneal injection of 1 mg of Olaparib per mouse formulated in 1:1:4 DMAC/Solutol/PBS (100 µl). The next day, mice were treated with 0.5 mg of Olaparib per mouse. Mice were then imaged as they had been on the first day. This treatment/imaging cycle was repeated for three more days, after which the animals were sacrificed. A total of 10 complete serial sessions were acquired for each mouse. Tumor SUV margins were drawn to span the entire tumor and were accomplished with the aid of the CT images using the Siemens Research Workplace v3.0 analysis application. SUV data from 18F-BO PET images underwent decay correction to the start of 18F-FDG PET imaging and used to correct 18F-FDG SUV data.
Results
Synthesis and Cellular Imaging
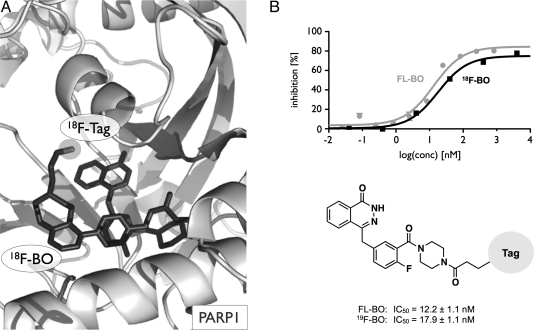
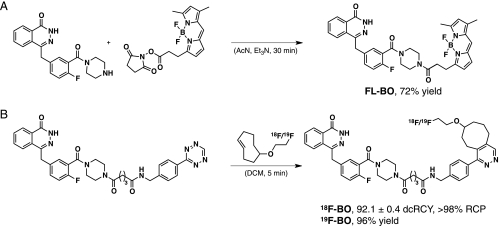
Before synthesis, we performed in silico modeling to determine which sites of the Olaparib scaffold would tolerate modifications. The docking platform [15] predicted (based on Penning et al. [16]) that the 2H-phthalazin-1-one group of 18F-BO would behave in an identical manner to that of Olaparib, namely by binding to the PARP-1-active site (Figures 1A and W1A). The 4-NH-piperazine functionality of Olaparib was shown to tolerate relatively bulky substituents without significantly affecting PARP-1 binding affinity. We thus modified this functionality through an NHS-ester reaction either with BODIPY FL succinimidyl ester to yield FL-BO or with tetrazine succinimidyl ester followed by 18/19F-trans-cyclooctene to yield 18/19F-BO (Figure 2). The identity and purity of the imaging agents were subsequently confirmed using NMR spectroscopy, HPLC, ESI-MS and high-resolution mass spectrometry. Using a PARP-1 activity assay, we then measured the IC50 values for FL-BO (12.2 ± 1.1 nM) and 19F-BO (17.9 ± 1.1 nM), which are similar to the one previously determined for Olaparib (AZD2281, 5 nM [7]) (Figures 1B and W1B).
Figure 1.
18F-BO and FL-BO. (A) Binding model depicting 18F-BO and PARP-1, with the 2H-phthalazin-1-one binding to the catalytically active site on PARP-1. (B) IC50 curves and values for 18F-BO (from Keliher et al. [8]) and FL-BO.
Figure 2.
Synthesis of imaging fluorescent and PET active imaging agents. (A) Synthesis of FL-BO: a fluorescent marker for PARP expression in vitro and in vivo. (B) Synthesis of 18F-BO: a PET agent allowing to measure PARP expression noninvasively in vivo [6,8].
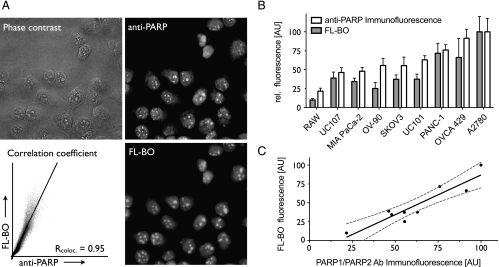
Next, we determined the intracellular uptake and localization of FL-BO. Figure 3A (Figure W2A) shows the typical staining patterns for cell lines incubated with FL-BO, fixed, permeabilized, and then costained with a PARP-1/2 antibody. The drug was found to localize predominantly in the nuclei, particularly in the nucleoli of cells, colocalizing with anti-PARP-1/2. The Pearson correlation coefficient for PANC-1 cells between the PARP antibody stain and FL-BO was Rcoloc. = 0.95. This correlation was similar for other ovarian and pancreatic cancer cell lines tested. We subsequently investigated whether the total cellular uptake of FL-BO correlated with PARP protein levels. Using different cell lines, we found a good correlation between PARP immunofluorescence and cellular FL-BO accumulation (R2 =0.858; Figures 3, B and C, and W2, B and C).
Figure 3.
Correlation of cellular FL-BO uptake and relative PARP expression. (A) In PANC-1 cells, there was an excellent correlation between intracellular FL-BO distribution and PARP-1/2 expression: phase contrast (top left), anti-PARP (top right), FL-BO (bottom right), and Pearson correlation coefficient of anti-PARP and FL-BO (bottom left). (B) Column representation of FL-BO uptake and anti-PARP immunofluorescence in different cell lines. (C) Correlation of FL-BO uptake and anti-PARP-1/2 immunofluorescence (dashed line indicates the 95% confidence band).
Intravital Imaging of FL-BO
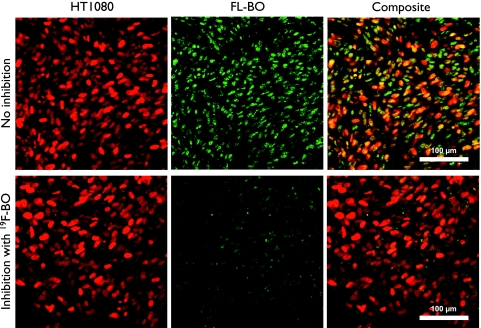
We next used a well-established window chamber model to determine the kinetics and cellular distribution of FL-BO at single-cell resolution in vivo [11]. Intravenous injection of FL-BO yielded a bright green fluorescent signal, which quickly diffused through the vessels' epithelial cells and resulted in a homogeneous nuclear staining of tumor cells (Figure 4, top row). To determine whether cellular accumulation could be inhibited in vivo, we also performed competition experiments with nonfluorescent 19F-BO (500 µg injected 90 minutes before FL-BO). Figure 4 shows that the inhibitor resulted in a marked decrease in uptake of the fluorescent imaging agent (Figure 4, bottom row).
Figure 4.
Intravital imaging of FL-BO. Top row (without prior injection of 19F-BO): HT1080 H2B mApple cells (red), FL-BO (green), and composite image. Bottom row (with prior injection of 19F-BO): HT1080 H2B mApple cells (red), FL-BO (green), and composite image. Note the inhibition of FL-BO uptake into tumor cells in vivo.
Whole-Body Imaging of 18F-BO
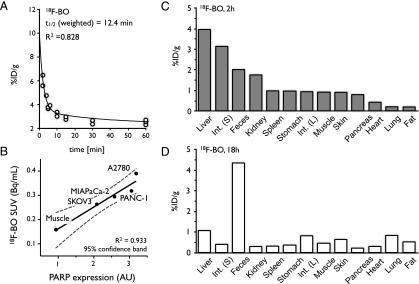
The blood half-life for 18F-BO was determined through serial bleeds, and the obtained data points were fitted using a biexponential decay curve. The resulting weighted half-life for 18F-BO was t1/2 = 12.4 minutes, with R2 = 0.828 (Figure 5A). Biodistribution studies at 2 hours after injection showed predominant hepatobiliary excretion of 18F-BO (3.9 ± 0.9 and 3.1 ± 1.6%ID/g for liver and small intestines, respectively; Figure 5C) with low values in most other host tissues. At 18 hours after injection, the highest amount of probe was found in the feces (Figure 5D).
Figure 5.
Pharmacokinetics of 18F-BO. (A) Blood half-life of 18F-BO after intravenous administration. (B) Correlation of PARP expression and 18F-BO uptake in four different ovarian and pancreatic tumor types as determined by immunoblot analysis. (C) Biodistribution of 18F-BO at 2 hours after intravenous administration. (D) Biodistribution of 18F-BO at 18 hours after intravenous administration.
18F-BO was next used to image PARP expression in vivo. For this, xenografts of four different human ovarian and pancreatic tumor models (SKOV3, MIA PaCa-2, PANC-1, and A2780, with ascending relative PARP expression profiles) were grown in nu/nu mice before tumoral uptake of 18F-BO was determined through PET imaging (Figures 5B and W3). The resulting SUVs (Bq/ml) for each of the four tumor types were subsequently quantitated by imaging and then correlated to PARP expression data obtained by Western Blot analysis. Trends for uptake values and Western blot analysis were similar, with A2780 having the highest uptake and expression, followed by PANC-1, MIA PaCa-2, and SKOV3 (Figures 5B and W3). Muscle tissue had both the lowest accumulation of 18F-BO and the lowest PARP expression.
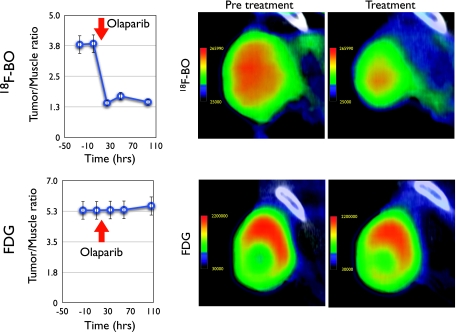
Finally, we determined whether 18F-BO could be used to measure therapeutic drug inhibition in vivo. For these experiments, mice bearing A2780 tumor xenografts were serially imaged both before and after Olaparib treatment (Figure 6). Tumoral 18F-BO tumor-muscle ratios were found to be significantly lower (P < .001) after single or multiple doses of Olaparib than before treatment in the same animal. As a comparison, we also obtained 18F-FDG scans to determine whether similar effects would be seen. Interestingly, we observed little change in tumor-muscle ratios of 18F-FDG after PARP treatment (Figure 6).
Figure 6.
Response of 18F-BO and 18F-FDG uptake to treatment with Olaparib measured in the same mouse. Left: Tumor/muscle plots at different time points before and after initiation of Olaparib treatment. Right: Representative PET-CT images of A2780 tumors (high PARP levels) before and after treatment. Note the changes with 18F-BO and the absence of changes with 18F-FDG. There were no appreciable CT changes in tumor volume during the 24-hour period after Olaparib injection.
Discussion
Our results show that, in ovarian cancer models, therapeutic doses of Olaparib inhibit PARP in vivo and that target inhibition can be quantitated by 18F-BO imaging within hours of initiating treatment. Our experiments were designed to 1) validate 18F-BO for in vivo PET imaging, 2) determine the robustness of our new imaging approach in different cancers, 3) directly compare 18F-BO imaging to the clinical standard of 18F-FDG PET imaging (because many cancers use glucose as an energy source [17,18]), and 4) determine the general suitability of the described platform for codeveloping bioorthogonally 18F-labeled companion diagnostics for therapeutic inhibitors. Interestingly, at the early time points investigated, therapeutic PARP inhibition had little effect on the 18F-FDG tumor/muscle imaging signal. In contrast, 18F-BO imaging showed profound changes. These results suggest that 18F-BO imaging could be of value to future clinical trials of PARP inhibitors. Specifically, 18F-BO imaging could be used for dose ranging, testing the comparative efficacy of combination treatments, evaluating treatment efficacy across patient subpopulations, and/or for enrolling (i.e., enriching for) likely responders to PARP inhibitor clinical trials.
PARP inhibitors are a new class of agent that have shown a certain amount of therapeutic efficacy, particularly in BRCA-related malignancies, notably breast and high-grade serous ovarian cancers [4]. For example, a phase 1 clinical study of Olaparib as a monoagent in breast cancer patients with BRCA mutations provided support for the theory of “synthetic lethality” [19]. In a follow-up study, in which the effects of combination therapy were evaluated, resistance to platinum correlated with decreased sensitivity to Olaparib [20]. However, a recent phase 2 clinical trial (ICEBERG 1) [21], using RECIST criteria as the metric of response, failed to show a dose response with Olaparib—suggesting that tumor response to therapy occurs through an alternative mode. In contrast, a more recent report demonstrated that administration of single-agent Olaparib in ovarian cancer patients with BRCA mutations was sufficient to induce tumor responses [5]. It is thus clear that the clinical response to PARP inhibition is varied and that currently accepted markers of such response (progression-free survival, RECIST, PARP inhibition in peripheral blood mononuclear cells, or hair follicles) are insufficiently sensitive (i.e., indirect and/or late). The availability of direct imaging tests capable of measuring PARP inhibition locally would thus be of enormous value in such settings.
The imaging agent used in the present study is based on the Olaparib scaffold with bioorthogonal modifications on the cyclopropane end—a region not essential for target binding [6,22]. Docking simulations showed that 18F-BO, like Olaparib and several other newer PARP inhibitors, binds to the catalytically active region of PARP-1 through its 2H-phthalazin-1-one group. The use of bioorthogonal 18F attachment has several advantages over direct fluorination, namely 1) the rapidity by which imaging agents can be developed, 2) the high synthetic yields, 3) the clean reaction products, 4) the short synthesis times, and 5) the ability to create companion fluorescent agents that can be readily tested through microscopic imaging [23]. Each of these is important in the rapid preparation and validation of companion imaging agents. Although companion imaging agents are structurally similar to their parent inhibitors, they differ importantly in their dosage. Compared with therapeutic doses, diagnostic doses of the imaging agent were approximately 700 times lower for PET imaging and 5 times lower for fluorescence imaging given the different inherent sensitivities of these techniques. In other words, the imaging agent has negligible effects compared with repeatedly administering the therapeutic parent compound.
We used a bioorthogonal approach to tag the small-molecule PARP inhibitor with 18F and convert it into a companion imaging agent. Orthogonal chemistry refers to a highly selective chemical reaction that is both fast and able to take place within living systems. Specifically, we used the trans-cyclooctene/tetrazine reaction because it is one of the fastest and most selective chemistries available under physiologic conditions. Additional advantages to this labeling platform include 1) the high translatability of fluorescently and PET-labeled compounds, 2) the standardized synthesis of 18F-labeled bioorthogonal precursors, and 3) the ability to rapidly screen potential targeted molecules of interest. Despite these advances and their benefits, we believe that 18F-BO and other such bioorthogonal small-molecule inhibitors could be improved further. For example, we anticipate the future use of hybrid fluorescent PET/optical tags [24,25]. These would not only enable the rapid translation of fluorescent targeted probes into PET probes but would also allow use of chemically identical molecules with both imaging modalities.
In summary, we describe a generic method for codeveloping bioorthogonally 18F-labeled companion diagnostics for therapeutic inhibitors. Using the example of PARP inhibition, we show that one such agent is able to accurately measure PARP expression levels in vivo and thus could be used as a tool to measure therapeutic inhibition within a day of treatment initiation. These agent(s) will likely be of value to the (pre)clinical testing of different PARP inhibitors as well as hopefully other emerging small-molecule inhibitors.
Supplementary Material
Acknowledgments
The authors thank Joshua Dunham and Jessica Truelove for image processing and generation; Rostic Gorbatov for surgery; Cesar Castro, Matthias Nahrendorf, and Yvonna Fisher-Jeffes for critical review of the article; and Ralph Mazitschek and Sarah Earley for many helpful discussions.
Footnotes
This study was funded in part by National Institutes of Health grants R01EB010011 and P50CA86355 (R.W.). K.S. Yang was supported by a National Institutes of Health grant T32-CA79443, and T. Reiner was supported by a grant from the German Academy of Sciences Leopoldina (LPDS 2009-24). The authors have no conflicts of interest to declare. The costs of publication of this article were defrayed, in part, by the payment of page charges. This article must therefore be hereby-marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.neoplasia.com
References
- 1.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13:1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 3.Comen EA, Robson M. Inhibition of poly(ADP)-ribose polymerase as a therapeutic strategy for breast cancer. Oncology (Williston Park) 2010;24:55–62. [PubMed] [Google Scholar]
- 4.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 5.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, Amnon A, Bell-McGuinn KM, Chen LM, Friedlander M, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of Olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2011;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 6.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed Engl. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menear KA, Adcock C, Boulter R, Cockcroft XL, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, et al. 4-[3-(4-Cyclopropane-carbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phth alazin-1-one: a novel bio-available inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem. 2008;51:6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 8.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. Chem Med Chem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkvoll KH, Rofstad EK, Brustad T, Marton P. A transparent chamber for the dorsal skin fold of athymic mice. Exp Cell Biol. 1984;52:260–268. doi: 10.1159/000163269. [DOI] [PubMed] [Google Scholar]
- 11.Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 2011;71:4608–4616. doi: 10.1158/0008-5472.CAN-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- 13.Makale M. Intravital imaging and cell invasion. Methods Enzymol. 2007;426:375–401. doi: 10.1016/S0076-6879(07)26016-1. [DOI] [PubMed] [Google Scholar]
- 14.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 15.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penning TD, Zhu GD, Gong J, Thomas S, Gandhi VB, Liu X, Shi Y, Klinghofer V, Johnson EF, Park CH, et al. Optimization of phenyl-substituted benzimidazole carboxamide poly(ADP-ribose) polymerase inhibitors: identification of (S)-2-(2-fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benzimidazole-4-carboxamide (A-966492), a highly potent and efficacious inhibitor. J Med Chem. 2010;53:3142–3153. doi: 10.1021/jm901775y. [DOI] [PubMed] [Google Scholar]
- 17.Locasale JW, Cantley LC, Vander Heiden MG. Cancer's insatiable appetite. Nat Biotechnol. 2009;27:916–917. doi: 10.1038/nbt1009-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 20.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 21.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, et al. Oral poly(ADP-ribose) polymerase inhibitor Olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 22.Reiner T, Earley S, Turetsky A, Weissleder R. Bioorthogonal small-molecule ligands for PARP1 imaging in living cells. Chembiochem. 2010;11:2374–2377. doi: 10.1002/cbic.201000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–991. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendricks JA, Keliher EJ, Wan D, Hilderbrand S, Weissleder R, Mazitschek R. Synthesis of 18F-BODIPY as bifunctional reporter for hybrid optical/PET imaging. Angew Chem Int Ed Engl. doi: 10.1002/anie.201107957. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Lin TP, Liu S, Huang CW, Hudnall TW, Gabbai FP, Conti PS. Rapid aqueous [18F]-labeling of a BODIPY dye for positron emission tomography/fluorescence dual modality imaging. Chem Commun (Camb) 2011;47:9324–9326. doi: 10.1039/c1cc13089g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.