Abstract
Cadherin/catenin-based adhesions coordinate cellular growth, survival, migration, and differentiation within a tissue by mechanically anchoring cells to their neighbors. They also intersect with diverse signaling pathways in development and cancer. Although the adhesive functions of adherens junction proteins are well characterized, their contribution to other signaling pathways is less well understood. Here, we show that ablation of α-catenin in the epidermis selectively induces apoptosis in suprabasal differentiating keratinocytes while sparing basal cell progenitors. This protection from death is coupled to elevated focal adhesion signaling, faster migration, and an altered distribution of growth factor receptors. We show that simultaneous depletion of α-catenin and focal adhesion kinase or p21-activated kinase eliminates basal cell protection as well as the elevated migration and proliferation of cells. The increased dependency of cells upon matrix interactions for their survival when cell–cell adhesions are destabilized has important implications for cancer progression and metastasis.
Keywords: focal adhesion kinase signaling, in utero transduction, receptor tyrosine kinase
To achieve tissue development and homeostasis, cells must integrate a diverse array of environmental signals from neighboring cells, extracellular matrix (ECM), and growth factors. Disruption of cell–cell adhesion can perturb the balance of these signals, leading to altered cell migration, proliferation, and survival (1). Adherens junctions (AJs) (reviewed in ref. 2) have particular importance for epidermal integrity. Ablation of both E-cadherin (the major epidermal transmembrane AJ protein) and the lesser-expressed P-cadherin perturbs tissue architecture, cell polarity, and epidermal barrier function (3). Cultured keratinocytes that lack α-catenin, a key AJ-associated regulator of actin cytoskeleton organization, fail to form mature AJs in response to calcium-induced cell–cell adhesion (4). In addition, whether by traditional mouse genetics or lentiviral targeting, epidermal depletion of α-catenin in vivo weakens junctions between keratinocytes; alters intercellular adhesion, cell polarity, and cytoskeletal organization; and enhances apoptosis (4, 5).
Cell–cell signaling converges with cell–ECM signaling at the level of F-actin regulation. Restricted to mitotically active basal cells of the epidermis, integrin-based focal adhesions (FAs) mediate dynamic adhesion to the underlying basement membrane. Upon integrin engagement, focal adhesion kinase (FAK) becomes activated and phosphorylates targets such as Paxillin. Paxillin can then recruit other effectors such as GIT–PKL–Pix–Pak complexes. The recruitment of effectors can activate Rac1 GTPase (6), which promotes migration by stimulating actin polymerization at the leading edge (7, 8). FAK activity also promotes FA disassembly, and, when FAK is absent, actin–FA complexes stabilize and cell migration declines (9).
Cell culture studies have described crosstalk between AJs and FAs with growth factor-induced transmembrane receptor tyrosine kinase (RTK) signaling (10, 11). Such interactions provide a means to couple adhesion to downstream RTK effectors, namely Src-Ras-Raf-MEK-MAPK (commonly called the Ras-MAPK pathway). Activation of Ras-MAPK signaling can be enhanced through direct or indirect association between RTKs and FA kinases such as FAK and Src (11). Raf and Mek can also be phosphorylated through Pak-, FAK-, and Src-dependent kinases (12), and, conversely, insulin-like growth factor (IGF) and epidermal growth factor (EGF)-dependent RTK signaling pathways can regulate cytoskeletal remodeling and cell migration by activating Rac1 and/or FAK, which both stimulate F-actin-myosin networks (13, 14). E-cadherin can also associate with IGF and EGF RTKs, but seems to suppress rather than enhance their signaling and proliferation (10, 15).
Intriguingly, epidermal ablation of the Ctnna1 gene encoding α-catenin is accompanied by increased Ras-MAPK–dependent proliferation (5). This poses the tantalizing possibility that the diverse downstream consequences of ablating AJ genes such as Ctnna1 in epidermis in vivo might be interrelated through common signaling pathways that involve alterations in cell–cell adhesion, ECM interactions, and RTK signaling. Here, we address this question using in utero epidermal-specific lentiviral delivery to early [embryonic day 9.5 (E9.5)] mouse embryos. By ablating expression of epidermal α-catenin alone, or together with components of the integrin signaling pathway, we discovered that conditional α-catenin loss (cKO) results in enhanced survival and migration of proliferative progenitors but also in elevated apoptosis of differentiating suprabasal cells. We trace this surprising result to the intersection between AJ, integrin-FAK/Pak, and RTK signaling pathways. Our findings provide insights into the complexities of these intersecting networks and their physiological relevance to tissue biology.
Results
Lack of α-Catenin During Epidermal Morphogenesis Induces Cytoskeletal Disorganization, Rupture of Cell–Cell Adhesions, and Suprabasal Apoptosis.
Previous in vivo studies on Ctnna1 cKO mice have relied mainly on immunofluorescence microscopy (IFM) of sagittal sections of skin. As judged by this analysis, cell–cell adhesion appeared to remain largely intact, with E-cadherin localized to cell–cell boundaries (4, 5). To examine this more closely, we transduced E9.5 embryos homozygous for the α-catenin floxed allele (Ctnna1fl/fl) with lentivirus carrying Cre recombinase, a technique that achieves specific targeting within the single-layered embryonic epidermis (5). Our test and Cre-transduced control [wild type (WT)] embryos also carried a Cre reporter allele, Rosa26-YFP (r26Yfpfl/+), so we could identify cells transduced with activated Cre recombinase by their YFP expression.
Whole-mount immunofluorescence imaging of E15.5 backskins revealed early differences. In control epidermis, both basal and suprabasal cells showed E-cadherin colocalized with F-actin, consistent with an intercellular junction-associated F-actin cortex (Fig. S1A). In Ctnna1 cKO epidermis, even though much of the F-actin still localized cortically, E-cadherin distribution was already irregular (Fig. S1B, arrowhead). Most notably, E-cadherin often clustered internally, particularly in suprabasal cells, and the typical honeycomb-like pattern of cells across epithelial sheets was perturbed. Moreover, when whole-mount IFM was applied to K14-Cre X Ctnna1fl/fl embryos (4), similar alterations were seen (not shown). Thus, we attributed these early differences not to the timing of α-catenin loss during embryogenesis, but rather to the increased resolution obtained by planar analyses.
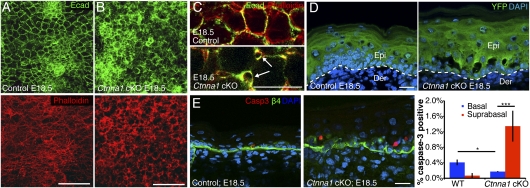
By the end of development (E18.5), epidermal perturbations in Ctnna1 cKO embryos were even more pronounced (Fig. 1 A and B). Disruptions in AJ-actin networks were now prevalent across the epithelial sheets. At higher magnification, intercellular gaps within the suprabasal layer were visible, as they were in E15.5 Ctnna1-null tissue (Fig. 1C, arrows). By coupling our analyses with sagittal sections of Ctnna1fl/fl;r26Yfpfl/+ embryos, it was clear that gaps were largely restricted to the basal–suprabasal interface in the Ctnna1fl/fl;r26Yfpfl/+ but not the r26Yfpfl/+ skins expressing Cre (Fig. 1D).
Fig. 1.
Morphological defects and suprabasal apoptosis in Ctnna1 cKO epidermis. (A and B) Projections of IFM confocal stacks of skin sections from E18.5 embryos, revealing disorganization within the 3D F-actin–AJ network of cKO epidermis. (C) Higher magnification of single E18.5 confocal sections revealing intercellular gaps within the cKO epidermal sheets (arrows). (D) Sagittal sections of E18.5 skins showing that cKO ruptures concentrate at the basal–suprabasal interface. YFP marks LV-Cre–infected cells. (E) Representative image and quantification of apoptotic cells in sagittal sections identified by active Caspase-3 immunostaining (red). Integrin β4 marks the base of cells within the basal layer. Error bars are ±SD. DAPI marks nuclei. [Scale bars (A and B): 50 μm; (D and E): 20 μm.]
As marked by active Caspase-3, apoptotic cells were consistently associated with these interlayer breaks (Fig. 1E). Interestingly, nearly all of the apoptotic cells in the Ctnna1 cKO epidermis localized suprabasally, despite the fact that both basal and suprabasal cells bordered the breaks. By contrast, the few apoptotic cells in control epidermis were found exclusively within the basal layer. Quantifications revealed that, surprisingly, apoptosis within the basal layer was even lower for the Ctnna1 mutant than for control tissue (Fig. 1E). Together, these findings suggest the existence of two types of survival cues: (i) ones that are transmitted when neighboring cells adhere (these are lost in AJ-deficient cells) and (ii) ones transmitted from adhesion to cell substratum (these are enhanced in AJ-deficient cells).
Focal Adhesion Signaling Is Enhanced in the Absence of α-Catenin.
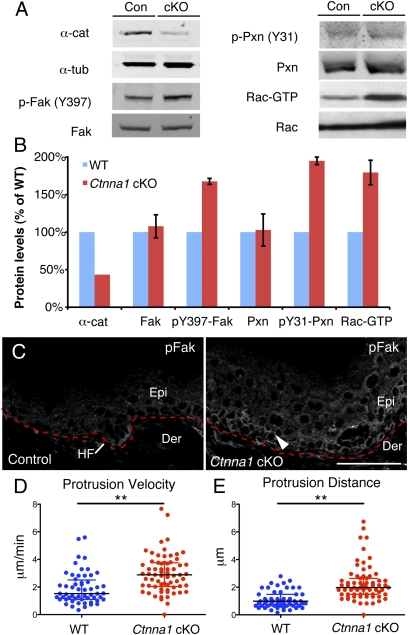
In seeking why basal cells specifically might enhance their survival cues in the absence of α-catenin, we focused on the possibility that FA-integrin signaling might be elevated. To pursue this hypothesis, we first performed immunoblot analysis on E15.5 epidermal lysates. Antibodies against the phosphorylated forms of two key FA components, FAK (Y397) and Paxillin (Y31), showed increased activation (170 and 195%, respectively) in Ctnna1 cKO relative to control epidermis (Fig. 2 A and B). The GTP-bound state of Rac1 was also enhanced. IFM provided further insights. In normal skin, FAK activity was highest at the leading edge of the down-growing hair follicle (Fig. 2C). In Ctnna1 cKO epidermis, FAK activity was comparatively higher throughout the basal layer. Together, these results suggest that, in the absence of α-catenin in vivo, FA and Rac1 signaling are enhanced specifically in the progenitor population.
Fig. 2.
Activation of focal adhesion signaling in the absence of α-catenin. (A) Immunoblots of in vivo skin lysates to assess the activation states of FAK, Paxillin, and Rac1. (B) Quantifications of data from A. (C) IFM reveals elevated activated (phosphorylated) FAK (arrowhead) at the leading front of the hair placode in control tissue and throughout the basal layer of Ctnna1 KO epidermis. (D and E) Quantifications of protrusion velocity (D) and protrusion distance (E) of keratinocytes migrating out of control and Ctnna1 cKO explants, as visualized by videomicroscopy over a 10-min interval. Black lines mark median ± interquartile range. Asterisks denote P < 0.01.
Increased Migration and Pak Activity in the Absence of α-Catenin.
To test whether the elevated integrin and Rac1 signaling in Ctnna1 cKO epidermis affected actin organization and migration, we analyzed membrane protrusion dynamics as a readout of actin cytoskeletal polymerization and Rac1 activity in epidermal explants. Videomicroscopy and kymograph analysis (exemplified in Fig. S2) revealed significant increases in both protrusion velocity and protrusion distance in Ctnna1-null vs. control (WT) epidermal cells (Fig. 2 D and E). When taken together with our biochemical studies showing an accompanying increase in activity of FAK, Paxillin, and Rac1, our results point to the view that, in the absence of α-catenin in vivo, FA and cytoskeletal dynamics are elevated specifically in epidermal progenitors, endowing them with enhanced migratory functions.
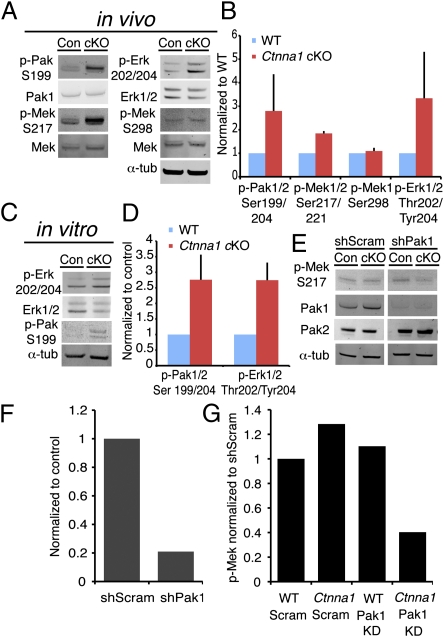
Prior studies on transformed (Cos-7) cells suggest that activated (phosphorylated) Paxillin can recruit and activate Pak, a downstream effector of Rac1 (8). Pak can phosphorylate the MAP-kinase kinase Mek (16), leading to a possible convergence between activated FA, Rac1, and MAPK signaling. To test whether these pathways might be elevated in Ctnna1 cKO, we performed immunoblots on epidermal lysates. We also observed an increase in Pak phosphorylation as well as an increase in Mek phosphorylation on its Pak target site in Ctnna1 cKO epidermis. Finally, we confirmed increased Erk1/2 phosphorylation, reflective of MAPK activation (Fig. 3 A and B).
Fig. 3.
Increased Pak activation in Ctnna1 cKO epidermis promotes Erk/MAPK phosphorylation. (A–E) Quantitative immunoblots of Pak1/2, Mek, and Erk1/2 phosphorylation levels from epidermal lysates from control and Ctnna1 cKO E18.5 embryos (A and B) and from their cultured keratinocytes (C and D). (E) Immunoblots of Mek phosphorylation upon in utero lentiviral transduction of Pak1 or scrambled shRNAs. (F) Quantifications of Pak1 knockdown efficiency. (G) Quantifications of relative Mek activity (phosphorylation) upon Pak1 knockdown.
To directly assess the importance of Pak signaling in these events, we used a chemical inhibitor of Pak activity, 2,2′-dihydroxy-1,1′-dinaphthyldisulfide (IPA-3) (17), and evaluated its effect on epidermal keratinocyte migration from skin explants. In control explants, keratinocytes grew out as a cohesive epithelial sheet over 18 h (Fig. S2 B and C). Treatment of control explants with 20 μM IPA-3 slowed this outward migration as would be expected if Pak influenced cytoskeletal dynamics. Ctnna1-null keratinocytes migrated outward more quickly from cKO explants and as nonadherent individual cells (Fig. S2 and below). Unexpectedly, IPA-3 also elicited frequent rounding and membrane blebbing of Ctnna1-null, but not control, keratinocytes emerging from the explants. This suggested that keratinocytes lacking α-catenin may rely more heavily upon the Pak signaling axis for their survival and migration.
Ctnna1-null keratinocytes also showed hyperactivation of Erk1/2 and Pak in vitro (Fig. 3 C and D). Whereas Pak1 seems to mediate Erk activation through phosphorylation of Mek on S217 (16), Pak2 has been reported to act as a negative regulator of Myc and the actin-depolymerizing factor Cofilin (18). Given these purportedly distinct functions, Pak1 seemed the best candidate for mediating the increased Mek/Erk activation in the Ctnna1 cKO epidermis. To test this, we specifically depleted Pak1 (but not Pak2) levels using a lentiviral shRNA (Fig. 3 E and F). Pak1 knockdown reduced Mek phosphorylation at S217 by 60% relative to control scrambled shRNA (Fig. 3G), whereas Mek phosphorylation was not altered by Pak1 depletion in WT keratinocytes. These findings revealed that WT keratinocytes possess some additional means of maintaining Ras/MAPK signaling that Ctnna1-null keratinocytes lack.
Distribution of Growth Factor Receptors Depends on α-Catenin.
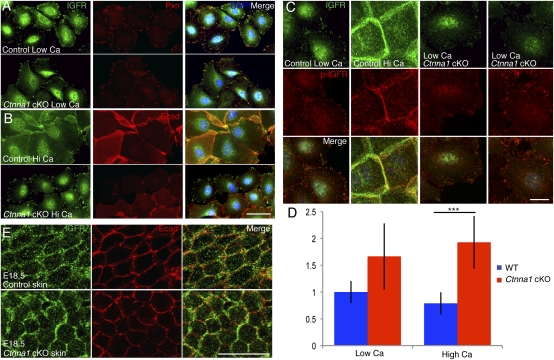
A variety of RTKs and cytoskeletal regulators localize to both FAs and AJs (19, 20), leading us to posit that a change in the stability of one adhesion type might shift the balance in localization of these proteins toward the other. Indeed, in keratinocytes under low-Ca2+ conditions, where AJs do not form, the RTK insulin growth factor receptor (IGFR) colocalized with Paxillin at FAs in both control and Ctnna1-null cells (Fig. 4A). Following a switch to high Ca2+ for 24 h, the IGFR distribution in control cells changed to sites of cell–cell contacts marked by E-cadherin (Fig. 4B). In contrast, IGFR remained at FAs in Ctnna1-null keratinocytes and showed no signs of E-cadherin colocalization, even as immature junctions formed in these cells. In contrast to IGFR, the epidermal growth factor receptor (EGFR) did not localize specifically to FAs under low-Ca2+ conditions in either control or Ctnna1-null cells (Fig. S3A). However, upon shifting cells to high Ca2+, EGFR redistributed to cell–cell adhesions in control cells, as seen for IGFR (Fig. S3B). In Ctnna1-null cells, even though some localization to cell–cell contacts was observed, EGFR did not colocalize specifically with E-cadherin. In general, Ctnna1-null cells also exhibited a higher number of focal adhesions than control cells under high-Ca2+ conditions but not under low-Ca2+ conditions (Fig. S3C). Taken together, these results revealed that localization of these critical epidermal RTKs is regulated by cell–cell adhesion and that this is compromised upon loss of α-catenin.
Fig. 4.
Loss of junctional IGFR in Ctnna1-null keratinocytes. (A and B). Keratinocytes from WT and Ctnna1 cKO embryos were cultured in either low- or high-calcium medium and immunolabeled for IGFR, Paxillin, and/or E-cadherin as indicated. (C and D) Representative IFM images and quantifications to determine relative phospho-IGFR and IGFR levels in WT and Ctnna1-null keratinocytes in low and high calcium. (E) Planar views of WT and Ctnna1 cKO epidermis after immunolabeling E18.5 embryos for IGFR and E-cadherin. [Scale bars (A and B): 50 μm; (C and E): 20 μm.]
Interaction with components of FAs has been shown to enhance RTK signaling in a number of cell types, whereas association with cadherin-based adhesions often has inhibitory effects (21, 22). To assess whether this might be the case for epidermal keratinocytes, we used quantitative IFM to probe the ratio of active (phosphorylated) IGFR to total IGFR in control and Ctnna1-null cells under conditions of low and high Ca2+. Interestingly, Ctnna1-null cells showed an increase in IGFR activity, which was particularly concentrated at FAs in low Ca2+ relative to control cells (Fig. 4 C and D). This difference was more pronounced upon a switch to high Ca2+, where IGFR localized to sites of cell–cell contact in control cells but remained at FAs in Ctnna1-null cells (Fig. 4 C and D). These findings suggested that IGFR activity in keratinocytes is at least in part modulated by its localization and association with AJs versus FAs.
In the case of EGFR, activation by EGF ligand is followed by rapid internalization of the ligand:receptor complex. This can attenuate signaling by targeting internalized receptors for lysosomal degradation but can also promote EGFR interaction with effectors such as Grb2 and Sos in early endosomes (23). To test whether the absence of α-catenin might alter this internalization behavior in keratinocytes, we stimulated control and Ctnna1-null serum-starved keratinocytes expressing GFP-tagged EGFR with a fluorescently labeled EGF and quantified internalization at subsequent time points. We found that 2 min after stimulation, both receptor and ligand accumulated at cell–cell junctions in WT cells, whereas internalization of the complex had already begun in Ctnna1-null cells (Fig. S3 D and F). By 20 min of stimulation, both control and mutant cells showed a primarily vesicular distribution of the receptor (Fig. S3 E and F). These data support the notion that, without α-catenin, ligand-induced EGFR internalization is stimulated in keratinocytes.
To explore the physiological relevance of these findings, we performed whole-mount IFM in vivo. In both control and Ctnna1 cKO E18.5 epidermis, IGFR and EGFR concentrated at intracellular puncta and cell–cell borders. In control tissue, cell border RTKs colocalized with E-cadherin. Interestingly, however, in Ctnna1-null tissue, cell border RTKs appeared to be enriched at sites where E-cadherin was reduced (Fig. 4E and Fig. S3G). As signaling through RTK pathways is a potent mechanism of Pak activation, we sought to determine whether the absence of α-catenin also had an effect on Pak1 localization. When AJ formation was induced in control keratinocytes by elevating Ca2+ levels, Pak1 localization shifted concomitantly with E-cadherin from a diffuse cytoplasmic to a junctional distribution (Fig. S4). This relocalization did not take place in Ctnna1-null keratinocytes, highlighting the importance of α-catenin and proper junction formation for Pak1 recruitment.
In Vivo Interplay Between Focal Adhesion and Adherens Junction Components.
We next addressed the physiological roles of these key FA proteins in the context of control and Ctnna1 cKO epidermis. To deplete PAK, we cloned Pak1 and Pak2 shRNAs into the LV-Cre vector and transduced Ctnna1fl/fl; r26Yfp/+ embryos. To deplete FAK, we transduced LV-Cre virus into Ctnna1fl/fl; FAKfl/fl; r26Yfp/+ embryos. We then measured the consequences to keratinocyte membrane protrusions and migration from ex vivo E18.5 skin explants.
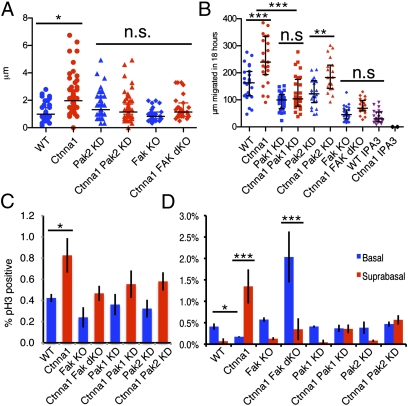
In control cells, reducing Pak2 and FAK did not appreciably affect membrane protrusivity levels; however, in Ctnna1-null cells, the levels were restored back to normal (Fig. 5A). When tissue explant outgrowth was monitored, directed migrations were clearly different (164 μm for WT versus 270 μm for Ctnna1 null after 18 h) (Fig. 5B, P < 0.001). Moreover, although depleting Pak2 had a modest effect on cell migration, the consequences of Pak1 or FAK depletion were profound. On a WT background, Pak1 and FAK depletion significantly reduced migration (P < 0.01), but, on the Ctnna1-null background, these effects were even stronger, nearly abolishing their migration advantage (Fig. 5B). In primary keratinocytes, depletion of these proteins elicited subtle changes in actin cytoskeleton organization, but did not rescue the AJ failure of Ctnna1-null cells to organize actin at the AJs (Fig. S5). These effects were consistent with those seen with IPA-3 (Fig. 5B and Fig. S2 B and C) and with the impaired explant outgrowth that we previously observed in FAK cKO mutant skin (9). Importantly, these genetic interactions also suggested that increased signaling through the FAK and Pak axis could contribute to the increased migration of Ctnna1-null cells out of an epithelial sheet.
Fig. 5.
In vivo depletion of FAK and Pak proteins rescues migratory, proliferative, and apoptotic phenotypes of Ctnna1 cKO epidermis. (A) Quantifications of membrane protrusion distances in keratinocytes emerging from skin explants. Explants were imaged by videomicroscopy over 10 min. (B) Quantifications of keratinocyte outgrowth distances after 18 h of culturing explants from the embryos indicated and treated as indicated. (A and B) Black lines mark median ± interquartile range. ***P < 0.001; **P < 0.01; *P < 0.05; n.s., no significant difference. (C and D) Frequencies of phospho-histone H3-positive (mitotic) basal epidermal cells (C) and phospho-caspase-3–positive (apoptotic) basal versus suprabasal epidermal cells (D) from WT, cKO, and/or KD embryos as indicated. Quantification is based on tissues from more than two animals and >15 tissue sections/animal. Error bars represent ±SD.
We next assessed whether the increased proliferation in the absence of α-catenin is dependent on signaling through FA proteins. To do so, we quantified the frequency of mitotic (phospho-histone H3-positive) epidermal cells in control and Ctnna1 cKO embryos following in utero depletion of Fak, Pak1, or Pak2. Their depletion on a WT background had no effect on proliferation (Fig. 5C). By contrast, depleting any of these three on a Ctnna1 cKO background erased much of the proliferative advantage of Ctnna1-null keratinocytes over their WT counterparts (Fig. 5C). These results point to enhanced crosstalk between FA signaling and MAPK regulation when α-catenin is absent.
Finally, we addressed the physiological consequences of these FA proteins on cell survival in the epidermis. To do so, we quantified the frequency of apoptotic cells in the basal and suprabasal layers of the epidermis in transduced control and Ctnna1 cKO embryos. In WT epidermis, apoptotic cells were found primarily in the basal layer, and depleting Fak, Pak1, or Pak2 did not alter this distribution bias. As documented above, apoptotic cells in Ctnna1 cKO skin were more frequently observed in suprabasal epidermal cells, whereas the basal layer contained fewer apoptotic cells than in WT. Interestingly, ablation of Fak and Ctnna1 simultaneously resulted in a striking reversal of this pattern, with the majority of apoptotic cells now localizing to the basal layer (Fig. 5D). Depleting Pak1 or Pak2 exhibited a lesser but similar effect, with an increase in basal apoptotic cells. Taken together, these results provide compelling evidence that FA signaling is essential to maintaining the survival of α-catenin–deficient basal cells and that survival and proliferation within the epidermis are governed by signaling components that shuttle between FAs and cell–cell contacts.
Discussion
The ability to properly coordinate cell survival, adhesion, and migration is of central importance for the development and maintenance of epithelial tissues and, when defective in cancers, causes tissue overgrowth. Our findings here suggest one possible mode for coregulating these pathways that senses whether α-catenin is present at AJs. We show that depleting α-catenin from embryonic epidermis rapidly induces previously unappreciated alterations in tissue morphology, cytoskeletal organization, and mechanical fragility at the basal and suprabasal juncture of the tissue, accompanied by enhanced apoptosis in the suprabasal layers and reduced apoptosis basally.
Although the exact mechanism leading to suprabasal induction of apoptosis remains to be determined, the most likely explanation is that cells undergo “anoikis,” defined as cell death due to loss of attachment (11). If the exclusive death of Ctnna1-null suprabasal cells occurs through common pathways to anoikis, it would follow that elevated FA signaling in the basal layer could provide protective, prosurvival signals that override the proapoptotic signals from lost cell–cell adhesion.
The elevation in FA signaling that accompanied Ctnna1 ablation provided an important clue to explain why apoptosis was actually reduced in the basal layer relative to control tissue. Indeed, when FAK, Pak1, or Pak2 was depleted in Ctnna1 cKO skin, the cell survival advantage conferred to basal cells was diminished. The potent effects of FAK on cell survival in the skin are particularly intriguing given, first, that FAK can associate with the established apoptosis-effector Trp53 and promote its ubiquitination and subsequent degradation (24) and, second, that Trp53 signaling is responsible for a significant portion of cell death occurring in Ctnna1 mutant skin (5).
In searching for mechanisms that might link α-catenin loss with both enhanced FA signaling and proliferation in the context of a tissue, we were intrigued by prior in vitro studies showing that interactions of RTKs with FA and AJ components typically result in opposing consequences (10, 25). Our results are consistent with these studies, particularly with the relative increase in phospho-IGFR that we detected in Ctnna1-null cells relative to WT and with the increased proliferation and Ras-Rac1-Mek-Erk/MAPK activation seen in cultured Ctnna1-null primary keratinocytes exposed to insulin and in skin epidermis lacking α-catenin (see also ref. 4).
These collective studies make it likely that elevated RTK signaling contributes to the elevated Ras-MAPK signaling that underlies the loss of α-catenin and accounts for the subsequent tissue dysplasia and progression to squamous cell carcinoma in situ (26) seen in these animals. Two studies lend further support to this view. The first study observed that epidermal ablation of the insulin receptor and IGFR leads to epidermal hypoplasia accompanied by an 80% decrease in Rac1 activity (27). The second study found that epidermal-specific deletion of Rac1 in postnatal skin inhibits migration as well as oncogenic Ras-induced tumor formation through a pathway implicating Pak1 and Mek (16, 28).
Other recent studies implicate additional proteins and/or pathways in explaining the enhanced proliferation resulting from α-catenin loss of function. One is the tumor-suppressor protein neurofibromatosis type-2 (also called Merlin) (29), which binds to α-catenin and, when depleted, results in destabilized cadherin-based adhesions and enhanced RTK, Rac1, and Pak activities (15, 30). Another is Yap1, a transcriptional effector of the Hippo growth control pathway, which appears to be negatively regulated by α-catenin (31). It is possible that Yap1 hyperactivation and/or Merlin inactivation within the basal layer of α-catenin–deficient epidermis also contribute to the increased RTK-MAPK, Rac1, and Pak activity that we observe in Ctnna1-null keratinocytes.
In summary, our results provide insights into the mechanisms that underlie the paradoxical links between loss of α-catenin and enhanced apoptosis, increased proliferation and migration, and enhanced susceptibility to cancer progression. The basal-specific enhancement of integrin-FAK-Rac1-Pak1 and RTK signaling, accompanied by diminished apoptosis, explains how the stem-cell compartment within the Ctnna1 cKO epidermis might gain a survival advantage and underscores the importance of cell-matrix adhesion in counteracting the otherwise dire consequences for the loss of this essential gene. This finding also suggests an explanation for why α-catenin's absence confers such a potent susceptibility to cancer progression.
Materials and Methods
Lentiviral Vectors and Constructs.
Lentiviral vectors were based on the pLKO.1 shRNA plasmid from the RNAi Consortium library (Sigma). Lentiviral constructs containing the puromycin resistance cassette were screened for knockdown efficiency in cultured keratinocytes, and the most efficient sequences were selected for use in vivo. The target sequences for shRNA hairpins are listed in SI Materials and Methods. Lentivirus production, concentration, and in utero microinjections were performed as described (5). EGFR-GFP expression vector was kindly provided by Sanford Simon (New York, NY) (32).
Mice.
The following mouse strains were used: CD1 (Charles River Laboratories); Gt(ROSA)26Sortm1(EYFP)Cos/+, referred to in the text as r26Yfp (Jackson Laboratories, donated by A. McMahon, Cambridge, MA); Ctnna1lox/lox; and FAKlox/lox (4, 33). Ctnna1lox/lox mice were bred with r26Yfp and/or FAKlox/lox mice to produce males that were doubly or triply homozygous for the genotypes. These males were mated with Ctnna1lox/lox or FAKlox/lox females for in utero LV-Cre–mediated excision and/or shRNA knockdown. Amniotic sacs of E9.5 embryos were injected with 109 cfu (1.5 μL) of lentivirus. Surgical procedures were limited to 30 min. The Rockefeller University Animal Care and Use Committee approved our animal experimentation protocols, carried out in the Comparative Biology Center (accredited by American Association for the Accreditation of Laboratory Animal Care International) on campus.
Explant Outgrowth.
Punch biopsies (3-mm) were harvested from E18.5 embryos and plated onto glass-bottom dishes (MatTek) coated with fibronectin (10 μg/mL). Explants were maintained in mixed Ca2+ (0.6 mM) media to support intercellular adhesion and incubated 24–48 h. For IPA-3 experiments, explants were cultured in the presence of 20 μM IPA-3 (Sigma) dissolved in DMSO or an equivalent volume of DMSO for control.
Statistics.
All quantitative data are expressed as mean ± SD. Differences between two groups were assayed using Student's t test. Differences between multiple groups (for the explant migration experiments comparing control and Ctnna1-null+/− FAK or Pak1/2) were assessed with two-way ANOVA followed by Tukey post hoc test, performed using Prism software (Graphpad). Migration data were plotted using Prism and represented as scatter plots showing median and interquartile range to illustrate population scatter.
Supplementary Material
Acknowledgments
We thank Markus Schober for his intellectual input and many helpful suggestions. We also thank Nicole Stokes for her assistance with in utero injections; Slobodan Beronja and Xiaoyang Wu for their comments on the manuscript; other E.F. laboratory members for discussions; The Rockefeller Comparative Bioscience Center for care and breeding of mice; and Alison North and The Rockefeller University Bioimaging center for assistance and advice with image acquisition. This work was supported by National Institutes of Health Grant R01-AR27883 (to E.F.). E.F. is an Investigator of The Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202120109/-/DCSupplemental.
References
- 1.Halbleib JM, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36(2):149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci USA. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 5.Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat Med. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 7.Kraynov VS, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 8.Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober M, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176:667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danen EHJ, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 12.Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 14.Menard RE, Mattingly RR. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell Signal. 2003;15:1099–1109. doi: 10.1016/s0898-6568(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 15.Curto M, Cole BK, Lallemand D, Liu C-H, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, et al. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 2010;29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 17.Deacon SW, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomar A, Schlaepfer DD. A PAK-activated linker for EGFR and FAK. Dev Cell. 2010;18(2):170–172. doi: 10.1016/j.devcel.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canonici A, et al. Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer. 2008;122:572–582. doi: 10.1002/ijc.23164. [DOI] [PubMed] [Google Scholar]
- 21.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4(4):E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 22.Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes. 2003;10(2):105–118. [PubMed] [Google Scholar]
- 23.Jiang X, Sorkin A. Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol Biol Cell. 2002;13:1522–1535. doi: 10.1091/mbc.01-11-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim S-T, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slack-Davis JK, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci USA. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stachelscheid H, et al. Epidermal insulin/IGF-1 signalling control interfollicular morphogenesis and proliferative potential through Rac activation. EMBO J. 2008;27:2091–2101. doi: 10.1038/emboj.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 29.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappoport JZ, Simon SM. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J Cell Sci. 2009;122:1301–1305. doi: 10.1242/jcs.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beggs HE, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.