Abstract
Nanofiber scaffolds have been useful for engineering tissues derived from mesenchymal cells, but few studies have investigated their applicability for epithelial cell-derived tissues. In this study, we generated nanofiber (250 nm) or microfiber (1200 nm) scaffolds via electrospinning from the polymer, poly-L-lactic-co-glycolic acid (PLGA). Cell-scaffold contacts were visualized using fluorescent immunocytochemistry and laser scanning confocal microscopy. Focal adhesion (FA) proteins, such as phosphorylated FAK (Tyr397), paxillin (Tyr118), talin and vinculin were localized to FA complexes in adult cells grown on planar surfaces but were reduced and diffusely localized in cells grown on nanofiber surfaces, similar to the pattern observed in adult mouse salivary gland tissues. Significant differences in epithelial cell morphology and cell clustering were also observed and quantified, using image segmentation and computational cell-graph analyses. No statistically significant differences in scaffold stiffness between planar PLGA film controls compared to nanofibers scaffolds were detected using nanoindentation with atomic force microscopy, indicating that scaffold topography rather than mechanical properties accounts for changes in cell attachments and cell structure. Finally, PLGA nanofiber scaffolds could support the spontaneous self-organization and branching of dissociated embryonic salivary gland cells. Nanofiber scaffolds may therefore have applicability in the future for engineering an artificial salivary gland.
Keywords: Biomimetic material, cell signaling, cell morphology, ECM, nanofibers, organ culture, PLGA, salivary gland, scaffold
1. Introduction
Xerostomia, or dry mouth, is a common characteristic of patients suffering from the autoimmune disease Sjögren's syndrome (SS), patients that undergo radiation regimens for treatment of head and neck cancers, and also in patients treated for other disorders since xerostomia can result as an adverse side effect of thousands of medications [1]. Saliva-secreting adult glands are comprised largely of acinar units composed of a single layer of secretory epithelial cells that are interconnected by a network of ductal epithelial cells that modify saliva made by the acinar cells and transport it to the mouth. Xerostomia can result when the acinar cells are destroyed during radiation therapy. Although treatment options are temporary, and no cure for xerostomia currently exists; tissue engineering strategies could offer long-term benefits for such patients.
The salivary epithelium is closely surrounded by mesenchymal-derived stromal tissue [2] that sits within a fibrillar extracellular matrix (ECM). Both the stromal-derived ECM and the specialized epithelial cell-associated ECM, the basement membrane, have been shown to extensively influence the growth, branching morphogenesis, and differentiation of the epithelium [7–9]. The basement membrane is a specialized ECM composed of two thin sheets of matrix proteins: a layer known as the basal lamina and an underlying layer of reticular fibers (lamina reticularus) [3]. SEM studies of developing rat SMGs show a three-dimensional (3D) thin fibrous meshwork (100–400 nm in thickness) directly beneath the epithelial cell plasma membranes [4] that comprises all of the layers of the basement membrane. The basement membrane is tightly linked to epithelial cell membranes through direct attachment via cell-surface receptor proteins (i.e. integrins and other transmembrane proteins) and functions to maintain the epithelial cytoarchitecture, in addition to multiple other signaling functions.
Many tissue engineering and organ regeneration approaches seek to use bio-inspired artificial electrospun nano- and microscale fiber scaffolds [5, 6] due to their structural similarity to the major components of tissue matrix proteins, such as fibronectin [7], collagen [8, 9], and laminin [10]. Such synthetic fiber scaffolds have been previously shown to promote in vivo-like cellular morphologies [11] primarily in mesenchymal-derived cells which interpret the nanofibers as an ECM and grow between the fibers. However, few studies have investigated the utility of nanofiber scaffolds as a surrogate for a basement membrane structure that would support epithelial cells. We previously fabricated uniform nanofiber (250 nm ± 50 nm) and microfiber (1200 nm ± 200 nm) scaffolds composed of the copolymer, poly (DL-lactide-co-glycolide) (PLGA). We showed that adult mouse submandibular salivary gland (SMG) ductal epithelial cells proliferated robustly as a monolayer on the surface of these scaffolds [12].
In the current study, we investigated whether nanofiber scaffolds could influence cell attachments and tissue morphology as the first step towards evaluating their suitability as scaffolds for future artificial salivary gland tissue engineering constructs. We hypothesized that the nanoscale architecture of the fiber scaffolds could influence cell-scaffold adhesions and direct an optimal cellular organization similar to in vivo salivary gland tissues. Cell-scaffold adhesions were examined via expression and localization of focal adhesion (FA) complexes, which are the major sensory mechanism by which cells physically interact with their extracellular environment [13]. Responses of cells to PLGA nanofiber scaffolds were compared to that of cells grown on microfiber scaffolds and on flat material control films. FAs were examined in embryonic salivary gland tissue explants, adult cell lines, and in primary embryonic cells grown on the scaffolds.
2. Materials and methods
2.1. Materials
Poly(D-lactide-co-glycolide) (PLGA), with a lactic to glycolic acid ratio of 85:15 and a molecular weight of 95,000 Da, was purchased from Birmingham Polymers (Pelham, AL) and hexafluoroisopropanol (HFIP), used to dissolve PLGA, was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against phospho-Tyr397-FAK (Cat. No 3283), phospho-Tyr118-paxillin (Cat. No 2541), total FAK (Cat. No 3285), and paxillin (Cat. No 2542) were from Cell Signaling (Danvers, MA). Anti-heparan sulfate proteoglycan (perlecan, Cat. No MAB1948) was from Millipore. Anti-collagen IV antibody (Cat. No AB756P) was from Millipore (Billerica, MA). Fibronectin rabbit polyclonal antibody (clone R5836) and laminin α5 polyclonal antibody were generously provided by Drs. Kenneth Yamada (NIH) and Jeffrey Miner (WUSTL), respectively. Anti-vinculin (Cat. No V9131), talin (Cat. No T3287) and vimentin (Cat. No V2258) antibodies were purchased from Sigma-Aldrich. Anti-GAPDH antibody (Cat. No 10R-G109a) was purchased from Fitzgerald Labs (Boston, MA) and anti-aquaporin-5 (Cat. No AQP-005) was purchased from Alomone labs (Israel). DAPI, SyBr green (to stain nuclei) and rhodamine–phalloidin (to stain actin) were obtained from Invitrogen. Donkey anti-species cyanine dye-conjugated AffiniPure F(ab')2 secondary antibodies were from Jackson Immunoresearch (West Grove, PA).
2.2. Fabrication of PLGA film and fiber scaffolds
PLGA nanofibers (average diameter of 250 ± 50 nm) and PLGA microfibers (average diameter of 1200 ± 200nm) were generated by electrospinning using a DOE approach, as described previously [12]. Briefly, PLGA dissolved in HFIP was dispensed through a 3 ml syringe via an automatic syringe pump. The needle was suspended vertically over a grounded aluminum collector plate at a fixed distance of 15 cm. and a voltage supply was wired to the metal needle with an alligator clip to produce a nonwoven mat of uniform nano- and microscale fibers. 12 mm round glass coverslips were pre-cleaned in ethanol and coated with Vectabond (Vector Laboratories, Burlingame, CA), as per manufacturer's protocols, to facilitate adhesion of PLGA. PLGA was then solvent cast using 60μl of 3% PLGA (w/v) dissolved in HFIP and dried for one hour at room temperature immediately before electrospinning in order to create an adhesive layer between the glass and the fibers and to prevent fiber detachment during culture. PLGA fibers were electrospun on top of such film-coated coverslips laid over aluminium foil on agrounded collector plate. PLGA material control films were generated by spin-coating 80μl of 5% PLGA (w/v) dissolved in chloroform onto Vectabond-treated glass coverslips for 1 minute at 1500 rpm, and then placed on a hotplate at 200°C until complete solvent evaporation occurred. Spin coating was used to create these PLGA films to ensure a smooth surface of even thickness. All scaffold surfaces were sterilized by UV irradiation for at least 1hr, washed and presoaked in sterile phosphate buffered saline (PBS) for 48 hrs, followed by cell culture media for 24hrs.
2.3. Cell and ex vivo SMG organ culture
SIMS cells, an immortalized adult mouse submandibular salivary gland ductal epithelial cell line [14, 15], were cultured in DMEM media with 10% fetal bovine serum (FBS) and 1× pen/strep in a humidified 37°C incubator maintained at 5% CO2 and 95% air. ParC10, an immortalized adult rat parotid gland acinar cell line was cultured in DMEM-F12 media supplemented with 2.5% FBS, growth factors and 50 μg/ml gentamycin, as previously described [16, 17]. Approximately two month-old adult timed-pregnant female mice (CD-1 strain) were obtained from Charles River Laboratories (Wilmington, MA) and adult or embryonic day 13 (E13) SMGs were harvested in accordance with protocols approved by the University at Albany IACUC and cultured ex vivo on floating porous polycarbonate (PC) Nuclepore Track-Etch membranes (0.1 μm, Whatman USA) or floating PLGA fiber mats at the air-media interface. PLGA fiber mats were mechanically anchored between two PDMS molds, each with a 12 mm diameter window in the center on individual 35mm tissue culture plates. Fibers were presoaked in serum-free DMEM/F12 media (Invitrogen) with 50 U/ml penicillin and 50 μg/ml streptomycin (1× pen/strep, Invitrogen) for 24 hrs, added to the bottom reservoir such that the fiber mats floated over the media to simulate the traditional SMG ex vivo culture method. Brightfield images were captured initially at 2 hrs and every 24 hrs thereafter using a Nikon E600 upright microscope fitted with a Photometrics CCD camera under 4× or 10× objectives and processed using MetaMorph software (V7.7.0.0, Molecular Devices, Sunnyvale, CA).
2.4. Immunocytochemistry (ICC) and confocal imaging
Cells or SMGs were fixed in freshly prepared 2 or 4% paraformaldehyde with 5% sucrose in 1× PBS, supplemented with phosphatase inhibitors (10 mM sodium fluoride, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate) for 20 or 30 minutes, respectively, and processed for ICC as previously described [18]. Where indicated, nuclei were stained with DAPI (1:5,000) or F-actin was detected using rhodamine-phalloidin (1:300) included in the secondary antibody solution. Samples were mounted on glass coverslips with mounting media (Fluor-Gel, EMS, Hatfield, PA) containing 1 mg/ml p-phenylenediamine (PPD) antifade solution, before imaging. Laser scanning confocal microscopy was performed using a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) and images were acquired at 20× or 63× magnification. Confocal images were processed in Leica LasAF software and all confocal images within a given experiment were captured using the same laser intensity and gain settings so that intensities could be compared across samples.
2.5. Scanning electron microscopy (SEM)
Fiber nanostructure was characterized using a Zeiss 1550 field emission SEM (Leo Electron Microscopy Ltd., Cambridge U.K.; Carl Zeiss, Jena, Germany). Using the incorporated digital annotation software, diameter measurements (15 per sample) were acquired, and arithmetic means and standard deviations were calculated for all fibers, as we reported previously [12]. For SEM imaging of cells on fibers, approximately 3 × 104 cells were seeded and allowed to adhere overnight to each surface before fixing and processing for SEM. Cells were fixed with 3% glutaraldehyde solution in 0.1 M phosphate buffer containing 0.1 M sucrose for 2 hrs at RT. The samples were then rinsed three times in PBS, dehydrated in a graded ethanol series and slowly infiltrated with hexamethyldisilazane (HMDS) for drying. Characterization of the cell-containing samples was also done using a Zeiss 1550 SEM. Prior to imaging, samples were mounted on 1cm2 stubs using carbon tape and were sputter-coated with ~5 nm gold-palladium to avoid sample charging.
2.6. Quantitative analyses of nuclear area and clustering coefficient
Confocal images (450 ×450 μm or 512×512 pixels) of DAPI-stained nuclei on the different substrates (n=20/sample type) were segmented using the watershed segmentation technique and used to extract: 1) the average nuclei area and, 2) the level of connectivity between nuclei. Using nuclei segmentation results, cell-graphs were constructed [19, 20] that enabled average clustering coefficient measurements. To reduce over-segmentation, we first binarized the images via global thresholding using Otsu's automatic threshold selection algorithm [21]. A topographic interpretation was obtained by taking the distance transform of the resulting binary image. This image was then inverted to construct the catchment basins and the watershed segmentation method was applied to construct watershed lines to divide these basins. Each basin corresponded to a unique nucleus in the image. Nuclei with an area of less than 25 μm2 were excluded to reduce any effects that might arise from pro-apoptotic cells or the highly condensed mesenchyme in adult SMGs.
1) Computation of nuclear area - Nuclear area was used as an indirect indicator of cell spreading on different substrates and compared to native adult SMG nuclei. After segmentation, the area for each nucleus within each image was calculated. The average nuclei size for the ith image and standard error is reported.
2) Computation of average clustering coefficient - Average clustering coefficient is a measure of the level of connectedness between nodes (nuclei) within the graph [22], which provides an estimate of how closely associated cells are. To compute this measurement, we constructed cell-graphs for each image using the cell nuclei as the vertices and, assuming the average-sized nuclei is circular in shape, established the edges between the nuclei using 5 times the radius of the average-sized nuclei as the distance threshold. We excluded nuclei whose clustering coefficients were zero, including isolated nuclei and those with neighbors that did not have any edges that connected them. The average clustering coefficient of the ith graph was computed as
where
is the clustering coefficient of the nth vertex, is the number of edges between the vertices that have a link with vertex n and kn is the number of vertices that have a link with vertex n.
2.7. Atomic force microscopy (AFM)
Stiffness measurements were performed using a Bruker Catalyst atomic force microscope driven by a Nanoscope V controller (Bruker AXS, Santa Barbara, CA). AFM probes used to record force/indentation data consisted of silicon cantilevers (nominal spring constant 4.5 N/m) mounted with 45 μm diameter polystyrene spheres (Novascan Technologies, Inc., Ames, IA). Actual spring constants were determined using the thermal tune method, and found to range from 4.02 N/m to 5.47 N/m. All measurements were performed on fully hydrated samples under fluid conditions. Young's modulus values were calculated for each recorded curve using the third-party analysis software PUNIAS (Protein Unfolding and NanoIndentation Analysis Software, www.punias@free.fr), which employs the Hertz model of continuum material mechanics. AFM measurements for microfibers may be considered `effective' moduli since the inherent scaffold topography of these samples constitutes a departure from the continuum material assumed by the Hertz model for calculating modulus.
2.8. Immunoblotting
Cells or SMGs were lysed for total protein by adding ice-cold RIPA buffer (50 mM HEPES, 150 mM NaCl, 10% Glycerol, 1.5 mM MgCl, 1.5 mM EGTA, 1% Triton-100, 1% Nadeoxycholate, 0.1% SDS, protease and phosphatase cocktail inhibitors, Roche) and vortexing every 5 minutes for 30 minutes followed by mild sonication. Cell debris was cleared by centrifugation at high speed (16,000 × g) for 20 minutes. Protein concentrations of the resulting supernatants were determined using a Micro-BCA assay kit (Pierce, Rockford, IL). Immunoblot analyses were performed using samples normalized for equal protein loading (5–10 μg total protein/lane), as previously described [18]. Blots developed on X-ray film were scanned (CanoScan, Canon, USA) and quantified densitometrically using Image J software (NIH). Representative blots and quantifications from experiments repeated three times are shown.
2.9. Time-lapse microscopy
Fluorescent time-lapse microscopy was performed on a Zeiss Z1 Cell Observer inverted microscope. Images were acquired every 10 minutes for 18 hrs using AxioVision software (V4.8, Carl Zeiss) using a 10× objective, processed in Axiovision software, and converted to movies (AVI/Cinepak codec) using Metamorph (Molecular Devices).
2.10. Statistical analysis
One-way analysis of variance (ANOVA) with Bonferroni's post-test was carried out to compare the means of different data sets within each experiment in GraphPad Prism 5 software. A value of p≤ 0.05 was considered to be statistically significant.
3. Results
3.1. Salivary gland branching morphogenesis and focal adhesion protein expression
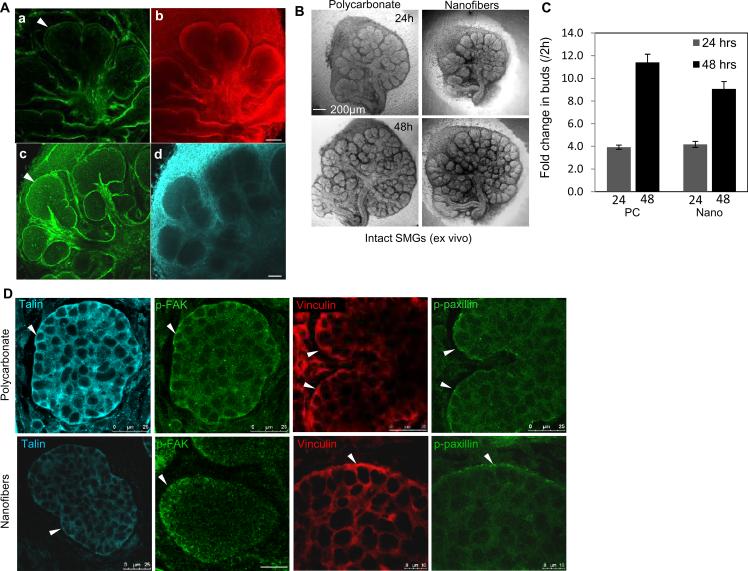
The embryonic mouse SMG is a classic model for studying the process of branching morphogenesis, tissue assembly, and regeneration [23, 24]. SMG organ branching morphogenesis and proliferation are strongly influenced by cues provided by fibrous basement membrane proteins, such as laminin [25], collagen [26] and the heparin sulfate proteoglycan perlecan [27] and fibronectin [28]. As detected by ICC and confocal microscopy, in developing E13 SMGs cultured ex vivo, these matrix proteins are in direct contact with outermost polarized epithelial cell layer (Fig. 1A). To determine if PLGA nanofiber scaffolds could support ex vivo embryonic SMG branching morphogenesis and epithelial cell proliferation, E13 SMGs were isolated and cultured on PLGA nanofibers or control polycarbonate membranes. SMGs cultured on nanofibers showed extensive budding and ductal outgrowth within 24 hrs (Fig. 1B). Morphometric analysis quantifying the fold change in the number of buds in SMGs on nanofibers at 24 hrs and 48 hrs, confirmed a significant increase over time (Fig. 1C), which was comparable to that seen on the polycarbonate membranes. Epithelial cell proliferation was assayed using nuclear staining for EdU, a thymidine analog (Supplementary Fig. 1A), which detected extensive proliferating nuclei within the buds of SMG explants grown on both nano- and microfiber scaffolds (Supplementary Fig. 1B), similar to control explants. These results indicate that nanofiber scaffolds can be used to support growth of SMG organ explants.
Fig. 1. PLGA fiber scaffolds support embryonic salivary gland branching morphogenesis and proliferation.
(A) Confocal images through the equatorial plane of E13 SMGs cultured ex vivo for 24 hrs and stained for basement membrane collagen IV (a), laminin α5 (b), perlecan (c) and fibronectin (d), scale bars = 50 μm. (B) Brightfield images of E13 mouse SMGs cultured ex vivo for 48hrs on fibers or polycarbonate membranes, scale bars = 200 μm. (C) Morphometric analysis of the fold change in the number of buds at 24 and 48 hrs, normalized to the number of buds at 2 hrs, Bars are mean ± S.E.M. from four different experiments. (D) Confocal images captured through the equatorial section of E13 SMGs grown on floating polycarbonate or PLGA nanofiber surfaces for 48 hrs ex vivo and stained for the FA proteins talin, p-FAK, vinculin and p-paxillin shows stronger localization along the basal cell membranes (white arrowheads) of the outer columnar cell layer in pro-acinar buds.
FA complexes are well established as the major adhesive and signal transducing components between the internal actin cytoskeleton and the external ECM and play a key role in sensing surface topography and mechanical properties of the extracellular environment [29, 30]. FA proteins, such as focal adhesion kinase (FAK) and the adaptor proteins, paxillin [13] and talin [31], can bind to and activate integrin β1 subunit cytoplasmic domains, along with vinculin [32], which connects integrins to actin filaments. Autophosphorylation on tyrosine 397 activates FAK creating a high-affinity binding site for Src and Src family kinases, which in turn activates other components of FA complexes, to initiate downstream signaling cascades [33]. Since our previous work demonstrated that FAK functions as a mechanosensor in glands undergoing branching morphogenesis [34], we examined FA expression in developing SMGs on polycarbonate and nanofiber scaffolds using ICC and confocal microscopy. Consistent with our previous work [34], strong focal basal membrane accumulations of p-FAK(Tyr397), talin, p-paxillin (Tyr118), and vinculin were detected in the outer epithelial cells of SMG explants cultured on either polycarbonate or nanofibers, with some additional diffuse cytoplasmic staining (Fig. 1D). This pattern of FA protein expression indicates that the organ explants cultured on nanofiber scaffolds continue to undergo dynamic morphogenesis as they do when cultured on polycarbonate scaffolds.
3.2. FA expression in adult SMG acinar epithelial cells
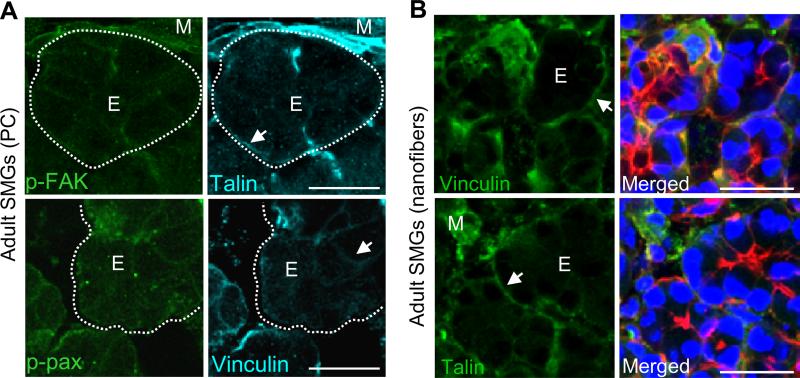
Since adult tissues do not undergo dynamic tissue remodeling to the extent that embryonic tissues do [35], we questioned whether focal adhesions would be required in adult acinar tissue. We examined the FA protein expression and localization within acinar epithelial cells in adult mouse SMG tissue explants cultured on polycarbonate membranes or on nanofiber scaffolds using ICC (Fig. 2). In comparison to the embryonic cells, differentiated adult acinar cells cultured on nanofibers showed reduced cytoplasmic staining for FA proteins, similar to explants cultured on control polycarbonate membranes (Fig. 2B). ICC and confocal imaging for the water channel protein, aquaporin-5 (AQP-5), which is primarily expressed by acinar cells [36] demonstrated that adult tissue explants can maintain their differentiation status when cultured on fiber scaffolds (Supplementary Fig. 2). Not surprisingly, strong staining for FA proteins was observed in the mesenchymal cells surrounding the acini, which are known to express high levels of these proteins (Fig. 2A). Together, these data suggest that FA expression is developmentally regulated with higher levels observed in morphogenetically active embryonic glandular tissues and lower levels detected in the adult salivary acinar cell population.
Fig. 2. FA formation and expression is low in adult SMG acinar epithelial cells.
(A) Confocal images of adult SMG tissue explants grown on polycarbonate (PC) for 48hrs ex vivo show low expression of the FA proteins p-FAK, talin, p-paxillin or vinculin within acinar epithelial cells (E, arrows). Staining is seen at the basal cell membranes (arrows) and in mesenchymal cells (M) surrounding the acini, which is outlined by dotted lines. (B) Confocal images of adult SMG tissue explants cultured for 48hrs ex vivo on nanofibers and stained for talin or vinculin (green) or co-stained with actin (red) and DAPI (nuclei, blue) also show low FA expression by acinar epithelial cells with stronger staining at the basal cell membranes (arrows) and in mesenchymal cells. Scale bars = 25 μm.
3.3. FA formation in acinar and ductal epithelial cells on nanofibrous scaffolds
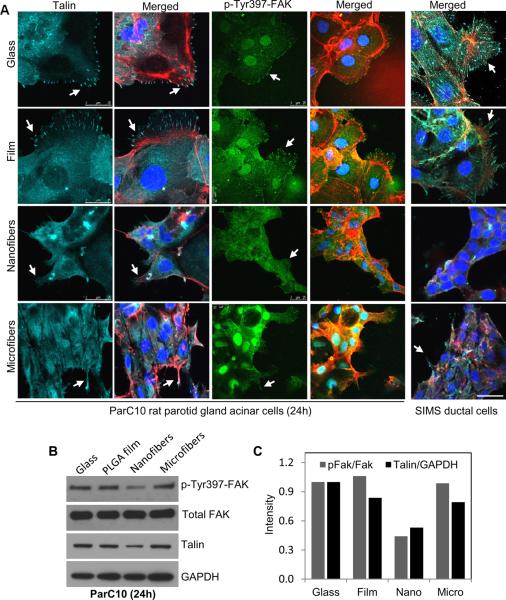
For application as a scaffold in engineered tissues, nanofiber scaffolds might serve as replacement for the basement membrane structure for an epithelial cell population. To investigate this possibility, an adult salivary gland acinar cell line was seeded on the nanofiber scaffolds. Although we previously reported that a salivary gland ductal cell line can attach and are viable on nanofiber scaffolds [12], the nature of the cell adhesions was not investigated. The composition and localization of FA proteins was examined in ParC10 acinar cells seeded on nanofiber scaffolds and compared to cells seeded on glass or on a material control (i.e. flat PLGA film). ICC and confocal imaging of cells grown on glass or film surfaces showed strong staining for p-FAK and talin localizing together in cellular protrusions where numerous FA complexes were formed, that associated with the actin cytoskeleton (Fig. 3A). In contrast, substantially reduced FA complex formation was detected in cells seeded on nanofibers, which paralleled a decrease in the protein levels of p-FAK and talin in these cells (Fig. 3B). To determine if this difference in FA formation was due to the fiber topography, we compared FA complexes formed by cells grown on microfibers (1200 nm average diameter) with those grown on nanofibers (250 nm average diameter). While cells on microfibers also showed decreased FA formation and p-FAK and talin levels compared to those on glass and film, cell protrusions which extended along the length of the microfibers, still stained positively for p-FAK and talin (Fig. 3 and Supplementary Fig. 3A), suggesting that cells grown on nanofibers may have a phenotype intermediate between that of cells grown on microfibers and on PLGA films. Interestingly, p-FAK was also observed in the nuclei of cells cultured on glass, film, and microfibers but little was detected in cells cultured on nanofibers. Western blot analysis was used to quantify the amounts of p-FAK and total talin within the whole population of cells seeded on each substrate. The ratio of p-FAK to total FAK and total levels of talin were lowest in cells grown on the nanofibers, while cells grown on microfibers showing intermediate levels of these proteins. A similar response in FA proteins was also observed in SIMS ductal cells, indicating that both acinar and ductal cell types exhibit decreased levels of FAK activation and talin when cultured on nanofibers.
Fig. 3. FA formation and protein expression is reduced in adult salivary gland acinar and ductal cell lines cultured on nanofiber scaffolds.
(A) Confocal images of ParC10 acinar or SIMS ductal epithelial cells grown on flat (glass or PLGA film) or fiber (nano or micro) surfaces and stained for talin (cyan), actin (red), p-Tyr397-FAK (p-FAK) (green) or nuclei (DAPI, blue), scale bars =25 μm. (B) Western blots and (C) densitometric quantification for p-FAK normalized to total FAK and talin normalized to GAPDH, indicate that talin and p-FAK expression is lowest on nanofiber surfaces.
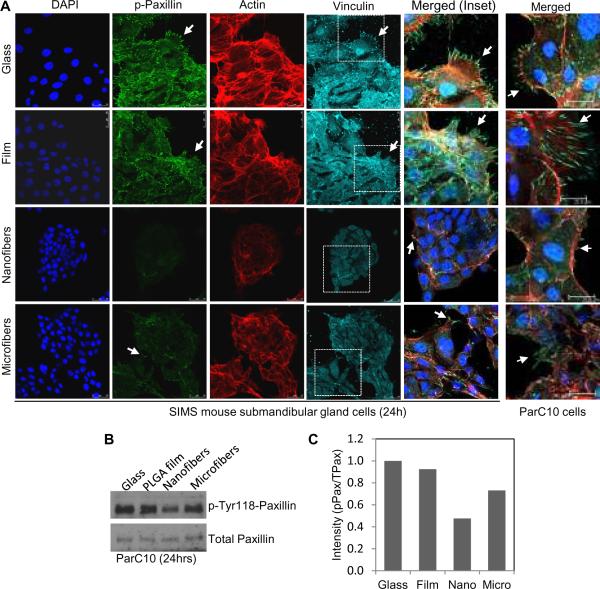
If the structures detected in the ParC10 cells and SIMS cells are FA complexes, these same structures should contain other key FA proteins, including paxillin and vinculin, which are recruited during maturation of FA complexes. Both SIMS ductal and ParC10 acinar cells grown on flat surfaces exhibited strong focal accumulations of p-paxillin (Tyr118) and vinculin (Fig. 4A). In contrast, these FA proteins were reduced in cells on nanofiber surfaces when compared to cells grown on other surfaces (Fig. 4 and Supplementary Fig. 3B). Fewer actin stress fibers were also detected in cells on nanofibers compared to other surfaces, which was consistent with the decrease in cell spreading and FA aggregate formation. The trend in decreasing FA formation in the order of glass> film> microfibers> nanofibers was confirmed using densitometric quantification of Western blots (Fig. 4B, C) to quantify these proteins in the entire cell population. These results suggest that both salivary acinar and ductal epithelial cell types can sense and respond to differences in scaffold fiber diameter by modulating FA protein expression activation and localization.
Fig. 4. Phosphorylated paxillin and total vinculin expression are reduced in salivary gland epithelial cells grown on nanofiber scaffolds.
(A) Confocal images of SIMS or ParC10 cells stained for nuclei (DAPI, blue), phospho-Tyr118-paxillin (p-paxillin) (green), actin (red) and vinculin (cyan) indicate FA complex aggregation at cell protrusions (arrows) on the flat or microfiber surfaces which are reduced on nanofiber surfaces. (B) Western blots and (C) densitometric quantification for p-paxillin normalized to total paxillin.
3.4. Influence of nanofiber scaffold topography on FA formation in acinar and ductal epithelial cells
Physical and chemical properties of the scaffold can influence FA formation and cell morphology [29]. Water contact angle measurements used to measure hydrophilicity of the pre-soaked scaffolds identified no significant differences (Supplementary Fig. 4A). Focal adhesions are known to respond to surface stiffness [13]. To determine if differences in scaffold stiffness between flat PLGA films or fiber scaffolds, might contribute to the observed differences in FAs, AFM was used to estimate average Young's moduli (E). AFM analysis yielded stiffness measurements within the MPa range for PLGA films and fibers (Table 1), all of which were orders of magnitude higher than the in vivo value for human SMGs, which has been reported to be 10.92 ± 3.1 kPa [37]. Since differences in scaffold thickness might also impact the formation of FAs, we measured the thickness of each scaffold using SEM (Table 1). The thickness of PLGA nanofibers were the thinnest (average thickness 1.2 ± 0.1 μm) and comparable to that of spin-coated PLGA films (1.2 ± 0.05 μm), while solvent cast film (9.1 ± 0.2 μm) and microfibers (12.1 ± 1.5 μm) were thicker, as expected. Since reduced FA formation persisted on the thinner and more rigid nanofiber scaffolds, in contrast to increased expression on the thicker but more compliant microfibers and films, nanotopography rather than substrate thickness or stiffness appeared to be the primary influence on FA formation. Decreased cell contact area or ligand spacing has been shown to progressively restrict cell extension and FA formation [38]. Consistently, our data also suggests that increased microfiber spacing (higher porosity) compared to the dense (low porosity) nanofiber spacing (Supplementary Fig. 4B) may likely contribute to the differences seen in cell morphology, spreading and FA formation. Together, these results indicate that structural differences provided by scaffold topography, rather than mechanical cues or other surface properties, are likely to regulate FA protein expression in salivary gland cells.
Table 1.
Young's modulus and thickness measurements for scaffolds
| Scaffold type | Avg. E (MPα) | No. of samples | Avg. no. of spots/sample | Avg. no. of measurements/spot | Avg. Thickness (μm) |
|---|---|---|---|---|---|
| Mean ± Std. dev. of Mean | |||||
|
| |||||
| PLGA Nanofibers | 11.0 ± 3.3† | 3 | 4 | 66 | 1.2 ± 0.1* |
| PLGA Microfibers | 0.30 ± 0.03 | 3 | 3 | 55 | 12.1 ± 1.5* |
| Spin-coated Film | 23.4 ± 6.2† | 2 | 4 | 72 | 1.2 ± 0.05 |
| Solvent cast Film | 9.3 ± 8.1 | 4 | 4 | 52 | 9.1 ± 0.2 |
PLGA, Poly-(DL)-lactide-co-glycolide
no statistical difference, between spin-coated film and nanofibers, p>0.05, ANOVA with Bonferroni's posthoc test.
Values represent fiber mat thickness only and do not include thickness of the underlying solvent cast film layer
3.5. Cell morphology and clustering of acinar and ductal cells on nanofiber scaffolds
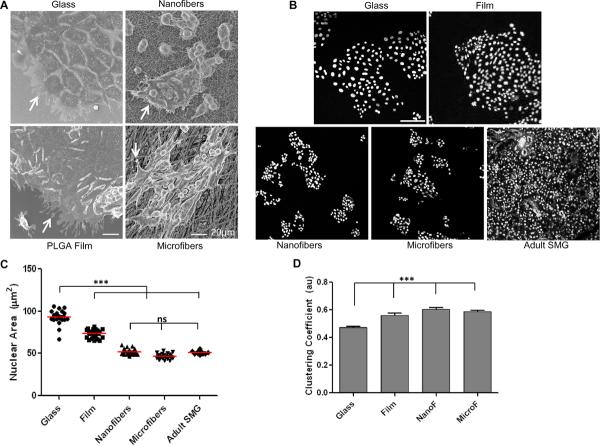
Activation of FA proteins such as FAK and paxillin are well known to regulate cell spreading and consequently morphology [39]. We therefore tested whether the differences in FA expression and formation might translate into differences in cell spreading on fiber or flat surfaces. Scanning electron micrographs showed that SIMS cells grown on nano or microfiber scaffolds adhered and proliferated robustly as a monolayer, without migrating into the pores of the fibers. These cells exhibited a more rounded and clustered morphology, in contrast to cells cultured on glass or PLGA film, which spread extensively within 24 hrs after seeding (Fig. 5A and Supplementary movies). To estimate the extent of cell clustering, we quantified the area of the nucleus, since nuclear structure is intimately integrated with the cellular cytoskeleton [38, 40]. The average nuclear area was quantified from segmented DAPI-stained confocal images of nuclei from cells seeded on different surfaces (Fig. 5B), and it showed a significantly higher average area for cells cultured on flat surfaces, as compared to cells cultured on either fiber surface (Fig. 5C). We found that the average nuclear area of cells cultured on fibers was also similar to those in adult glands (Fig. 5C), suggesting that a more in vivo-like cell morphology may be achieved in cells cultured on fiber scaffolds. Cell-graph methods (Supplementary Fig. 5) can be used to investigate the structural organization of cell nuclei and to quantify the level of connectedness between cells. The clustering coefficient from the graphs was computed, which showed an increasing trend in cell clustering on the fiber surfaces, as compared to flat surfaces (Fig. 5D). This rounded cell morphology, clustering, and well-organized cytoskeletal arrangement, as detected by cortical actin staining, was still evident at later time points (Supplementary Fig. 6). These results suggest that that fiber scaffold topography can significantly influence salivary gland epithelial cell adhesion and affect cellular morphology.
Fig. 5. Nanofiber scaffold topography directs epithelial cell morphology and clustering.
(A) Scanning electron micrographs show cell attachment, protrusions (arrows), and spreading of SIMS cells on glass, spin-coated PLGA film, nanofibers or microfibers, scale bars =20 μm. (B) Binarized segmented confocal images of DAPI-stained nuclei from SIMS cells on different surfaces or adult SMG tissue thin sections, scale bar = 100 μm. (C) Quantification of nuclear area as an indirect measure of cell spreading shows statistically significant differences between cells on flat versus fiber surfaces. No significant difference (ns) was detected between cells on fibers versus adult tissue, *p<0.05 was considered statistically significant using ANOVA followed by Bonferroni's post hoc tests to compare individual means. (D) Quantification of cell clustering using cell-graphs shows an increasing trend in cell clustering (au= arbitrary units) on fiber surfaces compared to glass (***p<0.0001, ANOVA).
3.6. Self-organization and branching morphogenesis of dissociated primary embryonic cells on PLGA fibers
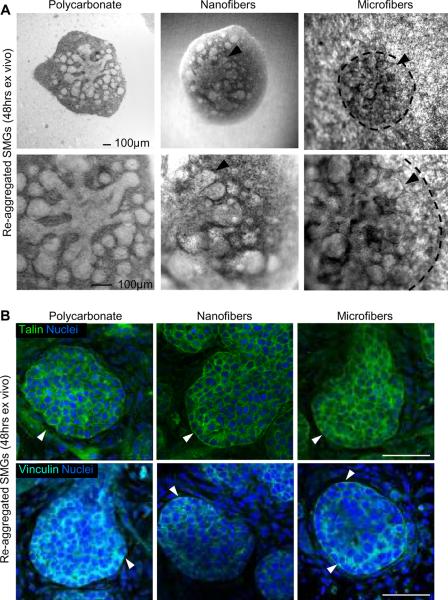
Since one route to generating an artificial gland will require growth, attachment, and organization of primary salivary gland cells or progenitors into acinar or ductal structures on an artificial scaffold, we derived a single-cell population from primary embryonic SMG tissue. These cells retain the ability to spontaneously self-organize and re-aggregate into gland-like structures that continue to undergo branching morphogenesis, similar to the intact organ [41]. These dissociated SMG cells were able to self-organize and form a tissue aggregate that formed buds and ducts within 24 hrs of culture on PLGA fiber scaffolds (Fig. 6A). There was cell-type specific separation as epithelial cells clustered together to form buds and ducts that branched extensively by 48 hrs and became surrounded by mesenchymal cells, as we previously reported [41]. No significant differences in cell re-aggregation or branching patterns of the aggregate tissues were apparent between nano- and microfiber scaffolds, and these structures were comparable to those formed on polycarbonate filters. ICC and confocal imaging of the reformed epithelial buds showed that these re-aggregated structures also expressed diffuse talin and vinculin FA proteins (Fig. 6B), which localized similar to intact developing glands (Fig. 1D), and consistent with our recent published data [34]. These results further support the applicability of nanofibers to function as suitable scaffold for primary salivary gland epithelial cells.
Fig. 6. Nanofiber scaffolds promote self-organization and branching of dissociated embryonic salivary gland cells.
(A) Brightfield images of spontaneously re-aggregated cell pellets from dissociated E13 mouse SMGs cultured ex vivo for 48 hrs on PLGA fibers show extensive bud outgrowth (arrowheads) and ductal elongation, similar to those cultured on a polycarbonate membrane, scale bars = 100 μm. The morphology of re-aggregated structures on microfibers is outlined by dashed lines to compensate for the interference with light microscopy. (B) Confocal images through the equatorial section of re-aggregated buds at 48 hrs stained for talin (green, top panels) or vinculin (cyan, bottom panels) and co-stained for nuclei (DAPI, blue) show diffuse cortical expression with stronger staining along the basal cell membranes at the bud periphery (arrowheads), similar to that observed in intact glands, scale bars = 50 μm.
4. Discussion
There is no current viable strategy for engineering a functional artificial salivary gland. While several studies have demonstrated that salivary gland cells can self-organize in matrigel [41–43], the resulting tissue constructs were closed-end tubes and spheres from which saliva could not be harvested. Another strategy would be to use a scaffold to direct cellular responses. We here report that a scaffold composed of nanofibers can stimulate cell self-organization in adult cells in a way that cell and tissue shape may be dictated by the scaffold.
In this study, we determined that the cells organize FA complexes differently on nanofiber scaffolds than cells cultured on flat or even microfiber substrates. Since FA complexes are well established as the major adhesive and signal transducing components between the internal actin cytoskeleton and the external ECM scaffold, they can sense the properties of such scaffolds. [34]. Cells on nanofiber scaffolds showed greatly reduced FA aggregate formation and decreased protein levels of activated FAK, paxillin, talin, and vinculin, which was similar to native adult SMG tissues, as reported here, and to cells cultured typically in 3D systems [44, 45]. Abundant expression of mature FA components such as talin, vinculin, and activated p-FAK and p-paxillin was detected in both acinar and ductal salivary epithelial cells cultured on glass or PLGA film surfaces and at cell protrusions on microfiber surfaces, which does not mimic the desired in vivo state and are likely artifacts of culture on these stiff 2D surfaces. The fact that the cells do not form similar focal adhesion complexes when cultured on the nanofiber scaffolds demonstrates that the cells take on a phenotype more similar to the cells in acinar structures in vivo when grown on this 3D scaffold. The associated cell clustering effect regulated by the nanofibers is a further benefit since epithelial cells, including morphogenetically active embryonic cells, function as connected sheets or clusters to effect tissue remodeling [46, 47]. Although FAK contains no nuclear localization sequence, it has been suggested to translocate to the nucleus by protein–protein interactions once activated and possibly interact with other nuclear proteins to regulate cell growth and morphology [48, 49]. Less nuclear p-FAK was observed in cells cultured on nanofibers than on microfibers or on flat surfaces. Since nuclear p-FAK was not detected in either embryonic or adult tissue, the absence of nuclear p-FAK in cells cultured on nanofiber scaffolds is likely the desired state and FAK localization may contribute to cellular organization and morphology. In contrast to epithelial cells, the highly condensed mesenchymal cells closely surrounding the acini in adult tissues showed higher levels of talin and vinculin, consistent with previously reported strong expression of FA proteins in fibroblasts [50]. Thus the reduced levels and diffuse localization of the FA proteins in epithelial cells cultured on nanofibers most closely resembled the pattern observed in adult epithelial tissues. Whether there are correlations between FA expression, activation and localization and acinar or ductal epithelial cell differentiation of cells on nanofibers, remains to be determined.
Our results indicate that nanofiber topography itself might prevent aggregation and maturation of FAs and also direct cell morphology and clustering. Since the elasticity of cellular matrices and scaffolds has been shown to strongly influence cell morphology and focal adhesions [29], we measured the stiffness of the scaffolds. Despite the significant technical challenges and limitations involved in mechanical testing of ultrafine nano- or microscale fibers [51], we were able to obtain representative elastic moduli for PLGA fiber scaffolds, which ranged from 11.0 ± 3.3 MPa for nanofibers to 0.3 ± 0.03MPa for microfibers. Since these values were well outside the range of stiffnesses that cells are typically exposed to in vivo, usually within the kPa range [52], and nanofibers were more stiff, or less elastic than microfibers, it is unlikely that scaffold stiffness can account for the differences in FA adhesion assembly detected on nano- and microfibers. Additionally, little difference was observed in wettability of the scaffolds. We conclude that scaffold topography, more so than mechanical or chemical properties, influences FA formation and acquisition of cell morphology in salivary gland cells. Scaffolds composed of materials that better reflect the stiffness encountered by cells in vivo may provide an additional benefit for regulation of cell behaviors, such as differentiation.
Interestingly, dissociated primary embryonic salivary gland cells grown on PLGA fiber scaffolds are capable of spontaneously self-organizing and re-aggregating into branched glandular structures resembling intact salivary glands that contain similar FA staining patterns. That the properties of self-organization and reassembly of primary glandular tissue are preserved by PLGA fiber scaffolds, is a significant advantage for engineering future 3D artificial tissue constructs using such ECM mimetic scaffolds. Taken together, the cellular phenotypes of increased cellular self-organization and clustering of embryonic cells and reduced FA formation observed in adult cells on the nanofiber surfaces are preferred traits to mimic the in vivo states of these tissues. In future work, it may be possible to design scaffolds that can direct organization and differentiation of stem or progenitor cell populations.
5. Conclusions
In this study, we identified nanofiber scaffolds composed of the nontoxic, bioabsorbable polymer, PLGA, as a material that directs assembly of salivary gland epithelial cells in vitro. Our results show that compared to cells cultured on planar PLGA film, glass, or on PLGA microfiber surfaces, cells cultured on the nanofiber scaffolds assembled the fewest FA complexes. Cells cultured on nanofibers significantly demonstrated decreased levels of the FA proteins, phosphorylated FAK and paxillin, and total levels of the FA proteins, talin and vinculin, which were abundant on planar surfaces. Our data show that nanofiber scaffolds are capable of promoting the most in vivo-like cell morphology, characterized by increased cell rounding and clustering and fewer cell protrusions in both acinar and ductal salivary gland epithelial cell types, in contrast to flat or microfiber surfaces. Further, PLGA fiber scaffolds were capable of supporting the growth and branching morphogenesis of intact embryonic submandibular salivary gland organ cultures and could also promote spontaneous self-organization of dissociated primary gland cells into branched gland-like structures.
Supplementary Material
Acknowledgements
The authors would like to thank Drs. Kenneth Yamada and Jeffrey Miner for antibodies, Drs. David Quissell and Mary Reyland for Par-C10 cells, and Daniel Malamud for SIMS cells. This work was supported by NIH grants R21 DE019197, R01 DE019244 and RC1 DE020402 to M.L, C06 RR015464, a postdoctoral Ruth L. Kirschstein National Research Service Award (F32DE020980) to S.S, an NSF-MRI DBI0922830 (J.C.) and C06 RR015464 (to the University at Albany, SUNY).
References
- [1].Ship JA. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002;8(2):77–89. doi: 10.1034/j.1601-0825.2002.2o837.x. [DOI] [PubMed] [Google Scholar]
- [2].Wang S, Cukierman E, Swaim WD, Yamada KM, Baum BJ. Extracellular matrix protein-induced changes in human salivary epithelial cell organization and proliferation on a model biological substratum. Biomaterials. 1999;20(11):1043–9. doi: 10.1016/s0142-9612(98)00255-5. [DOI] [PubMed] [Google Scholar]
- [3].LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med. 2007;232(9):1121–9. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- [4].Kadoya Y, Yamashina S. Ultrastructure of the basement membrane and its precursor in developing rat submandibular gland as shown by alcian blue staining. Cell Tissue Res. 1992;268(2):233–8. doi: 10.1007/BF00318791. [DOI] [PubMed] [Google Scholar]
- [5].Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20(6):573–88. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- [6].Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singer II. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979;16(3):675–85. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- [8].Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, et al. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J. 2007;92(6):2212–22. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998;122(1–2):119–22. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- [10].Neal RA, McClugage SG, Link MC, Sefcik LS, Ogle RC, Botchwey EA. Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng Part C Methods. 2009;15(1):11–21. doi: 10.1089/ten.tec.2007.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahmed I, Ponery AS, Nur EKA, Kamal J, Meshel AS, Sheetz MP, et al. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem. 2007;301(1–2):241–9. doi: 10.1007/s11010-007-9417-6. [DOI] [PubMed] [Google Scholar]
- [12].Jean-Gilles R, Soscia D, Sequeira SJ, Melfi M, Gadre A, Castracane J, et al. Novel modeling approach to generate polymeric nanofiber scaffolds for salivary gland cells. J Nanotechnol Eng Med. 2010;1(3):31008. doi: 10.1115/1.4001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, et al. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85(3–4):165–73. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [14].Laoide BM, Courty Y, Gastinne I, Thibaut C, Kellermann O, Rougeon F. Immortalised mouse submandibular epithelial cell lines retain polarised structural and functional properties. J Cell Sci. 1996;109(Pt 12):2789–800. doi: 10.1242/jcs.109.12.2789. [DOI] [PubMed] [Google Scholar]
- [15].Laoide BM, Gastinne I, Rougeon F. Tubular morphogenesis and mesenchymal interactions affect renin expression and secretion in SIMS mouse submandibular cells. Exp Cell Res. 1999;248(1):172–85. doi: 10.1006/excr.1999.4404. [DOI] [PubMed] [Google Scholar]
- [16].Turner JT, Redman RS, Camden JM, Landon LA, Quissell DO. A rat parotid gland cell line, Par-C10, exhibits neurotransmitter-regulated transepithelial anion secretion. Am J Physiol. 1998;275(2 Pt 1):C367–74. doi: 10.1152/ajpcell.1998.275.2.C367. [DOI] [PubMed] [Google Scholar]
- [17].Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol. 2008;45(1):65–75. doi: 10.1016/j.molimm.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336(2):169–82. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bilgin CC, Bullough P, Plopper GE, Yener B. ECM-Aware Cell-Graph Mining for Bone Tissue Modeling and Classification. Data Min Knowl Discov. 2009;20(3):416–38. doi: 10.1007/s10618-009-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lund AW, Bilgin CC, Hasan MA, McKeen LM, Stegemann JP, Yener B, et al. Quantification of spatial parameters in 3D cellular constructs using graph theory. J Biomed Biotechnol. 2009;2009:928286. doi: 10.1155/2009/928286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Systems, Man, and Cybernetics. 1979;9(1):62–6. [Google Scholar]
- [22].Gunduz C, Yener B, Gultekin SH. The cell graphs of cancer. Bioinformatics. 2004;20(Suppl 1):i145–51. doi: 10.1093/bioinformatics/bth933. [DOI] [PubMed] [Google Scholar]
- [23].Larsen M, Yamada KM, Musselmann K. Systems analysis of salivary gland development and disease. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):670–82. doi: 10.1002/wsbm.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74(7):349–64. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- [25].Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129(2):521–34. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakanishi Y, Nogawa H, Hashimoto Y, Kishi J, Hayakawa T. Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development. 1988;104(1):51–9. doi: 10.1242/dev.104.1.51. [DOI] [PubMed] [Google Scholar]
- [27].Bernfield MR, Banerjee SD, Cohn RH. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J Cell Biol. 1972;52(3):674–89. doi: 10.1083/jcb.52.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- [29].Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- [30].Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- [31].Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302(5642):103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- [32].Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179(5):1043–57. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11(11):802–14. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- [34].Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev Dyn. 2011;240(9):2069–83. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119(Pt 16):3376–84. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- [36].Larsen HS, Aure MH, Peters SB, Larsen M, Messelt EB, Kanli Galtung H. Localization of AQP5 during development of the mouse submandibular salivary gland. J Mol Histol. 2011;42(1):71–81. doi: 10.1007/s10735-010-9308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011;197(3):532–6. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- [38].Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- [39].Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, et al. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174(2):277–88. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci. 1997;94(3):849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13(4):721–35. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]
- [42].Chen MH, Chen YJ, Liao CC, Chan YH, Lin CY, Chen RS, et al. Formation of salivary acinar cell spheroids in vitro above a polyvinyl alcohol-coated surface. J Biomed Mater Res A. 2009;90(4):1066–72. doi: 10.1002/jbm.a.32167. [DOI] [PubMed] [Google Scholar]
- [43].Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75(4):318–24. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- [44].Damianova R, Stefanova N, Cukierman E, Momchilova A, Pankov R. Three-dimensional matrix induces sustained activation of ERK1/2 via Src/Ras/Raf signaling pathway. Cell Biol Int. 2008;32(2):229–34. doi: 10.1016/j.cellbi.2007.08.029. [DOI] [PubMed] [Google Scholar]
- [45].Hamamura K, Jiang C, Yokota H. ECM-dependent mRNA expression profiles and phosphorylation patterns of p130Cas, FAK, ERK and p38 MAPK of osteoblast-like cells. Cell Biol Int. 2010;34(10):1005–12. doi: 10.1042/CBI20100069. [DOI] [PubMed] [Google Scholar]
- [46].Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- [47].Friedl P, Zallen JA. Dynamics of cell-cell and cell-matrix interactions in morphogenesis, regeneration and cancer. Curr Opin Cell Biol. 2010;22(5):557–9. doi: 10.1016/j.ceb.2010.08.024. [DOI] [PubMed] [Google Scholar]
- [48].Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. Embo J. 2000;19(12):2911–23. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yi XP, Wang X, Gerdes AM, Li F. Subcellular redistribution of focal adhesion kinase and its related nonkinase in hypertrophic myocardium. Hypertension. 2003;41(6):1317–23. doi: 10.1161/01.HYP.0000072772.74183.5F. [DOI] [PubMed] [Google Scholar]
- [50].Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- [51].Tan EP, Ng SY, Lim CT. Tensile testing of a single ultrafine polymeric fiber. Biomaterials. 2005;26(13):1453–6. doi: 10.1016/j.biomaterials.2004.05.021. [DOI] [PubMed] [Google Scholar]
- [52].Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124(Pt 1):9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.