Abstract
As a short-lived radical that diffuses across membranes, rather than interacting with membrane-bound receptors, nitric oxide (NO) represents a significant departure from synthetically derived radiosensitizers. An endogenous compound, NO may equal or surpass its molecular cousin, oxygen, as a hypoxic radiosensitizer, through pleiotropic phenotypic effects on tumor perfusion, cell signaling, mitochondrial respiration, the fixation of radiation-induced damage, and the radioprotection of normal tissue. However, unlike oxygen, in the context of radiosensitization, the clinical role and utility of NO are poorly understood, with often contradictory and controversial reported effects: whether NO functions as a radiosensitizer may ultimately be contextual to the tumor microenvironment. This may make NO manipulation an ideal candidate for a personalized radiosensitization approach tailored to specific patient and tumor types/microenvironmental characteristics. Effective delivery of NO both systemically and directly to the tumor may be critical to the success of this approach. Compounds that release NO or NO precursors have the potential to drive innovation and result in a new fertile branch of the radiosensitizer tree.
Introduction
Do I contradict myself?
Very well then I contradict myself,
(I am large, I contain multitudes.)
Walt Whitman, Song of Myself
“NO” may be the most basic and primal word in the English language but its semantic interpretation is influenced by a complex interplay of contextual factors. The meaning of this seemingly simple negative word is thus shaded with subtlety and ultimately depends on factors such as inflection, tone, use of gesture, phrasing, and volume. Similarly, in hypoxic radiosensitization, the actions of the deceptively simple molecule, NO or nitric oxide, seem to be very much dependent on the “biologic” context, consisting of diverse parameters such as dose, oxygenation status, delivery method, vessel architecture, site of administration, and the tumor microenvironment. The basic molecular structure of NO belies the central importance of its subtle and protean biology. As one of the oldest and most primitive molecules, NO may have evolved from a critical defense mechanism [1] against ozone toxicity to subserve more complex and diverse functions. A vasodilator, neurotransmitter, antimicrobial, and immune regulator, NO is a “master regulator” [2], consummate multitasker and jack of all trades that possesses more than 36 different functions [3] with activity in every major organ system. A master flip-flopper as well, the molecule is nearly impossible to pin down.
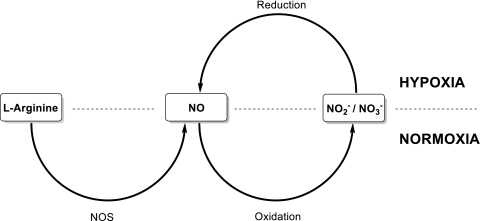
NO is generated endogenously by NO synthase (NOS) in mammals from the oxidation of l-arginine to l-citrulline (Figure 1). NO can also be formed independent of NOS by the reduction of nitrate and nitrite catalyzed by reductive enzymes such as deoxyhemoglobin and nitrate reductase. NOS can be categorized into two functional classes: constitutive (cNOS) and inducible (iNOS), based on their sensitivity to calcium [4]. As a gaseous short-lived free radical that is uncharged and therefore freely diffusible across tissues and cell membranes, NO is able to bind to its most sensitive known target, soluble guanylate cyclase, to stimulate the production of cyclic GMP, which is responsible for downstream NO-mediated signaling.
Figure 1.
The nitrate-nitrite-NO pathway. NO is generated from the precursor l-Arginine by the enzyme NOS under normoxic conditions. Under these conditions, NO is oxidized to nitrite and nitrate. Under hypoxia, nitrite is reduced by a variety of NOS-independent processes to form NO.
Unlike the specific and highly selective lock-and-key fit used by most other types of signal transducers, NO can react both directly and indirectly with multiple other partners. Indirectly, NO has the potential to generate multiple species with distinct biochemical properties, resulting in variable and unpredictable biologic effects. In addition, NO is both rapidly synthesized and metabolized, making it an ideal signaling molecule for rapid, transient responses.
In the context of radiosensitization, given the complexity, promiscuity, and pluripotency of this molecule [3], it is perhaps not surprising that the literature is contradictory, with some studies demonstrating measurable benefits of the combination of NO with radiotherapy, whereas others demonstrate a deleterious effect. NO is a paradox, alternately acting as a prooxidant and an antioxidant, a radiosensitizer and a radioprotector, and a dual regulator of apoptosis, both inducing and preventing apoptosis. This has led to an intense debate, in which both sides can claim evidence of support, as to whether NO is good or bad, friend or foe [5]. As a “foe,” increased levels of NOS have led to poorer survival in cervical cancer patients after radiotherapy [5]. It also has been reported that iNOS is expressed in several human cancers including breast, pancreatic, head and neck, and gynecologic tumors [6], which strongly implies that NO may be an important promoter of tumor growth and metastasis [7]. Paradoxically, perhaps, tumor shrinkage and decreased blood flow were associated with administration of the NOS inhibitor, N-nitro-l-arginine (l-NNA) in a phase 1 study of non-small cell lung carcinoma, as assessed by dynamic contrast-enhanced computed tomography. These data would suggest that NO donation could increase both tumor perfusion and tumor growth [8].
As a “friend,” NO has been widely reported as a hypoxic cell radio-sensitizer [9], in its effects on systemic and hypoxic vasodilation, red cell rheology, and decreased oxygen use. These are discussed more fully below.
In reality, then, it might be more accurate to consider NO from a multidimensional perspective, to explore the combined effects of NO local concentration, the biologic milieu, and interactions with oxygen on NO-mediated activity. At low concentrations, NO promotes tumor cell survival and angiogenesis, whereas at higher levels, when homeostatic balance is perturbed, NO can act as an antitumor agent [10]. For example, NO can combine with superoxide anions (O2-) to form toxic peroxynitrite (ONOO-), triggering apoptosis through direct and indirect mechanisms and contributing to radio-sensitizing effects. This notion of a dose threshold and threshold effects is central to NO induced cytotoxicity [11].
Therefore, the focus of the current article is on NO and its relationship to oxygen and radiosensitization; relevant aspects of NO physiology will be highlighted in an attempt to place its dual, seemingly contradictory, function into a wider context and to explore the potential utility of NO modulation as a promising, but currently unrealized, means of improving radiation sensitivity.
The Oxygen Effect
From the early role of NO in the evolution of life [1], to its discovery by the chemist Joseph Priestley, credited with isolating oxygen, the history of NO is closely intertwined with oxygen. Oxygen is the primary determinant mover of radiosensitization: the biologic effect of XRT is increased by a factor of 2 to 3 in the presence of oxygen, whereas radioresistance is associated with hypoxia. According to “the oxygen fixation hypothesis,” DNA is indirectly damaged by reactive oxygen species from the radiolysis of water molecules. The permanence of this damage is dependent on subsequent modification of DNA radicals. Under aerobic conditions, oxygen reacts through its two unpaired electrons with DNA radicals to form DNA peroxy radicals, thus preventing DNA repair. Under hypoxia, the DNA radical is quenched through thiol-mediated hydrogen atom donation and DNA crosslinks can be repaired (Table 1).
Table 1.
Mechanisms of NO Radiosensitization Effects
| Treatment | Systemic Effects | Local Effects |
| NO donors (tumor-type dependent) | Vasodilation and steal effect | Increased oxygenation |
| Red blood cell rheology modification | Release of NO by RBCs under hypoxic conditions | |
| Local Oxygen sparing through NO-mediated mitochondrial effects | ||
| TSP-1/CD47 modulation | Increased TSP-1 inhibits NO generation leading to vasoconstriction | Increased tumor blood flow through anti-steal effect |
| Insulin and electrical stimulus | Increased iNOS activity and NO | Increased tumoral blood flow |
| Focused low-dose radiation | None | Increased tumor NO through eNOS up-regulation |
| Hypoxia-activated NO donors | None | Increased local NO concentration, modulation of tumor blood flow |
Therefore, one approach to increase radiosensitivity is to improve the oxygen status of tumors [12]. Reoxygenation, however, is compromised by limited diffusion of oxygen inside tumors due to consumption by actively respiring cells and the distance of peripheral blood vessels to the center of the tumor. Highly electron-affinic compounds such as the nitroimidazoles, behaving as oxygen mimics, penetrate further than oxygen. However, although these molecules were demonstrated to be effective radiosensitizers, the nitroimidazoles suffer from dose-limiting neurotoxicities that preclude their clinical use [12].
Nitric Oxide
NO has an electron affinity comparable to oxygen. NO acts as a hypoxic radiosensitizer in its own right, mimicking the effects of oxygen on fixation of radiation-induced DNA damage [13]. However, NO has an extremely short circulating half-life, lasting only milliseconds that is not compatible with its long duration of action and far-reaching effects. Consequently, NO may exert cytotoxicity through the bystander effect, mediated by diffusion into the tumor interstitium and across tumor cell membranes [14]. Consequently, radio enhancement may occur with only marginal fluxes in NO.
However, increased radiosensitivity cannot be attributed exclusively to the NO molecule itself, but is combined with the oxygen effect [15]: The cytotoxic effects of NO derive both from the reaction of NO with O2- to form the peroxynitrite anion ONOO- and from modulation of oxygen consumption and delivery.
In the anthropomorphic characterization of reactive nitrogen species, NO has been viewed as good, superoxide as bad, and peroxide as ugly [5]. The short half-life [16] of NO may be partly explained by its rapid reaction with superoxide to form the peroxynitrite ion. Peroxynitrite causes apoptotic or necrotic cell death through nitration of tyrosine residues in proteins, lipid peroxidation, oxidation of critical thiols [17], DNA strand breakage, and activation of the nuclear enzyme poly(ADP-ribose) polymerase, leading to NAD+ depletion and energy failure [18]. At physiologic pH, peroxide combines with NO to form nitrogen dioxide and hydroxyl radicals [19], before oxidizing to nitrate, most often considered inert, and nitrite, which can be cycled back to NO and other reactive nitrogen oxide species [17] through hypoxia-mediated reduction.
Increased NO-Mediated Tumor Oxygenation
Vasodilatation and the Steal Effect
The administration of NO donors to control tumor blood flow is controversial. Some studies have reported improved tumor oxygenation and radiosensitization through increased blood flow, whereas others are consistent with the promotion of tumor growth and decreased tumor perfusion [20].
These apparently contradictory results could be attributed to the idiosyncrasies of the tumor microenvironment resulting in blood flow redistribution through steal or anti-steal effects. Because tumor blood vessels are fully dilated, a “steal effect” involves a relocation of blood away from the tumor as a result of NO-mediated systemic vasodilation. An anti-steal effect increases tumor blood flow and oxygenation through preferential vessel relaxation or systemic vasoconstriction [21].
Results from a phase 2 clinical study suggest that NO mediates vasodilation in tumors. Prostate cancer patients who had failed primary therapy were treated with low-dose, sustained delivery of glyceryl trinitrate resulting in a significant decrease in PSA. The authors suggested that, although low-dose NO had no direct cytotoxic effect, NO decreased the emergence of a more malignant phenotype, including invasion and metastases, presumably by decreasing tumor hypoxia through improved tumor blood flow [10]. A possible alternative and intriguing rationale for these observations is that the sustained delivery of NO paradoxically resulted in inhibition of NO signaling through tachyphylaxis [22]. The tachyphylaxis may have resulted from inhibition of guanylate cyclase through a feedback mechanism [23]. In this case, the results may have been due to a vasoconstrictive intratumoral effect because the patency of tumor vessels is highly dependent on constitutive NO production [24].
Red Cell Rheological Effects
Recent studies have demonstrated that renitration of stored blood with exogenous NO donors can reverse the rheological effects (crenation) on RBCs due to hypoxia and acidosis and thereby improve tissue perfusion and oxygenation on transfusion [25]. By analogy, NO should restore normal red cell shape and normalize blood viscosity in the relatively hypoxic, malformed, and tortuous tumor microvessels, increasing overall flow through the tumor. These hemodynamic changes would be expected to result in a radiosensitizing effect by improved oxygen delivery through relief of hypoxia.
Hypoxia and Hypoxic Vasodilation
The presence of hypoxia in the microenvironment would be expected to influence the susceptibility of tumors to nitrovasodilation with the two types of hypoxia, chronic and acute, responding differently. Long-term or diffusion-limited hypoxia is related to the maximum distribution distance of oxygen in actively respiring tumor tissue, creating an O2 tension gradient as the distance from vessels increases. Acute or transient hypoxia involves a temporary restriction of blood flow to areas of the tumor by transiently constricted or blocked vessels, resulting in local ischemia.
The vasculature of acutely hypoxic tumors may be more susceptible than chronically hypoxic tissues to the vasoactive and rheological properties of NO through relief or prevention of temporary flow stasis.
The hypoxia marker, pimonidazole, a 2-nitroimidazole, has been used to estimate hypoxia and perfusion in tumors [26]. In some tumors, a predominance of pimonidazole-positive areas in the vicinity of blood vessels indicates transient perfusion and acute hypoxia.
NO reacts with a highly conserved reduced cysteine at position 93 on the β chain of hemoglobin to form an S-nitrosothiol (SNO). This has implications for selective hypoxic vasodilation and, by extension, radiosensitization through increased oxygenation. It has been proposed that under normoxic conditions, NO binds to this cysteinyl residue on deoxyhemoglobin to form S-nitrosohemoglobin (SNOHb). Under hypoxic conditions, the allosteric transition in S-nitrosohemoglobin from the R (oxygenated) to the T (deoxygenated) conformation transfers NO to a cysteine in the membrane of the red cell. The red cell thus serves as the NO gatekeeper, sequestering NO equivalents in normoxia and dispensing them “altruistically” in hypoxia [27].
An alternative hypothesis posits that it is nitrite, rather than SNOHb, which is responsible for preserving NO bioactivity in the circulation [28]. In any event, whether the carrier is nitrite or SNOHb, NO bioactivity can be exported selectively to hypoxic zones of the tumor, dilating mature blood vessels and thereby facilitating O2 delivery.
Oxygen-Sparing Effects
Reoxygenation of tumors is linked to decreased oxygen consumption [29]. NO and its oxidative product nitrite have been reported to increase tumor oxygenation through inhibition of mitochondrial respiration [30] by binding reversibly to cytochrome c oxidase in complex IV [2]. Oxygen is thus redistributed [31] away from the electron transport chain toward nonrespiratory oxygen-dependent targets like hypoxia-inducible factor 1 (HIF-1). HIF-1, consisting of two subunits, HIF-1α and HIF-1β, is the master regulator [32] of hypoxic stress. Under well-oxygenated conditions, HIF-1α is in stasis—continuously synthesized and degraded. At low O2 concentrations, HIF-1α accumulates to heterodimerize with HIF-1β and activates the expression of HIF-dependent target genes such as VEGF, contributing to radioresistance [12]. By contrast, NO-mediated tumor reoxygenation increases tumor radio-sensitivity through resumption of HIF-1α degradation. These NO-elicited events can also generate peroxynitrite that induces oxidative stress and apoptosis [33] through different mechanisms, including DNA cross-linking.
Radioprotection
The above notwithstanding, NO has also been described as a radio-protectant. Liebmann et al. [34] demonstrated that pretreatment with NO donors enhanced the survival of mice to whole body irradiation and resulted in hemopoietic protection. Maxhimer et al. [35] reported radioprotection of soft tissue and prevention of apoptosis in irradiated muscle in vivo, presumably by increasing NO levels through inhibition of CD-47 expression. There are a number of potential mechanisms of healthy tissue radioprotection that are not necessarily mutually exclusive with simultaneous tumor radiosensitization. These include neutralization of radical nitrogen and oxygen species [36]. NO is a free radical with an unpaired electron in the highest orbital, making it highly reactive with other free radicals; therefore, by definition, NO can act as an antioxidant through electron transfer to quench free radicals. In addition, NO induces hypotension which results in decreased blood flow to bone marrow through the vascular steal phenomenon. Hence, paradoxically, the generation of hypoxia protects the cells in the bone marrow from the effect of radiation. Finally, NO can also inhibit apoptosis depending on cell types and environmental conditions.
NO-Based Radiosensitization Strategies
Background
In 1957, Howard-Flanders [37] demonstrated radiosensitization of hypoxic bacteria with NO gas. In the early 1990s, Mitchell et al. [38] reported that an NO donor drug, diethylamine NO (DEA/NO), sensitized hypoxic mammalian cells to irradiation as effectively as oxygen. De Ridder et al. [13] confirmed these radiosensitizing effects with both DEA/NO and spermine nonoate (SPER/NO), and the radiosensitivity of experimental tumors after application of the NO donor SIN-1 was demonstrated by Jordan et al. [39].
However, in contrast to these results, the NOS inhibitor nitro-l-arginine was found to induce tumor radioresistance [39]. In the same series of experiments, Jordan et al. [39] also demonstrated increased tumor blood flow and in vivo radiosensitization after intraperitoneal administration of isosorbide dinitrate. In contrast, using the NO donor nitroprusside, Thews et al. [40] described decreased tumor perfusion in rats bearing subcutaneous tumors, which correlated with a fall in mean arterial blood pressure, suggesting the presence of a vascular steal phenomenon. Similarly, Shan et al. [41] also reported that intravenous injection of DEA/NO lowered mean arterial blood pressure and decreased tumor blood flow and oxygenation.
Clearly, these publications demonstrate that the effects of NO donors on tumor blood flow and radiosensitization are not consistent or predictable. In addition, the potential for dose-limiting hypotension and vascular steal limits the clinical utility of NO donors as radio-sensitizers of both currently approved NO donors (organic nitrates including glyceryl trinitrate, isosorbide mononitrate, and dinitrate) and the direct NO donors that include sodium nitroprusside and SIN-1.
A number of strategies to optimize the activity/toxicity profile of NO manipulation have been studied. These include hypoxia-activated NO donors, thrombospondin 1/CD-47 modulation, and indirect activation of endogenous NOS (Table 2).
Table 2.
Direct and Indirect NO-Based Radiosensitization Strategies
| Direct | Indirect |
Hypoxia-activated NO donors
|
NO response manipulation strategies
|
Indirect activation of endogenous NOS
|
NO Manipulation Strategies for Radiosensitization
Hypoxia-Activated NO Donors
-
S-nitroso-N-acetylpenicillamine and S-nitrosoglutathione:
The nitrosonium ion (NO+) donors, S-nitroso-N-acetylpenicillamine (SNAP) and S-nitrosoglutathione (GSNO), generate NO bioreductively under hypoxic conditions, leading to in vitro radiosensitization [42]. The mechanism is thought to include delivery and release of NO through transnitrosylation steps. However, the above NO+ donors have also been associated with stabilization of HIF-1α [43], leading to the induction of HIF-1α target genes, and radioresistant phenotypes, which would limit their clinical utility.
-
RRx-001
RRx-001, a nonexplosive pernitro compound possessing a novel pharmacophore that originated in the defense industry, is a small-molecule NO donor that is currently in a phase 1 clinical trial. Preclinical studies demonstrated profound radiosensitization in vivo at nontoxic doses that was accompanied by a significant increase in tumor blood flow for up to 72 hours. At these doses, normal GI epithelium was not sensitized to the effects of radiation and may even have been protected [44].
Thrombospondin 1/CD-47 modulation. In contrast to directly modulating NO levels, thrombospondin 1 (TSP-1), acting through its receptor CD-47, antagonizes the effects of NO. TSP-1 expression is frequently suppressed in tumors, preventing NO regulation and thereby promoting NO proangiogenic effects. However, some tumors induce an increase in systemic TSP-1 derived from nontumorigenic stromal cells. The systemic increase in TSP-1 limits NO-driven responses in normal tissue and thereby induces an anti-steal effect by increasing tumor perfusion to the detriment of healthy tissue circulation [45]. Whereas local NO production drives tumor angiogenesis, systemic NO-mediated vasodilation preferentially enhances normal tissue perfusion at the expense of the tumor, similar to the steal effect. Therapeutic concepts to modulate the TSP-1/CD47 interaction have been described in the literature but have not advanced into formal development [46].
Indirect activation of endogenous NOS
-
Insulin and electrical stimulus
Jordan et al. [15] reported that insulin infusion and electrical stimulation of the host tissue radiosensitized experimental tumors in vivo through increased NO [13] production from the endothelial isoform of NOS (eNOS). The authors speculate that both treatments resulted in tumor reoxygenation from increased blood flow and/or reduced oxygen consumption due to decreased mitochondrial respiration. The increase in tumor oxygenation, and the radiosensitizing effect, was completely abolished in eNOS knockout mice.
-
Lipid A analog, ONO-4007
ONO-4007 is a synthetic analog of the lipid A moiety of gram-negative bacterial lipopolysaccharide. Preclinical data have demonstrated that the compound radiosensitizes with considerably less toxicity than lipopolysaccharide in animal models of malignancy [13]. The mechanism of action is thought to involve activation of the interferon-γ pathway and induction of NOS [47]. In a phase 1 trial, an MTD dose was reached, and although no objective responses were seen, disease stabilization was observed in five patients for the duration of the study (18 weeks) [48]. No information on a phase 2 trial is available.
Discussion and Conclusions
Like the word “NO,” the use of NO, even as an electrically neutral molecule, in radiotherapy is highly charged. The challenge of how to reconcile the therapeutic potential of NO with its seeming duality is perhaps best addressed by considering that the effects of oxygen may be mediated through NO [12]. More than merely a conduit for oxygen, however, NO is a cooperative partner that actively facilitates the delivery and use of oxygen and regulates the toxicity of oxygen reactive intermediaries in both normal and tumor tissues.
From this perspective, the relationship between oxygen and NO recapitulates elements of a feedback control that can be manipulated with pathophysiological and therapeutic implications. As the intermediary between the host macroenvironment and the tumor micro-environment, NO is the effector of this feedback interaction. This function requires a careful balancing act with the radiobiological outcome critically dependent on the concentration and location of both species.
The inconsistent results of tumor reoxygenation and NO manipulation as separate radiosensitization strategies may be related to the failure to explore integrated interventions that exploit NO and oxygen together in the context of the tumor microenvironment. This renders NO manipulation an ideal candidate for a personalized systems biology type of approach tailored both to the NO/O2 axis of specific patients and biologic features of tumors.
However, more nonclinical basic research under carefully defined and controlled conditions is required to fully understand and delineate the role of NO in the context of radiosensitization and to demonstrate that manipulation of NO may be beneficial for improving radiation response. The NO-mediated radiosensitization approaches described in this review are either well established and clinically explored or groundbreaking but still at a very early, nonclinical stage with the exception of RRx-001, a novel hypoxia-activated NO donor, currently in phase 1 trials. This promising approach has the potential of exploiting NO for radiosensitization while avoiding systemic NO-based effects.
Currently, the understanding of the clinical role and utility of NO in the context of radiosensitization is poorly understood and the effects are contradictory. Whether NO directly or indirectly functions as a radiosensitizer may ultimately be contextual to the tumor micro-environment and the specific architecture of the vasculature, particularly the presence or absence of smooth muscle coverage that allow the vessel to respond to a vasoactive stimulus through endogenous or external NO delivery.
As effective radiosensitization strategies represent an unmet need in the treatment of cancer, the development of new agents that specifically and selectively deliver NO to the tumor enabling local exposure affecting vasodilation, mitochondrial respiration, and red blood cell rheology, but without adverse systemic effects, represent an exciting future for NO-driven radiosensitizers.
References
- 1.Feelisch M, Martin JF. The early role of nitric oxide in evolution. Trends Ecol Evol. 1995;10:496–499. doi: 10.1016/s0169-5347(00)89206-x. [DOI] [PubMed] [Google Scholar]
- 2.Sarti P, Avigliano L, Gorlach A, Brune B. Superoxide and nitric oxide—participation in cell communication. Cell Death Differ. 2002;9:1160–1162. doi: 10.1038/sj.cdd.4401099. [DOI] [PubMed] [Google Scholar]
- 3.Gow AJ. The biological chemistry of nitric oxide as it pertains to the extrapulmonary effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3:150–152. doi: 10.1513/pats.200506-058BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan PA, Conroy B, Spedding AV. Expression of inducible nitric oxide synthase and p53 in oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:624–629. doi: 10.1067/moe.2000.108800. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Torbenson M, Wang Q, Ro JY, Becich M. Expression of inducible nitric oxide synthase in paired neoplastic and non-neoplastic primary prostate cell cultures and prostatectomy specimen. Urol Oncol. 2003;21:117–122. doi: 10.1016/s1078-1439(02)00208-9. [DOI] [PubMed] [Google Scholar]
- 7.Jadeski LC, Hum KO, Chakraborty C, Lala PK. Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int J Cancer. 2000;86:30–39. doi: 10.1002/(sici)1097-0215(20000401)86:1<30::aid-ijc5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Ng QS, Goh V, Milner J, Stratford MR, Folkes LK, Tozer GM, Saunders MI, Hoskin PJ. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 2007;8:111–118. doi: 10.1016/S1470-2045(07)70001-3. [DOI] [PubMed] [Google Scholar]
- 9.Worthington J, Robson T, O'Keeffe M, Hirst DG. Tumour cell radiosensitization using constitutive (CMV) and radiation inducible (WAF1) promoters to drive the iNOS gene: a novel suicide gene therapy. Gene Ther. 2002;9:263–269. doi: 10.1038/sj.gt.3301609. [DOI] [PubMed] [Google Scholar]
- 10.Heaton JP, Adams MA, Graham CH, Emerson L, Siemens RD. Evidence for the use of low dose nitric oxide in the treatment of rising PSA associated with biochemical failure following radical prostatectomy. J Urol. 2003;169(Suppl. S):1082. [Google Scholar]
- 11.Wang C, Trudel LJ, Wogan GN, Deen WM. Thresholds of nitric oxide-mediated toxicity in human lymphoblastoid cells. Chem Res Toxicol. 2003;16:1004–1013. doi: 10.1021/tx0340448. [DOI] [PubMed] [Google Scholar]
- 12.Oronsky BT, Knox SJ, Scicinski J. Six degrees of separation: the oxygen effect in the development of radiosensitizers. Transl Oncol. 2011;4:189–198. doi: 10.1593/tlo.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Ridder M, Verellen D, Verovski V, Storme G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide. 2008;19:164–169. doi: 10.1016/j.niox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, Billiar TR, Blumberg D. Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res. 2004;64:8015–8021. doi: 10.1158/0008-5472.CAN-04-2212. [DOI] [PubMed] [Google Scholar]
- 15.Jordan BF, Sonveaux P, Feron O, Gregoire V, Beghein N, Dessy C, Gallez B. Nitric oxide as a radiosensitizer: evidence for an intrinsic role in addition to its effect on oxygen delivery and consumption. Int J Cancer. 2004;109:768–773. doi: 10.1002/ijc.20046. [DOI] [PubMed] [Google Scholar]
- 16.Ducrocq C, Blanchard B, Pignatelli B, Ohshima H. Peroxynitrite: an endogenous oxidizing and nitrating agent. Cell Mol Life Sci. 1999;55:1068–1077. doi: 10.1007/s000180050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 18.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez GR, Di Mascio P, Bonini MG, Augusto O, Briviba K, Sies H, Maurer P, Rothlisberger U, Herold S, Koppenol WH. Peroxynitrite does not decompose to singlet oxygen (1ΔgO2) andnitroxyl (NO-) Proc Natl Acad Sci USA. 2000;97:10307–10312. doi: 10.1073/pnas.190256897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan BF, Misson P, Demeure R, Baudelet C, Beghein N, Gallez B. Changes in tumor oxygenation/perfusion induced by the no donor, isosorbide dinitrate, in comparison with carbogen: monitoring by EPR and MRI. Int J Radiat Oncol Biol Phys. 2000;48:565–570. doi: 10.1016/s0360-3016(00)00694-5. [DOI] [PubMed] [Google Scholar]
- 21.Trotter MJ, Chaplin DJ, Olive PL. Effect of angiotensin II on intermittent tumour blood flow and acute hypoxia in the murine SCCVII carcinoma. Eur J Cancer. 1991;27:887–893. doi: 10.1016/0277-5379(91)90140-9. [DOI] [PubMed] [Google Scholar]
- 22.Agvald P, Adding LC, Gustafsson LE, Persson MG. Nitric oxide generation, tachyphylaxis and cross-tachyphylaxis from nitrovasodilators in vivo. Eur J Pharmacol. 1999;385:137–145. doi: 10.1016/s0014-2999(99)00720-7. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs AJ. Soluble guanylate cyclase: an old therapeutic target re-visited. Br J Pharmacol. 2002;136:637–640. doi: 10.1038/sj.bjp.0704779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77–89. doi: 10.1023/a:1005908805527. [DOI] [PubMed] [Google Scholar]
- 25.Bonaventura J. Clinical implications of the loss of vasoactive nitric oxide during red blood cell storage. Proc Natl Acad Sci USA. 2007;104:19165–19166. doi: 10.1073/pnas.0708871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen HL, Haustermans KM, Sprong D, Blommestijn G, Hofland I, Hoebers FJ, Blijweert E, Raleigh JA, Semenza GL, Varia MA, et al. HIF-1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2002;54:1537–1549. doi: 10.1016/s0360-3016(02)03935-4. [DOI] [PubMed] [Google Scholar]
- 27.Sonveaux P, Lobysheva II, Feron O, McMahon TJ. Transport and peripheral bioactivities of nitrogen oxides carried by red blood cell hemoglobin: role in oxygen delivery. Physiology (Bethesda) 2007;22:97–112. doi: 10.1152/physiol.00042.2006. [DOI] [PubMed] [Google Scholar]
- 28.Gladwin MT, Schechter AN. NO contest: nitrite versus S-nitrosohemoglobin. Circ Res. 2004;94:851–855. doi: 10.1161/01.RES.0000126697.64381.37. [DOI] [PubMed] [Google Scholar]
- 29.Sonveaux P, Jordan BF, Gallez B, Feron O. Nitric oxide delivery to cancer: why and how? Eur J Cancer. 2009;45:1352–1369. doi: 10.1016/j.ejca.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, De Ridder M, Verovski VN, Sonveaux P, Jordan BF, Law K, Monsaert C, Van den Berge DL, Verellen D, Feron O, et al. Activated macrophages as a novel determinant of tumor cell radioresponse: the role of nitric oxide-mediated inhibition of cellular respiration and oxygen sparing. Int J Radiat Oncol Biol Phys. 2010;76:1520–1527. doi: 10.1016/j.ijrobp.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 33.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 34.Liebmann J, DeLuca AM, Coffin D, Keefer LK, Venzon D, Wink DA, Mitchell JB. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994;54:3365–3368. [PubMed] [Google Scholar]
- 35.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 37.Howard-Flanders P. Effect of nitric oxide on the radiosensitivity of bacteria. Nature. 1957;180:1191–1192. doi: 10.1038/1801191a0. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JB, Wink DA, DeGraff W, Gamson J, Keefer LK, Krishna MC. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res. 1993;53:5845–5848. [PubMed] [Google Scholar]
- 39.Jordan BF, Beghein N, Aubry M, Gregoire V, Gallez B. Potentiation of radiation-induced regrowth delay by isosorbide dinitrate in FSaII murine tumors. Int J Cancer. 2003;103:138–141. doi: 10.1002/ijc.10786. [DOI] [PubMed] [Google Scholar]
- 40.Thews O, Kelleher DK, Vaupel P. No improvement in perfusion and oxygenation of experimental tumors upon application of vasodilator drugs. Int J Oncol. 2001;19:1243–1247. doi: 10.3892/ijo.19.6.1243. [DOI] [PubMed] [Google Scholar]
- 41.Shan SQ, Rosner GL, Braun RD, Hahn J, Pearce C, Dewhirst MW. Effects of diethylamine/nitric oxide on blood perfusion and oxygenation in the R3230Ac mammary carcinoma. Br J Cancer. 1997;76:429–437. doi: 10.1038/bjc.1997.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssens MY, Verovski VN, Van den Berge DL, Monsaert C, Storme GA. Radiosensitization of hypoxic tumour cells by S-nitroso-N-acetylpenicillamine implicates a bioreductive mechanism of nitric oxide generation. Br J Cancer. 1999;79:1085–1089. doi: 10.1038/sj.bjc.6690173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park YK, Ahn DR, Oh M, Lee T, Yang EG, Son M, Park H. Nitric oxide donor, (+/-)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1α by inhibiting von Hippel-Lindau recruitment and asparagine hydroxylation. Mol Pharmacol. 2008;74:236–245. doi: 10.1124/mol.108.045278. [DOI] [PubMed] [Google Scholar]
- 44.Ning S, Bednarski M, Oronsky B, Scicinski J, Saul G, Knox S. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials; In Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 April 2–6; Orlando, Florida. 2011. American Association for Cancer Research, Philadelphia, PA, Abstract no. 676. [DOI] [PubMed] [Google Scholar]
- 45.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for antiangiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 47.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 2007;19:397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 48.de Bono JS, Dalgleish AG, Carmichael J, Diffley J, Lofts FJ, Fyffe D, Ellard S, Gordon RJ, Brindley CJ, Evans TR. Phase I study of ONO-4007, a synthetic analogue of the lipid A moiety of bacterial lipopolysaccharide. Clin Cancer Res. 2000;6:397–405. [PubMed] [Google Scholar]