Abstract
Background Although generic anti-retroviral drugs are in common use throughout the developing world, studies comparing their clinical effectiveness with that of proprietary formulations are lacking.
Methods We analysed observational data from a large cohort of adults on anti-retroviral therapy (ART) to assess potential differences between generic and proprietary zidovudine (ZDV) formulations in post-90-day mortality, ‘programme failure’ (a composite of death, follow-up losses and withdrawals) and other clinical outcomes. We accounted for drug exposure in three ways: an ‘initial dispensation’ approach that categorized patients according to the first prescription; ‘time-varying’ approach that attributed an outcome to the formulation taken at the time of event; and ‘predominant exposure’ approach that considered only those with >75% exposure to either brand or generic ZDV. Proprietary formulations were used as the reference group in all adjusted Cox proportional hazard regressions.
Results Among 14 736 patients eligible for analysis, 7277 (49%) initiated a generic formulation of ZDV and 7459 (51%) initiated a proprietary formulation. When categorized according to initial dispensation, no difference in post-90-day mortality was observed between the two groups [adjusted hazard ratio (AHR): 0.93, 95% confidence interval (CI): 0.77–1.12]. Similar findings were noted when drug formulation was treated as a time-varying exposure (AHR: 1.15, 95% CI: 0.89–1.48) when analysis was limited to those with a predominant exposure to one formulation or the other (AHR: 0.59, 95% CI: 0.24–1.49). Results were consistent across all approaches when programme failure was considered as an outcome. No longitudinal differences were detected between formulations for CD4 response, weight change and haemoglobin concentration. Generic ZDV formulations were associated with slight decreases in single-drug substitution.
Conclusions In this large programmatic cohort of adults starting ZDV-based first-line therapy, clinical outcomes appeared similar among patients on generic or proprietary formulations. These findings support continued use of generic anti-retroviral drug formulations in resource-constrained settings.
Keywords: HIV, AIDS, anti-retroviral therapy, ART, scale-up, PEPFAR, Emergency Plan, sub-Saharan Africa, generic, proprietary, anti-retroviral drugs
Introduction
The worldwide majority of patients receiving anti-retroviral therapy (ART) now live in sub-Saharan Africa.1 Among the most important steps in achieving this historic benchmark has been the substantial price reduction in anti-retroviral drugs. Once deemed too expensive for widespread use in poor nations, anti-retroviral drugs have become increasingly affordable in recent years, owing to both the price negotiations with proprietary manufacturers and the emergence of a wide variety of new generic formulations.2–6
The World Health Organization (WHO) recommends a limited formulary of triple-drug anti-retroviral regimens as first-line therapy for treatment of adults and adolescents in most developing world settings.7 The WHO also recommends that all anti-retroviral drugs undergo continuous quality assessment, and has established a ‘prequalification programme’ to assist host governments in their drug procurement.8 The US government, which is the largest single donor to the global AIDS mitigation effort,9 does not recognize the WHO pre-qualification process and instead requires approval by the US Food and Drug Administration (FDA) for any anti-retroviral drugs purchased with American funds.10 To date, 133 generic formulations have been approved in this manner, dating back to December 2004.11 Despite these rigorous approval processes, questions persist regarding the ongoing quality and bioequivalence of many generic formulations,12 particularly since continuing ‘post-approval’ evaluations have proven difficult to enforce.
The Zambian government’s ART programme in the Lusaka Urban District is a well-documented cohort with large patient numbers, careful prospective data collection and commingling of both proprietary and generic formulations.13,14 We sought to compare the field effectiveness of proprietary and generic first-line ART regimens in a field setting. To ensure maximum comparability (see rationale below), we limited our analysis to patients initiating a ZDV-containing regimen.
Methods
Clinical care
Details of the clinical care procedures followed in the Lusaka Urban District have been described in detail elsewhere.13–16 We emphasize here a few aspects that require further explication for the current report. During the study period, ART eligibility was determined according to the Zambian national guidelines, which closely followed recommendations by the WHO: CD4+ cell count <200/µl and WHO clinical stage 4, or CD4+ cell count <350/µl and WHO clinical stage 3.17 First-line anti-retroviral prescriptions comprised three drugs. These were either ZDV or stavudine (d4T), plus lamivudine (3TC) plus either nevirapine (NVP) or efavirenz (EFV). We started ZDV- or d4T-based regimens based upon drug availability, but typically did not initiate ZDV in patients whose haemoglobin was <10 g/dl.
The initial follow-up schedule for those starting ART included six visits during the first 3 months, with special focus on adherence and detecting adverse events. For individuals receiving a ZDV-containing regimen, haemoglobin levels were monitored at post-initiation weeks 2, 4 and 8. CD4+ cell counts were performed every 6 months. In our setting, virological monitoring is not routinely used; however, viral load testing is available to clinicians when discordant immunological and clinical evaluations require adjudication.18 Contact tracing by community health workers is scheduled for all ART patients when they remain delinquent for clinical appointments after 10 days.19 Patient death was ascertained by reports from clinical facilities, home-based care organizations and follow-up visits by community health workers.
Anti-retroviral drugs were dispensed monthly. In each of the 18 Lusaka facilities, clinical and pharmacy encounters occurred in physically separate areas. Clinicians wrote prescriptions, but did not indicate whether they were to be filled with generic or proprietary drugs. Prescriptions were filled on-site by pharmacy technicians. Efforts were made to continue a given patient on the same regimen and formulation at each subsequent visit. However, switching between proprietary and generic formulations was common, and depended upon what agents were available in the pharmacy when the patient presented. Programme data were collected on standardized care forms and transcribed on-site into an electronic medical record and patient tracking system adopted by the Zambian Ministry of Health.20
Analysis cohort and comparison groups
We included all ART-naïve adult patients (>16 years of age) initiating care at public sector facilities in the Lusaka Urban District. As already noted, patients switched commonly between generic and proprietary formulations for pharmacy stock (and not clinical) reasons. Similar amounts of proprietary and generic ZDV were available through most of the evaluation period, although generic dispensation predominated towards the end of our observation period. This facilitates what we believe to be a valid comparison of ZDV-related outcomes among patients prescribed proprietary versus generic formulations. In contrast, there was much less proprietary d4T available (<10% of all d4T prescriptions) and the dispensation of proprietary d4T was not random, as it was not available in a proprietary fixed-dose combination. For this reason, we have limited this analysis to patients initiating a ZDV-containing regimen. We included in this analysis adults initiating ART between programme inception and 1 July 2007, when the Zambian Ministry of Health changed recommended first-line ART to tenofovir-containing regimens.21 Observation continued until the data freeze date of 30 November 2010.
Outcomes and analytical methods
Although early mortality (i.e. deaths within 90 days of starting therapy) is high in our setting,13,14 the individual components of a drug regimen are unlikely to be related to early death. For this reason, we focused on post-90-day mortality as our primary outcome measure. Consistent with previous work,21,22 we also examined the association between ZDV formulation (i.e. proprietary or generic) and ‘programme failure’, a composite outcome that considered death, follow-up losses and formal withdrawals from the ART clinic. Patients receiving ART who were >60 days late for a pharmacy or clinical appointment (and who were not known to have died or formally withdrawn from the programme) were considered lost to follow-up.23
As with past analyses,22 we considered three analytical approaches. In the ‘initial dispensation analysis’, a given patient’s drug exposure was determined by his or her second month’s dispensation. (We excluded from the analysis any patient who contributed 30 person-days because prescriptions made during the first month of therapy, when the NVP dose is being escalated,24 typically commingle proprietary and generic formulations in the same month, making classification impossible.) Under the initial dispensation assumption, a patient who received an initial generic dispensation would be categorized as exposed exclusively to generic drug, even if he or she switched to a proprietary formulation at some point during therapy. In the ‘time-varying exposure’ analysis, a given patient’s drug exposure(s) are allowed to vary over time in the Cox model. Thus, an individual patient who switches formulations may contribute person-time to both categories of exposure in the proportional hazards regression. Finally, in an attempt to further understand the effects of switching between formulations, we characterized patients’ ‘predominant drug exposure’ during their first year on therapy as mostly (i.e. ≥75% of dispensations) generic or proprietary, and then examined our outcomes of interest in the 365 days that followed. Those who did not meet this dispensation threshold—i.e. those with 75% of either generic or proprietary ZDV formulations—were excluded from this exploratory analysis, as were individuals contributing <12 months of follow-up time.
Over the observation period, anti-retroviral drugs were purchased from manufacturers by the Zambian government and donors such as the US President’s Emergency Plan for AIDS Relief (PEPFAR). Although we could reliably classify formulations as generic or proprietary, we were unable to further categorize regimens by their different manufacturer or, for generic formulations, their qualification status from WHO or FDA. Regardless of the manufacturer, ZDV was most commonly dispensed as a two-drug co-formulation with 3TC. Occasionally patients received generic fixed-dose combinations of ZDV + 3TC + NVP, but this was far less frequent. ZDV was rarely given as a single pill in combination with ART. We categorized regimens based only on the ZDV formulation, even though the dispensation of other regimen components (e.g. NVP and EFV) could vary between proprietary and generic formulations as well.
When assessing baseline characteristics among analysis groups, we compared continuous variables with a Wilcoxon rank sum test and compared dichotomous and categorical variables with the Pearson χ2-test statistic. In the initial dispensation analysis and the predominant drug exposure analysis, we fitted Kaplan–Meier curves to examine survival functions and used the log-rank test to examine statistical difference among groups. For all three analytical approaches, we estimated hazard ratios (HRs) for mortality using Cox proportional hazards regression and tested the proportional hazards assumption for potential interaction between each variable and time in a given model using the likelihood ratio test. We adjusted for potential confounders shown to be associated with our outcomes of interest in the previous work.13 Many of these characteristics were routinely collected at baseline (i.e. within 1 month of ART initiation). We assessed adherence using the medication possession ratio (MPR), a metric that has been linked to patient survival, immunological recovery and virological outcomes in our setting.18,25 MPR is calculated by dividing the cumulative number of days a patient is late for pharmacy visits by the total number of days on therapy, and then subtracting this percentage from 100%. In the initial dispensation (first 90 days) and the predominant exposure (first 365 days) analyses, MPR was measured over an initial window period. Adherence was not considered in the time-varying analysis. We performed all reported proportional hazard regressions with a stratification by calendar year—to account for the possibility that care delivery may have improved (or declined) over time. Patients switching to a d4T-containing regimen and/or to a protease inhibitor-based regimen were censored at the time of regimen modification. When drug information was missing for a specific dispensation, it was carried over from the last patient interaction.
We studied other clinical outcomes according to an individual’s initial dispensation. As a surrogate for ZDV toxicity, we calculated the rate of single-drug substitution—from ZDV to d4T—for patients allocated to the proprietary and generic categories, and described these trends using adjusted time-to-event analyses. The prevalence of clinically significant anaemia in our setting (i.e. haemoglobin <8.0 g/dl) at 90 days was also measured among patients initiating proprietary and generic ZDV formulations. Finally, we compared the median longitudinal change in CD4+ cell count, haemoglobin levels and weight for the two comparison arms. We used SAS version 9.1.3 (SAS Institute, Cary, NC, USA) for all analyses. This reporting of programmatic data was approved by the Institutional Review Boards of the University of Zambia (Lusaka, Zambia) and the University of Alabama at Birmingham (Birmingham, AL, USA).
Results
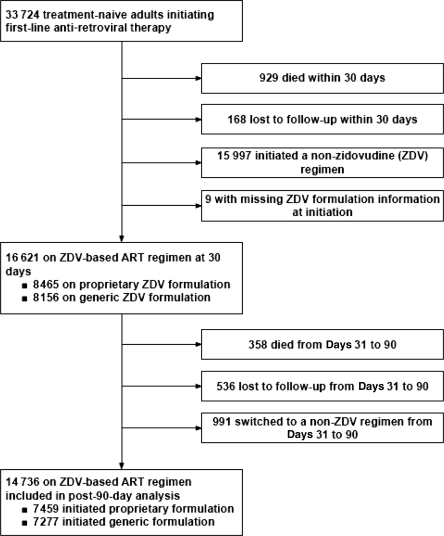
Between 26 April 2004 and 1 July 2007, we enrolled 33 724 HIV-1 seropositive treatment-naïve adults (>16 years of age) for entry into the Lusaka District HIV Care and Treatment Program (Figure 1). Of these, 929 died and 168 were lost to follow-up prior to 30 days on treatment (the point at which we were able to reliably characterize the initial drug exposure as either generic or proprietary). An additional 15 997 patients initiated a non-ZDV-containing regimen and 9 initiated ZDV, but available data did not allow classification as generic or proprietary drug exposure. The remaining 16 621 patients achieved at least 30 days of follow-up on a ZDV-containing first-line regimen. Between 31 and 90 days on therapy, an additional 358 patients died, 536 were lost to follow-up and 991 switched to a non-ZDV-containing regimen. Thus, 14 736 patients were available for the post-90-day (primary) analysis, contributing a median of 1373 days [interquartile range (IQR): 592–1798] of observation time (Figure 1). Demographic and medical characteristics, categorized according to a patient’s initial ZDV formulation, are shown in Table 1.
Figure 1.
Cohort profile of patients initiating ART between May 2004 and July 2007 (Lusaka, Zambia)
Table 1.
Baseline characteristics of adults initiating generic or proprietary ZDV-containing anti-retroviral regimens between May 2004 and July 2007 (Lusaka, Zambia)
| Patients initiating a proprietary formulation (N = 7459) |
Patients initiating a generic formulation (N = 7277) |
||||
|---|---|---|---|---|---|
| N | Value | N | Value | P value | |
| Age | 7459 | 35 (30, 41) | 7277 | 35 (30, 41) | 0.29 |
| 16–29 years | 1749 | 23.4% | 1681 | 23.1% | 0.96 |
| 30–34 years | 1866 | 25.0% | 1819 | 25.0% | |
| 35–39 years | 1573 | 21.1% | 1545 | 21.2% | |
| ≥40 years | 2271 | 30.4% | 2232 | 30.7% | |
| Male | 3267 | 43.8% | 3265 | 44.9% | 0.19 |
| BMI (kg/m2) | 6536 | 20.5 (18.7, 22.8) | 6576 | 20.3 (18.4, 22.6) | <0.01 |
| ≥16 | 6239 | 95.5% | 6267 | 95.3% | 0.67 |
| <16 | 297 | 4.5% | 309 | 4.7% | |
| Baseline CD4+ cell count (cells/mm3) | 7279 | 139 (78, 202) | 7050 | 139 (76, 200) | 0.46 |
| ≥200 | 1875 | 25.8% | 1782 | 25.3% | 0.32 |
| 50–199 | 4357 | 59.9% | 4192 | 59.5% | |
| <50 | 1047 | 14.4% | 1076 | 15.3% | |
| Baseline haemoglobin concentration | 6494 | 11.8 (10.8, 13.0) | 6433 | 11.8 (10.8, 12.9) | 0.32 |
| ≥10.0 | 5289 | 91.6% | 5180 | 90.2% | 0.03 |
| 8.0–9.9 | 441 | 7.6% | 498 | 8.7% | |
| <8.0 | 46 | 0.8% | 62 | 1.1% | |
| Active tuberculosis at enrolment | 988 | 13.2% | 942 | 12.9% | |
| WHO clinical stage | |||||
| Stage I or II | 2692 | 36.3% | 2519 | 34.7% | 0.11 |
| Stage III | 4136 | 55.7% | 4150 | 57.2% | |
| Stage IV | 597 | 8.0% | 589 | 8.1% | |
| Adherence over first 90 days (%) | 7459 | 100 (92, 100) | 7277 | 100 (92, 100) | 0.50 |
| 95–100 | 974 | 13.1% | 936 | 12.9% | 0.96 |
| 80–94 | 1536 | 20.6% | 1468 | 20.2% | |
| <80 | 984 | 13.2% | 957 | 13.2% | |
| Observation time, days, median (IQR) | 7459 | 1519 (798, 1889) | 7277 | 1329 (758, 1791) | <0.01 |
| Number of switches between formulations, median (IQR) | 7459 | 3 (1, 5) | 7277 | 2 (0, 5) | <0.01 |
| Time to first switch, days, median (IQR) | 6327 | 122 (73, 207) | 4833 | 88 (58, 143) | <0.01 |
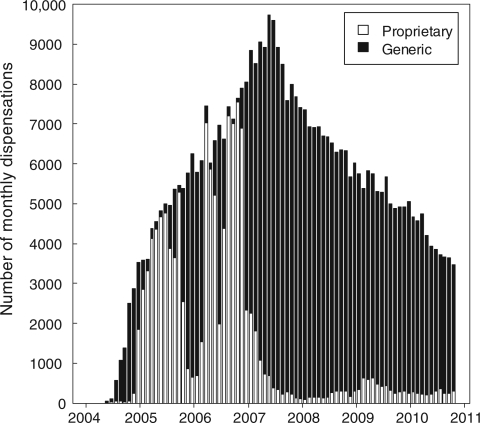
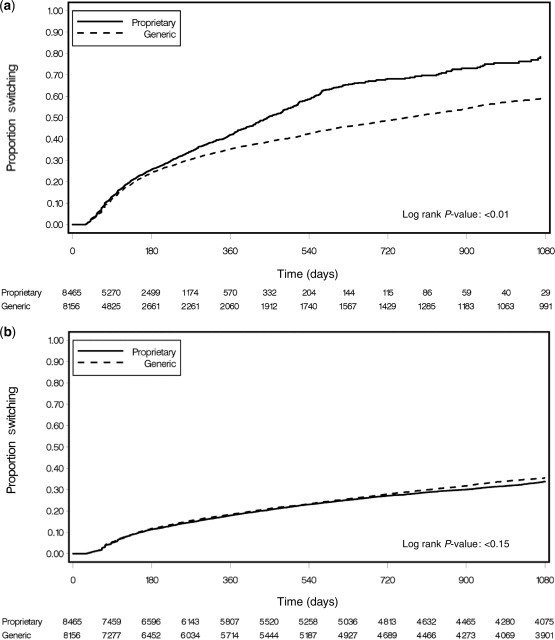
Among 14 736 patients in the analysis dataset, 13 642 (93%) started a regimen of ZDV + 3TC + NVP and 1094 (7%) started a regimen of ZDV + 3TC + EFV. A total of 7459 (51%) patients initiated a proprietary ZDV formulation (median follow-up: 1519 days; IQR: 798–1889), whereas 7277 (49%) initiated a generic formulation (median follow-up: 1329 days; IQR: 758–1791). Patients were prescribed a total of 119 295 monthly prescriptions for proprietary ZDV and 321 349 prescriptions for generic ZDV (Figure 2). The majority of patients (11 160, 76%) were switched from a proprietary formulation to a generic formulation or vice versa by their pharmacist; 6327 switched from brand to generic (median time to first switch: 122 days, IQR: 73–207), whereas 4833 switched from generic to brand (median time to first switch: 88 days, IQR: 58–143). A Kaplan–Meier analysis showing time to first switch is shown in Figure 3a.
Figure 2.
Dispensation of generic and proprietary formulations of ZDV from May 2004 to November 2010 (Lusaka, Zambia)
Figure 3.
Kaplan–Meier analysis showing time to first switch between proprietary and generic formulations (a) and time to single-drug substitution from ZDV to other anti-retroviral agent, a surrogate marker for drug toxicity (b) among patients enrolled from May 2004 to July 2007 (Lusaka, Zambia)
Survival
When we considered those who remained active and in care for at least 90 days, 983 patients died over 49 136 patient-years of follow-up, a rate of 2.0 per 100 patient-years [95% confidence interval (CI): 1.9–2.1]. The hazard of death associated with generic formulations did not appear different from that of proprietary ZDV across the varying statistical approaches: initial dispensation (AHR: 0.93, 95% CI: 0.77–1.12), time-varying (AHR: 1.15, 95% CI: 0.89–1.48) or predominant exposure (AHR: 0.59, 95% CI: 0.24–1.49). The baseline CD4+ count and the WHO stage were consistently associated with the mortality outcome, whereas the association between death and other covariates [e.g. sex, body mass index (BMI) and adherence] varied with approach (Table 2).
Table 2.
Factors associated with post-90-day mortality among adults initiating generic or proprietary ZDV-containing anti-retroviral regimens between May 2004 and July 2007 (Lusaka, Zambia)
| Initial dispensation | Time-varying | Predominant exposure | |
|---|---|---|---|
| Adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
| Drug formulation dispensed | |||
| Proprietary | 1.0 | 1.0 | 1.0 |
| Generic | 0.93 (0.77–1.12) | 1.15 (0.89–1.48) | 0.59 (0.24–1.49) |
| Age (years) | |||
| 16–29 | 1.0 | 1.0 | 1.0 |
| 30–34 | 0.89 (0.69–1.13) | 0.95 (0.71–1.27) | 0.76 (0.43–1.35) |
| 35–39 | 1.07 (0.84–1.37) | 1.13 (0.85–1.51) | 1.29 (0.76–2.19) |
| ≥40 | 1.22 (0.97–1.53) | 1.34 (1.02–1.74) | 1.47 (0.90–2.41) |
| Sex | |||
| Female | 1.0 | 1.0 | 1.0 |
| Male | 1.46 (1.24–1.72) | 1.38 (1.14–1.68) | 0.89 (0.62–1.28) |
| BMI (kg/m2) | |||
| ≥16 | 1.0 | 1.0 | 1.0 |
| <16 | 2.19 (1.67–2.86) | 2.22 (1.64–3.01) | 1.01 (0.44–2.32) |
| Baseline CD4+ cell count (cells/mm3) | |||
| ≥200 | 1.0 | 1.0 | 1.0 |
| 50–199 | 1.15 (0.93–1.42) | 1.22 (0.96–1.56) | 1.20 (0.77–1.87) |
| <50 | 1.62 (1.27–2.07) | 1.70 (1.28–2.25) | 1.76 (1.03–3.01) |
| Baseline haemoglobin concentration | |||
| ≥10.0 | 1.0 | 1.0 | 1.0 |
| 8.0–9.9 | 1.46 (1.13–1.90) | 1.66 (1.25–2.21) | 1.23 (0.68–2.21) |
| <8.0 | 1.35 (0.63–2.92) | 0.97 (0.31–3.02) | 2.00 (0.49–8.07) |
| Tuberculosis at enrolment | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 0.97 (0.78–1.22) | 1.13 (0.89–1.44) | 0.86 (0.52–1.44) |
| WHO clinical stage | |||
| Stage I or II | 1.0 | 1.0 | 1.0 |
| Stage III | 1.48 (1.21–1.81) | 1.58 (1.24–2.01) | 1.61 (1.05–2.46) |
| Stage IV | 2.43 (1.84–3.23) | 2.73 (1.97–3.79) | 2.69 (1.48–4.88) |
| Adherence (%) | |||
| 95–100 | 1.0 | N/A | 1.0 |
| 80–94 | 0.70 (0.56–0.87) | N/A | 1.05 (0.70–1.59) |
| <80 | 1.42 (1.14–1.76) | N/A | 2.43 (1.48–3.98) |
Programme failure
When we considered post-90-day programme failure as our outcome, 6995 patients met the definition for this analysis endpoint over the observation period (rate: 13.3 per 100 patient-years, 95% CI: 13.0–13.6). We were unable to detect any differences between generic and proprietary ZDV across our three statistical approaches: initial dispensation (AHR: 1.00, 95% CI: 0.93–1.07), time-varying (AHR: 0.91, 95% CI: 0.82–1.00) or predominant exposure (AHR: 0.85, 95% CI: 0.59–1.23). The association between programme failure and other covariates of interest resembled that of our mortality analyses (Table 3).
Table 3.
Factors associated with programme failure among adults initiating generic or proprietary ZDV-containing anti-retroviral regimens between May 2004 and July 2007 (Lusaka, Zambia)
| Initial dispensation | Time-varying | Predominant exposure | |
|---|---|---|---|
| Adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
| Drug formulation dispensed | |||
| Proprietary | 1.0 | 1.0 | 1.0 |
| Generic | 1.00 (0.93–1.07) | 0.91 (0.82–1.00) | 0.85 (0.59–1.23) |
| Age (years) | |||
| 16–29 | 1.0 | 1.0 | 1.0 |
| 30–34 | 0.78 (0.72–0.84) | 0.77 (0.70–0.84) | 0.64 (0.53–0.78) |
| 35–39 | 0.69 (0.63–0.75) | 0.69 (0.63–0.76) | 0.71 (0.58–0.86) |
| ≥40 | 0.66 (0.61–0.71) | 0.64 (0.59–0.70) | 0.69 (0.58–0.83) |
| Sex | |||
| Female | 1.0 | 1.0 | 1.0 |
| Male | 1.31 (1.24–1.40) | 1.29 (1.20–1.38) | 1.22 (1.06–1.41) |
| BMI (kg/m2) | |||
| ≥16 | 1.0 | 1.0 | 1.0 |
| <16 | 1.47 (1.30–1.67) | 1.49 (1.29–1.71) | 1.18 (0.86–1.63) |
| Baseline CD4+ cell count (cells/mm3) | |||
| ≥200 | 1.0 | 1.0 | 1.0 |
| 50–199 | 0.99 (0.92–1.06) | 0.96 (0.89–1.04) | 0.88 (0.75–1.03) |
| <50 | 1.01 (0.92–1.11) | 0.96 (0.86–1.07) | 0.91 (0.72–1.14) |
| Baseline haemoglobin concentration | |||
| ≥10.0 | 1.0 | 1.0 | 1.0 |
| 8.0–9.9 | 1.22 (1.10–1.36) | 1.30 (1.15–1.47) | 1.20 (0.93–1.55) |
| <8.0 | 1.40 (1.01–1.93) | 1.58 (1.11–2.23) | 1.64 (0.89–3.03) |
| Tuberculosis at enrolment | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 0.90 (0.82–0.99) | 0.92 (0.83–1.01) | 1.01 (0.82–1.24) |
| WHO clinical stage | |||
| Stage I or II | 1.0 | 1.0 | 1.0 |
| Stage III | 1.14 (1.07–1.22) | 1.12 (1.04–1.20) | 1.07 (0.91–1.25) |
| Stage IV | 1.37 (1.23–1.54) | 1.31 (1.16–1.49) | 1.17 (0.90–1.54) |
| Adherence (%) | |||
| 95–100 | 1.0 | N/A | 1.0 |
| 80–94 | 0.95 (0.87–1.02) | N/A | 1.42 (1.21–1.67) |
| <80 | 1.43 (1.31–1.56) | N/A | 2.86 (2.35–3.48) |
Other outcomes
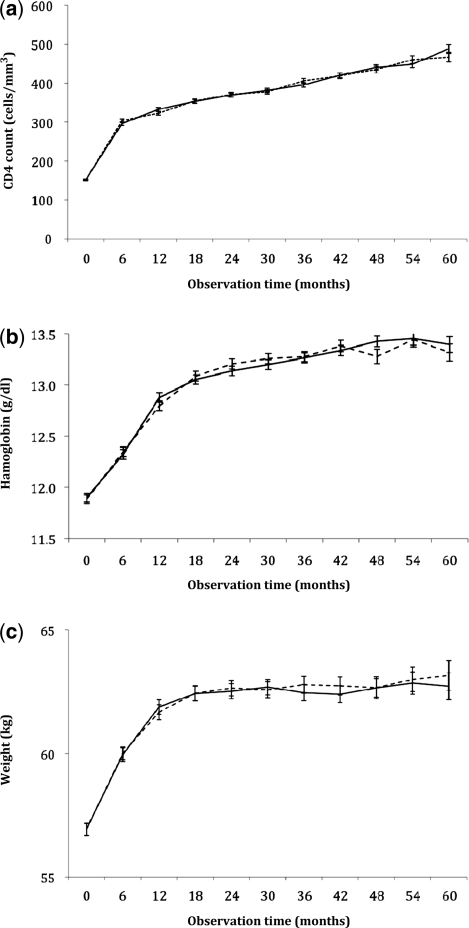
The rate of single-drug substitution, a surrogate marker for ZDV drug toxicity, was 10.5 (95% CI: 10.2–10.8) in our overall cohort (Figure 3b). In the adjusted Cox regression models, generic ZDV had slightly lower hazard of substitution when compared with proprietary formulations (AHR: 0.91, 95% CI: 0.84–0.99). The prevalence of diagnosed severe anaemia at 90 days likewise did not differ. Of the 2937 patients, 107 (4.7%) patients initiating a generic formulation and in whom haemoglobin information was available had at least one haemoglobin measurement <8.0 g/dl compared with 81 of 1938 (4.2%) patients initiating a proprietary formulation (P = 0.41). Patients initiating proprietary and generic formulations had similar rises in their median CD4+ counts, haemoglobin concentrations and weight over the observation period (Figure 4a–c).
Figure 4.
Median CD4+ cell (a), haemoglobin (b) and weight responses (c) over time among adults initiating generic and proprietary formulations of ZDV-based ART from May 2004 to July 2007 (Lusaka, Zambia). The solid lines represent patients initiating a proprietary formulation of ZDV, whereas the dotted lines represent those initiating a generic formulation of ZDV
Discussion
In this large analysis of programmatic data from a public sector ART programme in Lusaka, Zambia, patients who were dispensed generic drugs had similar outcomes to those who were dispensed proprietary drugs. Specifically, we were unable to detect any differences in mortality, programme failure, CD4+ lymphocyte response, haemoglobin response or weight gain that could be attributed to generic drug exposure.
The costs between proprietary and generic drugs continue to differ greatly. The per patient cost for ZDV-3TC co-formulations, an example central to the current analysis, is estimated at $231/year for branded drugs compared with $101–123/year for generic formulations.26 It is therefore not surprising that, as the volume of procured anti-retroviral drugs has increased dramatically worldwide, the proportion of generic formulations purchased has also skyrocketed. In a survey of 16 countries, Holmes et al. found that the proportion of generic drugs purchased through PEPFAR gradually increased from 14.8% in 2005 to 89.3% in 2008. (Programmes in Zambia followed this general trend, with notable increases in generic drug as a proportion of overall procurement: 42.8% in 2005, 56.5% in 2006, 91.3% in 2007 and 93.7% in 2008.) The total cost savings associated with this shift—from proprietary to generic formulations—was estimated at $323 million over the 4-year period.10
The effectiveness of generic anti-retroviral formulations thus remains an important scientific question, one with obvious public health implications. Pharmacological analyses of generic anti-retroviral drugs have generally confirmed bioequivalence when compared with their proprietary counterparts.27–33 In cohort studies, generic formulations have also been associated with consistently favourable treatment responses.34–45 Laurent et al., for example, demonstrated a high rate of virological suppression (80%) and favourable CD4+ treatment responses (median +83 cells/µl) among 60 patients on generic fixed-dose combination drugs at 24 weeks. Although the proportion with virological suppression decreased in the second year of therapy, overall outcomes appeared comparable with other treatment cohorts in the region.35 Idigbe et al.36 demonstrated encouraging virological responses at 24 weeks and immunological responses at 48 weeks among 50 Nigerian adults on ART. Pujari et al.37 reported similar outcomes in a 1291-patient cohort in India, where generic anti-retroviral formulations were used exclusively for treatment. No virological data were collected, but therapy resulted in significant and sustained CD4 response up to 24 months. In a cohort of 6861 patients across 21 Medecins Sans Frontieres sites, Calmy et al.38 reported similarly favourable health responses to fixed-dose combinations from non-proprietary manufacturers. To our knowledge, however, no study has directly compared clinical outcomes associated with generic and proprietary formulations.
A strength of this study lies in its large sample size. Our population of almost 15 000 patients allowed for powerful comparisons of the generic drug formulations vs their brand name counterparts, and could be expected to detect differences in their effectiveness. This is complemented by the specifics of drug prescription and dispensation in the Lusaka programme. There is no provision for the clinicians caring for patients to indicate whether a given prescription is to be filled with a particular formulation. Dispensation occurs in separate pharmacy areas and is driven by the availability of drugs, with an effort made to maintain a given patient on the same formulation from month to month. This permitted a unique natural experiment; whereas this was not a randomized trial, it was close to one, as initial exposure to proprietary versus generic formulations was essentially random.
Our data included a large number of patients who switched between proprietary and generic formulations. Although we maintain that this switching was essentially random early in the course of observation, procurement of generic ZDV formulations (vs brand name ones) increased dramatically after 2007 (Figure 2). This phenomenon might be expected to bias the results of our initial dispensation analysis towards the null hypothesis, since such crossover would make the two exposure groups more similar. To address this issue, we conducted two complementary statistical analyses, using (i) a time-varying approach that attributed patient outcomes to the ZDV formulation prescribed at the time of the event, and (ii) a predominant exposure categorization that limited comparisons to patients with exclusive or near exclusive exposures of either proprietary or generic ZDV. The consistency of findings across these two analyses was reassuring and suggests that, on a public health scale, proprietary and generic formulations may not differ with respect to clinical outcomes.
We note several limitations to our analysis. First, although the treatment programme has been ongoing since April 2004, subtle differences between the studied formulations could take longer periods to manifest. Virological monitoring could lead to earlier detection of treatment failure, but such testing is not routinely available in our setting. Secondly, like many cohorts in the region,46 a significant proportion of deaths may have been misclassified as follow-up losses. Such miscategorization would likely be random and not favour one allocation arm over the other; however, to confirm this assumption, we employed a composite outcome (i.e. programme failure) that considered those lost to follow-up as meeting a study endpoint. We were reassured to find minimal difference between our two outcomes of interest across the range of analytical approaches. Thirdly, we categorized ART as either generic or proprietary based solely on the ZDV formulation. For reasons of complexity, we did not consider these same characteristics for other components of the three-drug combination, in particular NVP or EFV. If a difference were to exist in the efficacy of these formulations, it would incorrectly bias our results towards the null hypothesis. Fourth, we were unable to further categorize generic ZDV formulations by manufacturer, the WHO pre-qualification or the FDA approval. Although such analyses could have provided important insight, a stratified analysis incorporating these factors would have magnified the central methodological challenge associated with this analysis: switching between drug formulations.
In Zambia, where an estimated 1 million adults and children are infected with HIV—all of whom will eventually require ART—even modest differences in drug prices could mean the difference between universal access and more limited services. Procurement of the cheapest effective medicines possible is critical to the national response to AIDS. We are encouraged to find that the use of generic formulations in this large patient cohort was associated with very favourable outcomes, and outcomes that were very much comparable to those achieved with proprietary formulations. Like that of many neighbouring countries, Zambian government’s decision to procure and prescribe these drugs was a sound one.
Funding
The work reported herein was supported by the Zambian Ministry of Health. Substantial additional support, including the purchase of anti-retroviral drugs approved by the US Food and Drug Administration, comes through a multi-country grant to the Elizabeth Glaser Pediatric AIDS Foundation from the US Centers for Disease Control and Prevention (CDC; U62/CCU12354). Support for data management was provided in part by a grant for Operations Research for AIDS Care and Treatment in Africa from the Doris Duke Charitable Foundation (2005047). Additional investigator salary support is provided by the University of Alabama at Birmingham Center for AIDS Research (P30-AI027767), National Institutes of Health (K01-TW06670) and a Doris Duke Clinical Scientist Development Award (2007061).
Acknowledgements
The CDC was not involved in data collection or management, but through its article ‘clearance’ process, it was involved in the review and approval of the article. Jeff Stringer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions. Conceived and designed the analysis: J.S.A.S., A.J.M., J.W.L. and B.H.C. Analyzed the data: M.J.G and J.W.L. Wrote the first draft of the article: J.S.A.S. Contributed to the writing of the manuscript: J.S.A.S., A.J.M., M.J.G., L.M., J.W.L., E.M.S., P.M., M.S.S., P.M., F.B.W., S.E.R. and B.H.C. I.C.M.J.E. criteria for authorship read and met: J.S.A.S., A.J.M., M.J.G., L.M., J.W.L., E.M.S., P.M., M.S.S., P.M., F.B.W., S.E.R. and B.H.C. Agree with manuscript results and conclusions: J.S.A.S., A.J.M., M.J.G., L.M., J.W.L., E.M.S., P.M., M.S.S., P.M., F.B.W., S.E.R. and B.H.C.
Conflict of interest: None declared.
KEY MESSAGES.
In the context of a large HIV treatment programme in Lusaka, Zambia, no detectable differences in mortality, programme failure or other clinical outcomes were observed when generic formulations of ZDV-based ART were compared with their proprietary counterparts.
These findings remained consistent across three statistical approaches for categorizing patient drug exposure.
Our results provide reassurance to many programmes in the sub-Saharan Africa region, which—for reasons of cost and availability—have relied heavily on generic manufacturers for their rapidly expanding ART programmes.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Wirtz VJ, Forsythe S, Valencia-Mendoza A, Bautista-Arredondo S. Factors influencing global antiretroviral procurement prices. BMC Public Health. 2009;9:S6. doi: 10.1186/1471-2458-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waning B, Kaplan W, King AC, Lawrence DA, Leufkens HG, Fox MP. Global strategies to reduce the price of antiretroviral medicines: evidence from transactional databases. Bull World Health Organ. 2009;87:520–28. doi: 10.2471/BLT.08.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinheiro E, Vasan A, Kim JY, Lee E, Guimier JM, Perriens J. Examining the production costs of antiretroviral drugs. AIDS. 2006;20:1745–52. doi: 10.1097/01.aids.0000242821.67001.65. [DOI] [PubMed] [Google Scholar]
- 5.Kumarasamy N. Generic antiretroviral drugs—will they be the answer to HIV in the developing world? Lancet. 2004;364:3–4. doi: 10.1016/S0140-6736(04)16605-1. [DOI] [PubMed] [Google Scholar]
- 6.Chien CV. HIV/AIDS drugs for Sub-Saharan Africa: how do brand and generic supply compare? PLoS One. 2007;2:e278. doi: 10.1371/journal.pone.0000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach–2010 Revision. Geneva, Switzerland: WHO Press; 2010. [PubMed] [Google Scholar]
- 8.World Health Organization. The WHO Prequalification Project. Fact sheet 278, August 2010. http://www.who.int/mediacentre/factsheets/fs278/en/index.html. (1 November 2011, date last accessed) [Google Scholar]
- 9.US President’s Emergency Plan for AIDS Relief. PEPFAR: A Commitment Renewed. (updated February 2009). http://www.pepfar.gov/documents/organization/114676.pdf. (1 November 2011, date last accessed) [Google Scholar]
- 10.Holmes CB, Coggin W, Jamieson D, et al. Use of generic antiretroviral agents and cost savings in PEPFAR treatment programs. JAMA. 2010;304:313–20. doi: 10.1001/jama.2010.993. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. President’s Emergency Plan for AIDS Relief: Approved and Tentatively Approved Antiretrovirals in Association with the President’s Emergency Plan. http://www.fda.gov/InternationalPrograms/FDABeyondOurBordersForeignOffices/AsiaandAfrica/ucm119231.htm. (1 November 2011, date last accessed) [Google Scholar]
- 12.Bartlett JA, Muro EP. Generic and branded drugs for the treatment of people living with HIV/AIDS. J Int Assoc Physicians AIDS Care. 2007;6:15–23. doi: 10.1177/1545109707299856. [DOI] [PubMed] [Google Scholar]
- 13.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 14.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 15.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS. doi: 10.1097/QAD.0b013e328307a051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambian Ministry of Health. Antiretroviral Therapy for Chronic HIV Infection in Adults and Adolescents: New ART Protocols, May 2007. Lusaka, Zambia: Printech Press; 2007. [Google Scholar]
- 18.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1031–35. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–17. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 20.Fusco H, Hubschman T, Mweeta V, et al. Electronic patient tracking supports rapid expansion of HIV care and treatment in resource-constrained settings [Abstract MoPe11.2C37] Paper presented at 3rd IAS Conference on HIV Pathogenesis and Treatment 2005. Rio de Janiero, Brazil. [Google Scholar]
- 21.Chi BH, Mwango A, Giganti M, et al. Early clinical and programmatic outcomes with tenofovir-based antiretroviral therapy in Zambia. J Acquir Immune Defic Syndr. 2010;54:63–70. doi: 10.1097/QAI.0b013e3181c6c65c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi BH, Mwango A, Giganti M, et al. Comparative outcomes of tenofovir-based and zidovudine-based antiretroviral therapy regimens in Lusaka, Zambia. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e31823058a3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi BH, Cantrell RA, Mwango A, et al. An empirical approach to defining loss to follow-up among patients enrolled in antiretroviral treatment programs. Am J Epidemiol. 2010;171:924–31. doi: 10.1093/aje/kwq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreiro P, Soriano V, Casas E, et al. Prevention of nevirapine-associated exanthema using slow dose escalation and/or corticosteroids. AIDS. 2000;14:2153–57. doi: 10.1097/00002030-200009290-00012. [DOI] [PubMed] [Google Scholar]
- 25.Chi BH, Cantrell RA, Zulu I, et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol. 2009;38:746–56. doi: 10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medecins Sans Frontieres. Untangling the Web of Antiretroviral Price Reductions: Drug Prices & Patent Status. http://utw.msfaccess.org./drugs (1 November 2011, date last accessed) [Google Scholar]
- 27.Dos Reis Serra CH, Mori Koono EE, Kano EK, Schramm SG, Armando YP, Porta V. Bioequivalence and pharmacokinetics of two zidovudine formulations in healthy Brazilian volunteers: an open-label, randomized, single-dose, two-way crossover study. Clin Ther. 2008;30:902–08. doi: 10.1016/j.clinthera.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Penzak SR, Acosta EP, Turner M, Tavel JA, Masur H. Antiretroviral drug content in products from developing countries. Clin Infect Dis. 2004;38:1317–19. doi: 10.1086/383575. [DOI] [PubMed] [Google Scholar]
- 29.Penzak SR, Acosta EP, Turner M, Tavel JA, Masur H. Analysis of generic nevirapine products in developing countries. JAMA. 2003;289:2648–49. doi: 10.1001/jama.289.20.2648-c. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran G, Perloff ES, von Moltke LL, Swaminathan S, Wanke CA, Greenblatt DJ. Analysis of generic antiretroviral formulations manufactured in India. AIDS. 2004;18:1482–84. doi: 10.1097/01.aids.0000131346.76289.27. [DOI] [PubMed] [Google Scholar]
- 31.Vezina HE, Henry K, Ravindran GD, et al. A randomized crossover study to determine bioequivalence of generic and brand name nevirapine, zidovudine, and lamivudine in HIV-negative women in India. J Acquir Immune Defic Syndr. 2006;41:131–36. doi: 10.1097/01.qai.0000199098.95967.ab. [DOI] [PubMed] [Google Scholar]
- 32.Byakika-Tusiime J, Chinn LW, Oyugi JH, Obua C, Bangsberg DR, Kroetz DL. Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults. PLoS One. 2008;3:e3981. doi: 10.1371/journal.pone.0003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosseinipour MC, Corbett AH, Kanyama C, et al. Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian adults. AIDS. 2007;21:59–64. doi: 10.1097/QAD.0b013e3280117ca0. [DOI] [PubMed] [Google Scholar]
- 34.Laurent C, Kouanfack C, Koulla-Shiro S, et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364:29–34. doi: 10.1016/S0140-6736(04)16586-0. [DOI] [PubMed] [Google Scholar]
- 35.Laurent C, Kouanfack C, Koulla-Shiro S, et al. Long-term safety, effectiveness and quality of a generic fixed-dose combination of nevirapine, stavudine and lamivudine. AIDS. 2007;21:768–71. doi: 10.1097/QAD.0b013e328045c4d7. [DOI] [PubMed] [Google Scholar]
- 36.Idigbe EO, Adewole TA, Eisen G, et al. Management of HIV-1 infection with a combination of nevirapine, stavudine, and lamivudine: a preliminary report on the Nigerian antiretroviral program. J Acquir Immune Defic Syndr. 2005;40:65–69. doi: 10.1097/01.qai.0000159516.39982.1b. [DOI] [PubMed] [Google Scholar]
- 37.Pujari SN, Patel AK, Naik E, et al. Effectiveness of generic fixed-dose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. J Acquir Immune Defic Syndr. 2004;37:1566–69. doi: 10.1097/00126334-200412150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–69. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 39.May SB, Barroso PF, Nunes EP, et al. Effectiveness of highly active antiretroviral therapy using non-brand name drugs in Brazil. Braz J Med Biol Res. 2007;40:551–55. doi: 10.1590/s0100-879x2007000400014. [DOI] [PubMed] [Google Scholar]
- 40.Kiertiburanakul S, Khongnorasat S, Rattanasiri S, Sungkanuparph S. Efficacy of a generic fixed-dose combination of stavudine, lamivudine and nevirapine (GPO-VIR) in Thai HIV-infected patients. J Med Assoc Thai. 2007;90:237–43. [PubMed] [Google Scholar]
- 41.Manosuthi W, Chimsuntorn S, Likanonsakul S, Sungkanuparph S. Safety and efficacy of a generic fixed-dose combination of stavudine, lamivudine and nevirapine antiretroviral therapy between HIV-infected patients with baseline CD4 <50 versus CD4 > or = 50 cells/mm3. AIDS Res Ther. 2007;4:6. doi: 10.1186/1742-6405-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T, Dai Y, Kuang J, et al. Three generic nevirapine-based antiretroviral treatments in Chinese HIV/AIDS patients: multicentric observation cohort. PLoS One. 2008;3:e3918. doi: 10.1371/journal.pone.0003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getahun A, Tansuphasawadikul S, Desakorn V, Dhitavat J, Pitisuttithum P. Efficacy and safety of generic fixed-dose combination of stavudine, lamivudine and nevirapine (GPO-vir) in advanced HIV infection. J Med Assoc Thai. 2006;89:1472–78. [PubMed] [Google Scholar]
- 44.Bourgeois A, Laurent C, Mougnutou R, et al. Field assessment of generic antiretroviral drugs: a prospective cohort study in Cameroon. Antivir Ther. 2005;10:335–41. [PubMed] [Google Scholar]
- 45.Zijenah LS, Kadzirange G, Rusakaniko S, et al. A pilot study to assess the immunologic and virologic efficacy of generic nevirapine, zidovudine and lamivudine in the treatment of HIV-1 infected women with pre-exposure to single dose nevirapine or short course zidovudine and their spouses in Chitungwiza, Zimbabwe. Cent Afr J Med. 2006;52:1–8. [PubMed] [Google Scholar]
- 46.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]