Abstract
Mammalian target of rapamycin (mTOR) controls lymphangiogenesis. However, the underlying mechanism is not clear. Here we show that rapamycin suppressed insulin-like growth factor 1 (IGF-1)- or fetal bovine serum (FBS)-stimulated lymphatic endothelial cell (LEC) tube formation, an in vitro model of lymphangiogenesis. Expression of a rapamycin-resistant and kinase-active mTOR (S2035T, mTOR-T), but not a rapamycin-resistant and kinase-dead mTOR (S2035T/D2357E, mTOR-TE), conferred resistance to rapamycin inhibition of LEC tube formation, suggesting that rapamycin inhibition of LEC tube formation is mTOR kinase activity dependent. Also, rapamycin inhibited proliferation and motility in the LECs. Furthermore, we found that rapamycin inhibited protein expression of VEGF receptor 3 (VEGFR-3) by inhibiting protein synthesis and promoting protein degradation of VEGFR-3 in the cells. Down-regulation of VEGFR-3 mimicked the effect of rapamycin, inhibiting IGF-1- or FBS-stimulated tube formation, whereas over-expression of VEGFR-3 conferred high resistance to rapamycin inhibition of LEC tube formation. The results indicate that rapamycin inhibits LEC tube formation at least in part by downregulating VEGFR-3 protein expression.
Introduction
Lymphangiogenesis, like angiogenesis, refers to the formation of lymphatic vessels from preexisting lymphatic vessels, which plays an important role in promoting tumor growth and metastasis [1]. For numerous types of solid tumors, for example, breast, colon, and prostate cancers and melanoma, the lymphatic system is the primary conduit for initial metastasis [1,2]. Metastatic cancer spread is enhanced by an increase of lymphangiogenesis in and around the primary tumor [1,2]. Clinically, the extent of lymph node metastasis has been regarded as a major indicator for the staging and the prognosis of most human cancers and used to determine therapeutic strategy [1]. Thus, targeting lymphangiogenesis is becoming an attractive and potential approach for cancer therapy.
The mammalian target of rapamycin (mTOR), a member of the phosphoinositide-3′ kinase (PI3K)-related kinase family, lies downstream of the insulin-like growth factor 1 (IGF-1) receptor-PI3K [3,4]. mTOR functions at least as two complexes (mTORC1 and mTORC2) in mammalian cells [3,4]. mTORC1 is composed of mTOR, mLST8 (also termed G-protein β-subunit-like protein, a yeast homolog of LST8), proline-rich Akt substrate 40 kDa, and raptor (regulatory-associated protein of mTOR) [5–11], whereas mTORC2 consists of mTOR, mLST8, mammalian stress-activated protein kinase-interacting protein 1, rictor (rapamycin-insensitive companion of mTOR), and protor (protein observed with rictor, also named proline-rich protein 5) [12–19]. mTORC1 is sensitive to rapamycin, growth factors, energy, amino acids, and redox levels and phosphorylates ribosomal p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) [5–11]. mTORC2 is only sensitive to prolonged (>24 hours) rapamycin exposure in certain cases and growth factors and phosphorylates Akt and serum and glucocorticoid-inducible kinase 1 [12–20]. In response to growth factors, energy, amino acids, and redox levels, mTORC1 regulates cell proliferation and growth by controlling protein synthesis and ribosome biogenesis through phosphorylation of 4E-BP1 and S6K1 [5–11]. Both mTORC1 and mTORC2 interact with a negative regulator DEPTOR [21]. Although the cellular functions of the mTOR complexes remain to be determined, current data indicate that mTOR plays a central role in the regulation of cell growth, proliferation, survival, differentiation, motility, and angiogenesis [3,4].
Clinical trials have demonstrated that rapamycin and its analogs (CCI-779, RAD001, and AP23573; termed rapalogs) are promising anticancer agents. Rapalogs share a common mechanism by which they form a complex with FK506 binding protein 12 and then bind to mTOR, selectively inhibiting its function [22]. Studies have demonstrated that rapamycin functions as an anticancer agent by inhibiting cell growth and proliferation, inducing apoptosis and autophagy, inhibiting cell motility and invasion, and preventing angiogenesis [22]. Recently, rapamycin has been found to exert a potent antilymphangiogenic effect in vitro and in vivo [23–25], suggesting that mTOR regulates lymphangiogenesis as well. However, so far, the underlying molecular mechanism remains largely unknown.
Vascular endothelial growth factor (VEGF) receptor 3 (VEGFR-3), also known as fms-like tyrosine kinase 4, is primarily expressed on the surface of the lymphatic endothelial cells (LECs) [26]. In response to VEGF-C/D binding, VEGFR-3 can be activated, leading to activation of the downstream signaling molecules, such as PI3K/Akt and mitogen-activated protein kinase pathways, which are crucial for LEC survival and lymphangiogenesis [27–30], as well as metastasis [1,2,31]. Thus, VEGFR-3 pathway has emerged as a novel target for cancer prevention and treatment.
Deregulation of IGF-1 signaling occurs frequently in a variety of tumors, and is correlated to malignant progression and poor prognosis [3,4]. Recent studies have demonstrated that IGF-1 and IGF-2 also induce lymphangiogenesis in vitro and in vivo [32,33], suggesting that IGFs may contribute to tumor lymphangiogenesis and lymphatic metastasis. Here, for the first time, we show that mTOR regulates IGF-1- or fetal bovine serum (FBS)-stimulated lymphangiogenesis in an in vitro model (tube formation) [34] by mediating protein expression of VEGFR-3, further highlighting that mTOR inhibitors can be exploited for prevention and treatment of tumor metastasis.
Materials and Methods
Cell Lines and Cultures
Mouse LECs and pleural mesothelial cells (MIM), derived from the mesenteric lymphatic tissue and the pleura of transgenic mice expressing SV40 large T antigen [35], respectively, were grown in antibiotic-free Dulbecco modified Eagle medium (DMEM)/F12 medium (Mediatech, Herndon, VA) supplemented with 10% FBS (HyClone, Logan, UT) at 37°C and 5% CO2. Human embryonic kidney 293 (American Type Culture Collection, Manassas, VA) and 293TD cells (Invitrogen, Carlsbad, CA) were growninantibiotic-free DMEM supplemented with 10% heat-inactivated FBS at 37°C and 5% CO2. For experiments where cells were deprived of serum, cell monolayers were washed with phosphate-buffered saline, and incubated in the serum-free DMEM/F12 medium (Mediatech).
Plasmids and Transfection
LEC clones stably overexpressing p3xFlag-VEGFR-3-TV1 and p3xFlag-TV1 plasmid (empty vector, as a control) were generated and used as described [34].
Recombinant Adenoviral Constructs and Infection
The recombinant adenoviruses expressing FLAG-tagged rapamycin-resistant and kinase-active mTOR (S2035T, designated mTOR-T), rapamycin-resistant and kinase-dead mTOR-T (S2035T/D2357E, designated mTOR-TE) and the control vector expressing green fluorescence protein (GFP) alone (Ad-GFP) were described previously [37]. All recombinant adenoviral constructs were amplified, titrated, and used as described [37].
Lentiviral Short Hairpin RNA Cloning, Production, and Infection
Lentiviral short hairpin RNAs (shRNAs) to VEGFR-3 and GFP were produced as described [34]. LECs, when grown to ∼70% confluence, were infected with the above lentiviral shRNAs in the presence of 8 µg/ml polybrene and exposed to 2 µg/ml puromycin after 24 hours of infection. In 5 days, cells were used for experiments.
Tube Formation Assay
Tube formation assay was performed as described [34]. Briefly, LECs, pretreated with or without rapamycin (100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, were trypsinized and then seeded into a 96-well plate (2 x 104/well) precoated with 40 µl (10 mg/ml) growth factor-reduced Matrigel (BD Biosciences, Billerica, MA), in DMEM/F12 medium supplemented with/without rapamycin (100 ng/ml) ± IGF-1 (10 ng/ml) or 2% FBS. After 2 to 3 hours of incubation at 37°C, the capillary tube structures were observed, and representative images were captured with an Olympus inverted phase-contrast microscope (Olympus Optical Co, Melville, NY) (200x) equipped with the Quick Imaging system. Tube length was quantified by ImageJ software (http://rsbweb.nih.gov/ij/; National Institutes of Health, Bethesda, MD). Briefly, three randomly selected fields of view were photographed in each treatment. Tube length was assessed by drawing a line along each tube and measuring the length of the line in pixels. The average of three fields was taken as the value for each treatment.
Assays for Cell Proliferation, Cell Motility, and Viability
The effects of rapamycin on LEC proliferation, motility, and viability were determined as described previously, using cell proliferation assay [36], single-cell motility assay [37], and one-solution assay [38], respectively.
Reverse Transcription-Polymerase Chain Reaction
The effect of rapamycin on VEGFR-3 mRNA expression was determined by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR), as described [34].
Determination of VEGFR-3 Protein Synthesis and Degradation
To determine the effect of rapamycin on VEGFR-3 protein synthesis, LECs, grown in 100-mm dishes to 70% confluence, were serum starved for 24 hours, and pretreated with or without rapamycin (100 ng/ml) for 22 hours, followed by stimulation with IGF-1 (10 ng/ml) or 2% FBS for 2 hours. Subsequently, the cells were briefly washed with phosphate-buffered saline twice and cultured in 3 ml of labeling medium (DMEM, without l-Met/l-Cys; Mediatech) containing 10 µM MG-132 for 10 minutes to deplete Met/Cys in the cells, in the presence or absence of rapamycin with or without IGF-1 or FBS. The cells were then pulsed with 0.3 mCi of 35S-Met/Cys (MP Biomedicals, Solon, OH) for 4 hours and lysed in the RIPA buffer (50 mM Tris, pH 7.2, 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, protease inhibitor cocktail [1:1000; Sigma, St Louis, MO]) followed by immunoprecipitation with antibodies to VEGFR-3 and GAPDH, respectively. The immunocomplexes were subjected to SDS-PAGE, transferred to a polyvinylidene fluoride membrane, and finally autoradiographed at -80°C. ImageJ was used to semiquantitate the intensities of the bands.
To determine the effect of rapamycin on VEGFR-3 protein degradation, LECs, grown in the growth medium to 90% confluence, were treated with 50 µg/ml cycloheximide (CHX; Sigma) in the presence or absence of rapamycin (100 ng/ml) for 0 to 12 hours, followed by Western blot analysis with antibodies to VEGFR-3 and β-tubulin (loading control), respectively.
Western Blot Analysis
Western blot analysis was performed as described previously [34]. The primary antibodies used included antibodies to mTOR (FRAP), S6K1, LYVE-1, fms-like tyrosine kinase 4 (VEGFR-3), GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), 4E-BP1 (Zymed Laboratories, South San Francisco, CA), p-S6K1 (T389), p-4E-BP1 (T70) (Cell Signaling, Beverly, MA), FLAG, and β-tubulin (Sigma). ImageJ was used to semiquantitate the intensities of the bands.
Statistical Analysis
Results were expressed as mean values ± SD. Data were analyzed by one-way analysis of variance followed by post hoc Dunnett t test for multiple comparisons. P < .05 was considered significant.
Results
Rapamycin Inhibits IGF-1- or FBS-Stimulated Tube Formation in LECs
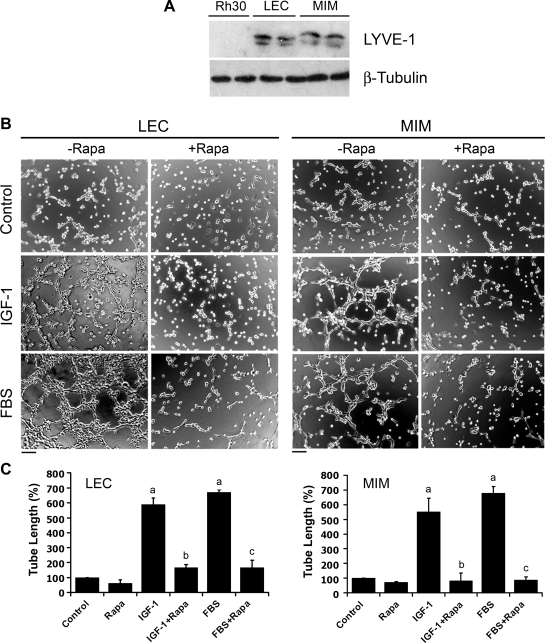
To elucidate the molecular mechanism by which mTOR regulates lymphangiogenesis, first of all, we investigated the effect of rapamycin, a selective inhibitor of mTOR, on capillary-like tube formation in LECs, an in vitro lymphangiogenesis model [34]. Two established LEC lines, LEC and MIM [35], were used. As shown in Figure 1A, a lymphatic specific marker, LYVE-1, was detectable in LEC and MIM cells, but not in a rhabdomyosarcoma (Rh30) cells (as a negative control), indicating that both LEC and MIM still possessed lymphatic characteristics through passages and could be used for the studies. We found that serum-starved LEC and MIM cells hardly formed the capillary tube structures on growth factor-reduced Matrigel within 2 hours (Figure 1B). Consistent with the previous in vivo findings [32,33], IGF-1 did stimulate lymphangiogenesis in vitro, as detected by the tube formation (Figure 1B). Here we found that IGF-1 (10 ng/ml) or 2% FBS stimulated the tube formation by approximately five-to seven-fold, which was significantly attenuated by pretreatment with rapamycin (100 ng/ml, for 24 hours; Figure 1C).
Figure 1.
Rapamycin inhibits IGF-1/FBS-stimulated tube formation in LECs. (A) Cell lysates from indicated cells were subjected to Western blot analysis with the indicated antibodies. (B and C) LEC and MIM cells were treated with rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by tube formation assay. Representative images are shown in B. Bar, 100 µm. The length of tube-like formation was evaluated by ImageJ software. Quantitative data are presented as mean ± SD (n = 3) in C. aP <.05, difference versus control group. bP < .05, difference versus IGF-1 group. cP < .05, difference versus FBS group.
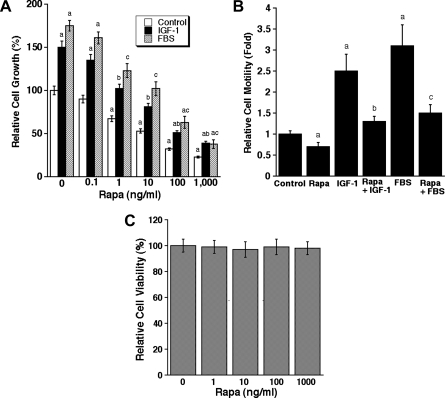
Because cell proliferation and migration are critical steps for lymphangiogenesis [1,2], we also examined the effects of rapamycin on these cellular events. As illustrated in Figure 2A, treatment with rapamycin for 72 hours inhibited proliferation in LECs in DMEM/F12 or the medium supplemented with IGF-1 (10/ng/ml) or FBS (10%) in a concentration-dependent manner. The half maximal inhibitory concentration (IC50) was 20 to 30 ng/ml. In addition, IGF-1 or 2% FBS stimulated LEC motility by approximately three-to four-fold, which was attenuated by rapamycin (100 ng/ml) nearly to the basal level (Figure 2B). However, treatment with rapamycin (100 ng/ml) for 24 hours did not obviously alter cell viability (Figure 2C), as detected by one-solution assay. Considering that one-solution assay cannot differentiate cell proliferation inhibition from cytotoxicity, trypan blue staining, a conventional method to detect cell viability, was used. We noticed that treatment with rapamycin (100 ng/ml) for 24 hours did not have an obvious effect on the cell viability in LEC and MIM cells, as approximately 96% of the cells excluded the dye.
Figure 2.
Rapamycin inhibits proliferation and motility in LECs. (A) LECs, grown in serum-free DMEM/F12 or the medium supplemented with IGF-1 (10 ng/ml) or FBS (10%), were exposed to rapamycin (0-1000 ng/ml) for 72 hours, followed by cell counting using a Beckman Coulter counter. (B) Cell motility of LECs was determined using the single-cell motility assay. (C) LEC viability was evaluated by one-solution assay. Quantitative data are presented as mean ± SD (n = 3) in A to C. aP < .05, difference versus control group. bP < .05, difference versus IGF-1 group. cP < .05, difference versus FBS group.
Rapamycin Inhibits IGF-1- or FBS-Stimulated LEC Tube Formation in an mTOR Kinase Activity-Dependent Manner
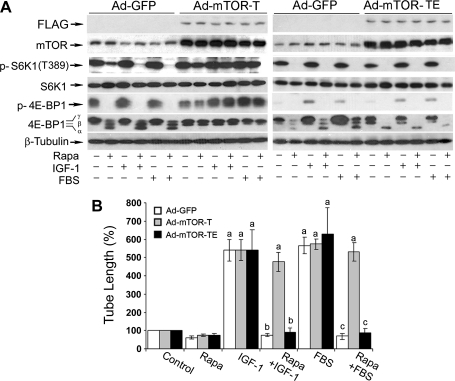
Because a kinase activity-independent function for mTOR in the regulation of cell differentiation has been reported [39], we next examined whether rapamycin inhibits LEC tube formation by inhibiting the kinase activity of mTOR. For this, LECs were infected with recombinant adenovirus expressing empty vector (GFP), FLAG-tagged rapamycin-resistant but kinase-active mTOR (S2035T, mTOR-T), or kinase-dead mTOR-T (S2035T/D2357E, mTOR-TE) for 24 hours, and then treated with or without rapamycin for 24 hours in the presence or absence of IGF-1 or 2% FBS. We found that the expression of mTOR-T, but not mTOR-TE or GFP, prevented rapamycin inhibition of phosphorylation of S6K1 (T389) and 4E-BP1 (Figure 3A), the two best-characterized downstream effector molecules of mTOR. The results show that mTOR-T functioned as a rapamycin-resistant mutant, and mTOR-TE as a kinase-dead mutant in LECs, as seen in other cell lines [37]. Interestingly, expression of mTOR-T, but not mTOR-TE or GFP, conferred high resistance to rapamycin inhibition of LEC tube formation (Figure 3B), suggesting that rapamycin inhibits IGF-1- or FBS-stimulated LEC tube formation in an mTOR kinase activity-dependent manner.
Figure 3.
Rapamycin inhibits LEC tube formation in an mTOR kinase activity-dependent manner. LECs were infected for 24 hours with Ad-GFP, Ad-mTOR-T, and Ad-mTOR-TE, respectively, and then serum-starved for 24 hours. Subsequently, the cells were treated with or without rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by Western blot analysis with the indicated antibodies (A) or tube formation assay (B). Quantitative data are presented as mean ± SD (n = 3) in B. aP < .05, difference versus control group. bP < .05, difference versus IGF-1 group. cP < .05, difference versus FBS group.
Rapamycin Inhibits Expression of VEGFR-3 Protein Expression
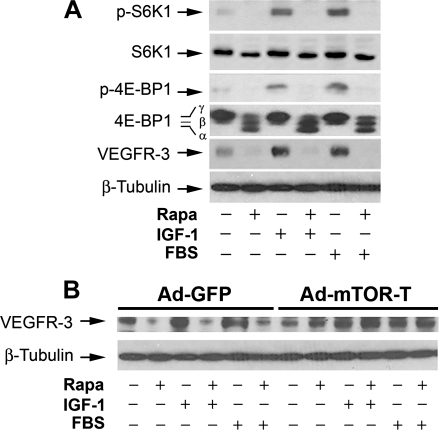
VEGFR-3 is primarily expressed in LECs and essential for lymphangiogenesis [1,26]. To determine whether mTOR regulates lymphangiogenesis through targeting VEGFR-3 signaling, LECs were exposed to rapamycin (100 ng/ml) for 24 hours, followed by Western blot analysis. As shown in Figure 4A, a considerable level of cellular VEGFR-3 protein was detectable in LECs grown in serum-free DMEM/F12 medium, and IGF-1 (10 ng/ml) or 2% FBS stimulated VEGFR-3 expression by approximately two-to three-fold. As expected, rapamycin treatment inhibited the basal or IGF-1/FBS-stimulated phosphorylation of S6K1 and 4E-BP1, two best-known substrates of mTOR [3,4]. Of interest, rapamycin downregulated the basal or IGF-1/FBS-stimulated expression of VEGFR-3 by more than 85%, suggesting that mTOR regulates VEGFR-3 protein expression.
Figure 4.
Rapamycin inhibits cellular protein expression of VEGFR-3 in LECs. LECs (A), or LECs infected for 24 hours with Ad-GFP and Ad-mTOR-T, respectively (B), were treated with rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by Western blot analysis with the indicated antibodies. β-Tubulin was used as a loading control.
To substantiate the above finding, LECs were ectopically expressing mTOR-T and GFP, respectively, by infection with corresponding recombinant adenoviruses. As shown in Figure 4B, expression of mTOR-T, but not GFP, prevented rapamycin down-regulation of VEGFR-3 expression, indicating that rapamycin inhibits VEGFR-3 protein expression in an mTOR-dependent manner.
Rapamycin Inhibits LEC Tube Formation by Downregulating VEGFR-3 Protein Expression
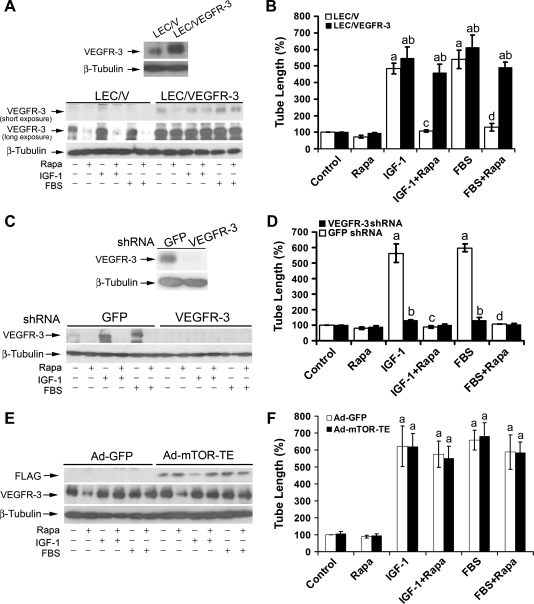
To determine the role of VEGFR-3 in rapamycin inhibition of LEC tube formation, LECs (LEC/VEGFR-3) stably overexpressing VEGFR-3 were generated. In comparison with the control cells (LEC/V) transfected with the empty vector, an approximately three-fold increase of VEGFR-3 protein expression was detected in LEC/VEGFR-3 cells (Figure 5A, upper panel). Treatment with rapamycin (100 ng/ml) for 24 hours reduced the basal or IGF-1/FBS-stimulated protein expression of VEGFR-3 by approximately 85% in LEC/V cells (Figure 5A, bottom panel). When LEC/VEGFR-3 cells were treated with rapamycin (100 ng/ml) for 24 hours, VEGFR-3 protein expression was downregulated by 70%, 50%, and 30% under serum-free, IGF-1, and FBS stimulation conditions, respectively, but the VEGFR-3 protein levels under any conditions were still higher than the basal level in the control (LEC/V) cells (Figure 5A, bottom panel). Of interest, overexpression of VEGFR-3 did not significantly influence the basal tube formation, but significantly increased IGF-1/FBS-stimulated tube formation and conferred high resistance to rapamycin inhibition of the tube formation in LECs (Figure 5B), suggesting that rapamycin inhibits LEC tube formation at least in part by suppressing VEGFR-3 protein expression.
Figure 5.
Rapamycin inhibits LEC tube formation by downregulating VEGFR-3 protein expression. (A and B) Overexpression of VEGFR-3 confers high resistance to rapamycin inhibition of LEC tube formation. Overexpression of VEGFR-3 was detected in pooled clones of LECs stably transfected VEGFR-3 (LEC/VEGFR-3) but not in the control cells transfected with an empty vector (LEC/V) by Western blot analysis (A, upper panel). LEC/V (control) and LEC/VEGFR-3 cells were treated with rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by Western blot analysis with the indicated antibodies (A, bottom panel) or tube formation assay (B). Quantitative results of tube formation are shown as mean ± SD (n = 3) in (B). aP <.05, difference versus control group. bP <.05, difference versus LEC/V group. cP < .05, difference versus IGF-1 group. dP < .05, difference versus FBS group. (C and D) Down-regulation of VEGFR-3 mimics the effect of rapamycin, inhibiting IGF-1/FBS-stimulated tube formation. Lentiviral shRNA to VEGFR-3, but not GFP, downregulated VEGFR-3 protein expression in LECs, as detected by Western blot analysis with the indicated antibodies (C, upper panel). LECs, infected with lentiviral shRNAs to VEGFR-3 and GFP (control), respectively, were treated with rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by Western blot analysis with the indicated antibodies (C, bottom panel) or tube formation assay (D). Quantitative results of tube formation are shown as mean ± SD (n = 3) in D. aP < .05, difference versus control group. bP <.05, difference versus GFP shRNA group. cP < .05, difference versus IGF-1 group. dP <.05, difference versus FBS group. (E and F) Overexpression of VEGFR-3 renders high resistance to rapamycin inhibition of tube formation in LECs expressing mTOR-TE. LEC/VEGFR-3 cells were infected for 24 hours with Ad-GFP and Ad-mTOR-TE, respectively, and then serum starved for 24 hours. Subsequently, the cells were treated with or without rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by Western blot analysis with the indicated antibodies (E) or tube formation assay (F). Quantitative data are presented as mean ± SD (n = 3) in F. aP < .05, difference versus control group.
To further verify the role of VEGFR-3 in rapamycin inhibition of LEC tube formation, we used RNA interference. Infection with lentiviral shRNA to VEGFR-3 knocked down the protein expression of VEGFR-3 by approximately 90%, comparing with the control infected with lentiviral shRNA to GFP (Figure 5C, upper panel). Silencing VEGFR-3 mimicked the effect of rapamycin, inhibiting IGF-1- or FBS-stimulated tube formation by approximately 80% (Figure 5D). Under this condition, no synergistic or additive inhibitory effect on the tube formation was observed by treatment with rapamycin (Figure 5D). The results suggest that rapamycin inhibits LEC tube formation primarily by suppressing VEGFR-3 protein expression.
In addition, we also observed that silencing VEGFR-3 inhibited FBS-or IGF-1-stimulated tube formation in LECs infected with recombinant adenoviral vectors expressing mTOR-T or GFP (control), regardless of the presence or absence of rapamycin (data not shown). The result indicates that silencing VEGFR-3 was able to overcome the resistance of cells expressing mTOR-T to inhibition of tube formation by rapamycin. However, when LEC/VEGFR-3 cells were infected with recombinant adenoviral vectors expressing mTOR-TE or GFP (control), considerable levels of VEGFR-3 were still detected in the cells stimulated with IGF-1- or FBS (Figure 5E), which was in agreement with the observation in LEC/VEGFR-3 cells (Figure 5A). Of note, overexpression of VEGFR-3 rendered high resistance to rapamycin inhibition of IGF-1- or FBS-stimulated tube formation in LECs expressing mTOT-TE or GFP (Figure 5F). Our findings strongly suggest that VEGFR-3 is essential for mTOR-mediated LEC tube formation.
Rapamycin Inhibits Protein Expression of VEGFR-3 by Inhibiting Protein Synthesis and Promoting Protein Degradation of VEGFR-3 in LECs
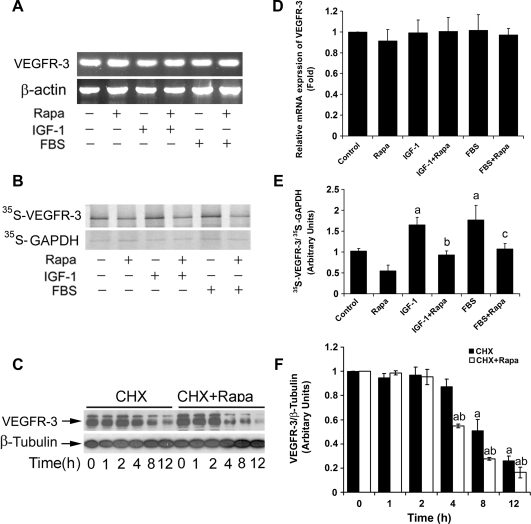
Rapamycin inhibition of protein expression of VEGFR-3may occur at transcriptional, translational, and/or posttranslational level. To address this question, firstly, semiquantitative RT-PCR was used to determine whether rapamycin affects VEGFR-3 mRNA expression. As shown in Figure 6A, stimulation with IGF-1 (10 ng/ml) or 2% FBS in the presence or absence of rapamycin (100 ng/ml, 24 hours) did not significantly alter mRNA expression of VEGFR-3.
Next, 35S-Met/Cys labeling was used to determine whether rapamycin affects VEGFR-3 protein synthesis. Serum-starved LECs were pretreated with IGF-1 (10 ng/ml) or 2% FBS in the presence or absence of rapamycin (100 ng/ml) for 24 hours and then pulsed with 35S-Met/Cys for 4 hours. By autoradiography, IGF-1 or FBS enhanced incorporation of 35S-Met/Cys into VEGFR-3 by approximately 1.5-fold, which was significantly (by 40%–45%) attenuated by rapamycin (Figure 6B), suggesting that rapamycin inhibits protein synthesis of VEGFR-3 in LECs.
Figure 6.
Rapamycin does not alter mRNA expression but inhibits protein synthesis and promotes protein degradation of VEGFR-3. (A) Rapamycin did not affect VEGFR-3 mRNA level. Total RNA was extracted from LECs treated with rapamycin (Rapa, 100 ng/ml) for 24 hours in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, followed by semiquantitative RT-PCR. β-Actin was used as a loading control. (B) Rapamycin inhibited protein synthesis of VEGFR-3 in LECs. LECs were pretreated with rapamycin (Rapa, 100 ng/ml) for 24 hours, in the presence or absence of IGF-1 (10 ng/ml) or 2% FBS, and then pulsed with 35S-Met/Cys for 4 hours, followed by immunoprecipitation with antibodies to VEGFR-3. The immunoprecipitates were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes, followed by autoradiography. GAPDH served as an internal control. (C) Rapamycin promoted protein degradation of VEGFR-3 in LECs. LECs, grown in 10% FBS-DMEM/F12 medium, were exposed to CHX (50 µg/ml), in the presence or absence of rapamycin (Rapa, 100 ng/ml) for 0 to 12 hours, followed by Western blot analysis with the indicated antibodies. Semiquantitative data for A, B, and C by densitometry using ImageJ are shown in D, E, and F, respectively. Results are means ± SD and are pooled from three independent experiments. aP < .05, difference versus control group. bP < .05, difference versus IGF-1 group. cP < .05, difference versus FBS group. dP < .05, difference versus CHX group.
In addition, to determine whether rapamycin influences protein degradation of VEGFR-3, LECs, grown in the complete growth medium, were exposed to CHX (50 µg/ml), an inhibitor of eukaryotic protein synthesis by preventing initiation and elongation on 80S ribosomes, in the presence or absence of rapamycin (100 ng/ml), for up to 12 hours, followed by Western blot analysis. We found that rapamycin strikingly promoted VEGFR-3 protein turnover rate. As shown in Figure 6C, a considerable level of VEGFR-3 protein was still detectable when the cells were treated with CHX alone for 8 hours. However, a lower level of VEGFR-3 protein was observed when the cells were treated with CHX + rapamycin only for 4 hours. Taken together, our results reveal that rapamycin did not alter the mRNA level, but inhibited the protein synthesis and promoted the protein degradation of VEGFR-3, thereby downregulating VEGFR-3 protein expression in LECs.
Discussion
Recent studies have shown that mTOR not only controls angiogenesis [40,41] but also regulates lymphangiogenesis [23–25]. mTOR controls angiogenesis by regulating expression of hypoxia-inducible factor 1α and VEGF-A (or VEGF) [40,41]. However, it is not known how mTOR regulates lymphangiogenesis. Here, for the first time, we present evidence that mTOR regulates IGF-1- or FBS-stimulated lymphangiogenesis in an in vitro model (tube formation) by mediating the expression of VEGFR-3 in LECs. This is supported by the observations that 1) rapamycin inhibits IGF-1- or FBS-stimulated LEC tube formation in an mTOR kinase activity-dependent manner; 2) rapamycin inhibited IGF-1- or FBS-stimulated VEGFR-3 protein expression; 3) overexpression of VEGFR-3 conferred high resistance to rapamycin inhibition of IGF-1- or FBS-stimulated LEC tube formation; and 4) down-regulation of VEGFR-3 mimicked the effect of rapamycin, blocking IGF-1- or FBS-stimulated LEC tube formation. These data are in line with the previous findings that blocking VEGFR-3 signaling alone by VEGFR-3 fusion protein [42,43], VEGFR-3-soluble form [29,44], or small-molecule inhibitors of VEGFR-3 [30,45,46] inhibited lymphangiogenesis. As deregulation of IGF-1 signaling occurs frequently in a variety of tumors and is associated with malignant progression and poor prognosis [3,4], our results strongly support the notion that mTOR inhibitors are a new class of antilymphangiogenic agents and that they may be explored for prevention and treatment of tumor metastasis.
VEGFs and their receptors are central controllers of angiogenesis and lymphangiogenesis [1]. Five VEGFs (VEGF or VEGF-A, placenta growth factor, VEGF-B, VEGF-C, and VEGF-D) and three VEGF receptors (VEGFR-1, -2, and -3) have been well documented in mammals [1]. VEGFR-1/2 and VEGFR-3 are primarily expressed on the surface of vascular and LECs, respectively [1]. VEGF-A binds to VEGFR-1/2, regulating vasculogenesis and angiogenesis, whereas VEGF-C/D binds to VEGFR-3, mediating lymphangiogenesis [1]. It has been described that rapamycin inhibits lymphangiogenesis by inhibiting expression of VEGF-C in rat lymphatic metastasis-prone cell line B13LM, a tumor cell line [24]. However, in the present study, we failed to detect expression of VEGF-C or -D in immortalized murine LECs, which were isolated from the mesenteric lymphatic tissue of transgenic mice expressing SV40 large T antigen [35], even without rapamycin treatment (data not shown). As a positive control, we did observe that rapamycin downregulated protein expression of VEGF-C in prostate (PC-3) and breast (MDA-MB231) carcinoma cells (data not shown). Our data are in agreement with the previous findings that VEGFs are generally produced and secreted by tumor cells but not by the vascular or LECs [1,2]. The results further indicate that rapamycin inhibition of IGF-1- or FBS-stimulated LEC tube formation in our model was primarily through blocking VEGFR-3 protein expression rather than through inhibiting VEGF-C/D expression. However, we also found that overexpression of VEGFR-3 alone was not able to induce LEC tube formation under serum starvation without stimulation with IGF-1 or FBS, suggesting that a certain level of VEGFR-3 activity in the cells is essential for the tube formation. Also, FBS seemed to stimulate LEC tube formation more potently than IGF-1. This is probably due to the presence of more growth factors (platelet-derived growth factors [PDGFs], fibroblast growth factor 2, IGFs, etc.) or hormones (e.g., insulin) in the FBS. In addition, we noticed that overexpression of VEGFR-3 failed to completely rescue the tube formation inhibited by rapamycin, suggesting that rapamycin inhibits IGF-1- or FBS-stimulated LEC tube formation probably involving other signaling molecules as well. More studies are needed to address this issue.
In addition to VEGF-C/D, several growth factors have also been reported to induce lymphangiogenesis [32,33,47–51]. However, the mechanisms underlying these effects are various among the growth factors. For instance, the basic fibroblast growth factor 2 induced lymphatic vessel growth in the mouse cornea, which occurred indirectly by induction of VEGF-C expression and activation of VEGFR-3 signaling [47,48]. PDGF-BB or hepatocyte growth factor promoted lymphangiogenesis, through direct activation of hepatocyte growth factor receptor (also known as c-Met) or PDGF receptors, respectively, rather than through activation of VEGFR-3 [49,50]. Similarly, IGF-1 induced lymphangiogenesis by direct activation of IGF-1 receptor, which was independent of VEGF-C/-D/VEGFR-3 system as well, because a soluble VEGFR-3, a known lymphangiogenic antagonist, was unable to block IGF-1-induced corneal angiogenesis or lymphangiogenesis in vivo [32]. In this study, however, we found that IGF-1 stimulates LEC tube formation through up-regulation of VEGFR-3 protein expression, which is regulated through an mTOR-dependent mechanism. It has been described that IGF-1 induces angiogenesis by indirect up-regulation of VEGF expression [51], implying dependence on VEGFR signaling. Although we cannot rule out the discrepancy being due to different experimental models used, likely, IGF-1 may regulate angiogenesis and lymphangiogenesis through VEGFR-dependent and -independent mechanisms. Further studies are required to determine how IGF-1 activates VEGFR-3 signaling.
In this study, we found that rapamycin dramatically decreased the basal or IGF-1/FBS-stimulated VEGFR-3 expression in LECs. To understand the underlying mechanism, RT-PCR, 35S-Met/Cys labeling, and VEGFR-3 protein degradation assay were performed. We found that IGF-1, FBS, or rapamycin did not obviously alter VEGFR-3 mRNA level. However, rapamycin was able to inhibit protein synthesis and promote protein degradation of VEGFR-3. This is in agreement with other observations that mTOR mediates Cap-dependent translation initiation of cyclin D1, VEGF, and others [3–5]. Currently, we do not know how rapamycin promotes VEGFR-3 degradation. Most recent studies have demonstrated that mTOR regulates DEPTOR protein level through SCF(βTrCP) E3 ubiquitin ligase-dependent mechanism [52–54]. New studies may gain insight into whether rapamycin downregulates VEGFR-3 by a similar mechanism.
In summary, here we have shown that rapamycin inhibits IGF-1/FBS-stimulated LEC tube formation in an mTOR kinase activity- dependent manner, supporting the idea that mTOR regulates lymphangiogenesis. Mechanistically, rapamycin inhibits LEC tube formation at least in part through downregulating VEGFR-3 protein expression. The results suggest that mTOR inhibitors are potent antilymphangiogenic agents and that they can be explored for the prevention and treatment of tumor metastasis.
Abbreviations
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- DMEM
Dulbecco modified Eagle medium
- FBS
fetal bovine serum
- GFP
green fluorescence protein
- IGF-1
insulin-like growth factor 1
- LEC
lymphatic endothelial cell
- mTOR
mammalian target of rapamycin
- mTOR-T
rapamycin-resistant and kinase-active mTOR (S2035T)
- mTOR-TE
rapamycin-resistant and kinase-dead mTOR (S2035T/D2357E)
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- PI3K
phosphoinositide-3′ kinase
- S6K1
ribosomal p70 S6 kinase 1
- VEGF
vascular endothelial growth factor
- VEGFR-3
VEGF receptor 3
Footnotes
This work was supported in part by the National Institutes of Health (CA115414 to S. Huang), the American Cancer Society (RSG-08-135-01-CNE to S. Huang), and the National Natural Science Foundation of China (no. 81102473 to Y. Luo).
References
- 1.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem. 2010;17:698–708. doi: 10.2174/092986710790514471. [DOI] [PubMed] [Google Scholar]
- 3.Proud CG. mTOR signalling in health and disease. Biochem Soc Trans. 2011;39:431–436. doi: 10.1042/BST0390431. [DOI] [PubMed] [Google Scholar]
- 4.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 9.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca BD, Smith EM, Lee VH, Mackintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 15.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce LR, Huang X, Boudeau J, Pawłowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, Hegg JW, Bandhakavi S, Griffin TJ, Kim DH. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor β expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 20.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum-and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 21.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Iadevaia V, Proud CG. Differing effects of rapamycin and mTOR kinase inhibitors on protein synthesis. Biochem Soc Trans. 2011;39:446–450. doi: 10.1042/BST0390446. [DOI] [PubMed] [Google Scholar]
- 23.Huber S, Bruns CJ, Schmid G, Hermann PC, Conrad C, Niess H, Huss R, Graeb C, Jauch KW, Heeschen C, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 2007;71:771–777. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi S, Kishimoto T, Kamata S, Otsuka M, Miyazaki M, Ishikura H. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 2007;98:726–733. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer T, Tritsaris K, Hübschmann MV, Gibson J, Nisato RE, Pepper MS, Dissing S. IL-20 activates human lymphatic endothelial cells causing cell signalling and tube formation. Microvasc Res. 2009;78:25–32. doi: 10.1016/j.mvr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 28.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 32.Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor down-regulation. Oncogene. 2010;29:2517–2527. doi: 10.1038/onc.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y, Zhou H, Liu L, Shen T, Chen W, Xu B, Han X, Zhang F, Scott RS, Alexander JS, et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene. 2011;30:2098–2107. doi: 10.1038/onc.2010.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ando T, Jordan P, Wang Y, Jennings MH, Harper MH, Houghton J, Elrod J, Alexander JS. Homogeneity of mesothelial cells with lymphatic endothelium: expression of lymphatic endothelial markers by mesothelial cells. Lymphat Res Biol. 2005;3:117–125. doi: 10.1089/lrb.2005.3.117. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Shen T, Luo Y, Liu L, Chen W, Xu B, Han X, Pang J, Rivera CA, Huang S. The antitumor activity of the fungicide ciclopirox. Int J Cancer. 2010;127:2467–2477. doi: 10.1002/ijc.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, Zhou H, Han X, Huang S. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem. 2010;285:38362–38373. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med. 2011;50:624–632. doi: 10.1016/j.freeradbiomed.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erbay E, Chen J. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem. 2001;276:36079–36082. doi: 10.1074/jbc.C100406200. [DOI] [PubMed] [Google Scholar]
- 40.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 41.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 42.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 43.Karpanen T, Wirzenius M, Mäkinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Ylä-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 45.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 46.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, Chang H, Robinson C, Hunter K, Dobrzanski P, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- 47.Kubo H, Cao R, Brakenhielm E, Mäkinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang LK, Garcia-Cardeña G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goad DL, Rubin J, Wang H, Tashjian AH, Jr, Patterson C. Enhanced expression of vascular endothelial growth factor in human SaOS-2 osteoblast-like cells and murine osteoblasts induced by insulin-like growth factor I. Endocrinology. 1996;137:2262–2268. doi: 10.1210/endo.137.6.8641174. [DOI] [PubMed] [Google Scholar]
- 52.Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, et al. mTOR drives its own activation via SCF (βTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell. 2011;44:290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(βTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell. 2011;44:304–316. doi: 10.1016/j.molcel.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan S, Skaar JR, Kuchay S, Toschi A, Kanarek N, Ben-Neriah Y, Pagano M. mTOR generates an auto-amplification loop by triggering the βTrCP- and CK1α-dependent degradation of DEPTOR. Mol Cell. 2011;44:317–324. doi: 10.1016/j.molcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]