Abstract
Regulated exocytosis is a fundamental process that every secretory cell uses to deliver molecules to the cell surface and the extracellular space by virtue of membranous carriers. This process has been extensively studied using various approaches such as biochemistry, electro-physiology, and electron microscopy. However, recent developments in time-lapse light microscopy have made possible imaging individual exocytic events hence advancing our understanding of this process at a molecular level. In this review, we focus on intravital microscopy a light microscopy-based approach that enables imaging subcellular structures in live animals, and discuss its recent application to study regulated exocytosis. Intravital microscopy has revealed differences in regulation and modality of regulated exocytosis between in vitro and in vivo model systems, unraveled novel aspects of this process that can be appreciated only in in vivo settings, and provided valuable and novel information on its molecular machinery. In conclusion, we make the case for intravital microscopy being a mature technique that can be used to investigate the molecular machinery of several intracellular events under physiological conditions.
Keywords: Regulated exocytosis, light microscopy, intravital microscopy, actin, cytoskeleton, in vivo imaging, exocrine secretion
Regulated exocytosis
Regulated exocytosis is one of the processes that secretory cells use to deliver molecules to the cell surface and the extracellular space. Molecules destined for secretion are synthesized in the endoplasmic reticulum, transported to the Golgi apparatus, processed, and sorted into membranous carriers that are constitutively released from the trans-Golgi network (1). These carriers, that can be vesicular or tubular in shape, are transported in a cytoskeleton-assisted fashion to the cell periphery where they dock to the plasma membrane. This step is followed by the fusion of the two lipid bilayers and the formation of the fusion pore, which permits the release of soluble cargo molecules into the extracellular space (2). Docking and fusion are triggered by specific extracellular signals that are transduced intracellularly by proteins at the plasma membrane, such as G protein-coupled receptors, tyrosine kinase receptors, and voltage-dependent calcium channels (1, 3). In addition, organelles that are not in the secretory pathway, such as lysosome-related or endosome-derived vesicles can also undergo regulated exocytosis using similar mechanisms (4, 5).
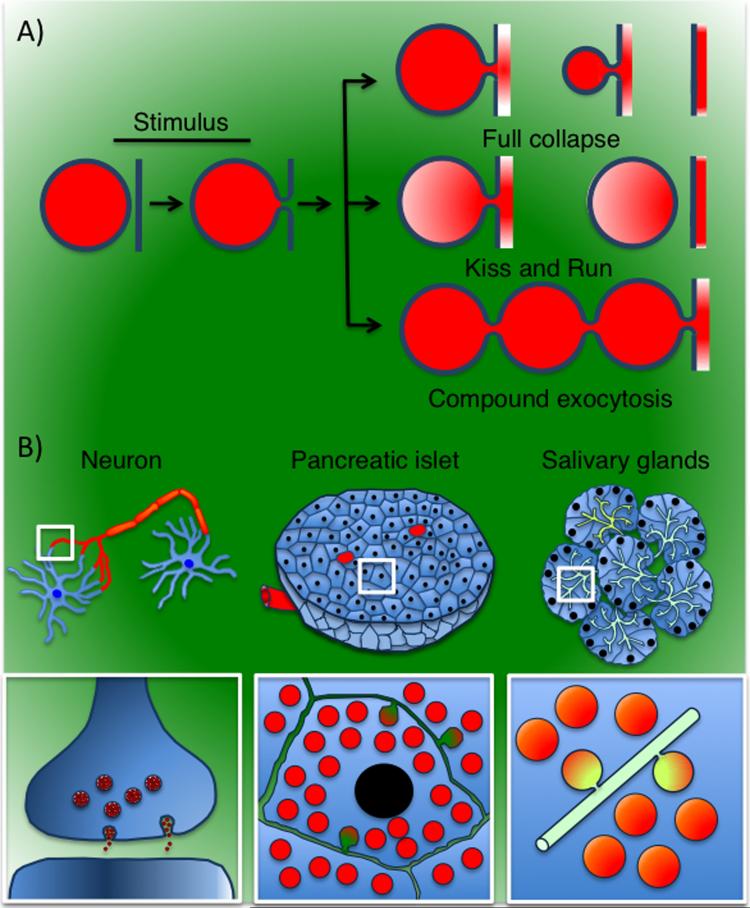
The modality and the kinetics of regulated exocytosis vary considerably among the numerous types of secretory cells. Indeed, exocytic membranes can undergo three different processes after fusion with the plasma membrane: 1) full collapse, where they are completely absorbed into the plasma membrane (6, 7), 2) kiss and run, where they detach from the plasma membrane after the initial opening of the fusion pore and the partial release of cargo molecules (8), and 3) compound exocytosis, where they fuse with the plasma membrane (primary fusion event) serving as a docking site for other exocytic membranes (secondary fusion events) generating a string of interconnected vesicles (Fig. 1A) (7, 9, 10). This diversity in modality of regulated exocytosis may reflect the fact that the organization of the plasma membrane, the morphology of the membranous carriers, and the nature of the cargo molecules, differ among the various secretory organs. For example, in neurons, neurotransmitters are transported in small vesicles (50-100 nm in diameter) that are released from the axon terminal into the synaptic cleft within few milliseconds (11). In endocrine glands, molecules destined to secretion are transported in vesicles (60-300 nm in diameter) that are released at the plasma membrane into the extracellular space, diffuse through the stroma, and eventually reach the bloodstream (12). In exocrine glands, molecules are stored in large vesicles (1-1.5 μm in diameter) that are released at specialized domains of the plasma membrane, typically the apical poles, which form ductal structures leading directly to the external environment (Fig. 1B).
Figure 1. Regulated exocytosis.
A – Modality of regulated exocytosis. Upon stimulation, exocytic vesicles fuse with the plasma membrane and the fusion pore opens. Exocytic vesicles can undergo: 1) full collapse, 2) kiss and run, or 3) compound exocytosis. B - Different architecture of the secretory organs. In neurons, small synaptic vesicles release their content at the pre-synaptic terminals into the synaptic cleft (left). In endocrine glands, such as the pancreatic islets, molecules are released in the extracellular space and reach the bloodstream (center). In exocrine glands, such as the salivary glands, cargo molecules are released into a series of canaliculi and ducts that are connected to the oral cavity (right)
Light microscopy: a powerful tool to study regulated exocytosis
Regulated exocytosis has been primarily studied using a variety of biochemical, immunological or electrochemical assays aimed at measuring the amount of secreted molecules. Although these approaches offer an accurate view of the secretory activity at the single cell level, they do not provide any mechanistic information on the exocytic steps or on the morphology and fate of individual exocytic vesicles. Alternatively, regulated exocytosis has been investigated by measuring membrane capacitance. This approach enables studying in great detail individual fusion events at the plasma membrane, thus providing accurate information on the opening of the fusion pore and the size of fusing vesicles. However, its major limitations are the inability to distinguish between endocytic and exocytic events, and to provide morphological information on exocytic vesicles before or after the fusion step. The latter issue has been addressed by using electron microscopy, which does not allow studying the dynamics of the exocytic events.
These approaches have been complemented by the recent advancements in time-lapse light microscopy and the development of fluorescent protein technology. Here we briefly describe few of the techniques that have been successfully used to study specific steps of regulated exocytosis (Table 1). Among them, total internal reflection microscopy (TIRF), has permitted to image the exocytic steps that occur in close proximity to the plasma membrane (13). For example, the machinery regulating docking and fusion of the exocytic vesicles transporting the glucose transporter 4 (GLUT4) has been thoroughly dissected by TIRF. Studies in primary rat adipocytes expressing GFP-tagged GLUT4 have revealed that under basal conditions GLUT4-containing vesicles traffic towards the plasma membrane in a microtubule-dependent fashion, whereas insulin stimulation promotes their tethering and fusion with the plasma membrane (14). Furthermore, some of the components regulating the opening of the fusion pore have been identified by using the pH sensitive probe VAMP2-pHluorin targeted to the GLUT4-containing vesicles (15). Similar approaches have been used to study the exocytosis of dense core vesicles in bovine chromaffin cells, PC12 cells, and primary cultured hippocampal neurons (13, 16, 17), lytic granules in cytotoxic T lymphocytes (4), and insulin granules in pancreatic beta cells (18). However, TIRF does not allow imaging exocytic vesicles that are distant from the plasma membrane and its use is restricted to cell cultures adherent on glass. In this respect, spinning disk confocal microscopy has been successfully applied to follow the fate of exocytic vesicles before and after the fusion step. For example, the machinery regulating the exocytosis of the Weibel-Palade (WP) bodies in cultured human endothelial cells has been dissected by using a combination of spinning disk confocal and correlative electron microscopy (19). The fusion of the WP bodies with the plasma membrane and the kinetics of release of two cargo molecules, the von Willebrand factor (VWF) and the leukocyte receptor P-selectin, was analyzed in details. Interestingly, the extrusion of the VWF was facilitated by the recruitment of a dynamic actin ring and the actin motor myosin IIb around the WP bodies (20). The use of the spinning disk has been extended to ex-vivo preparations. Indeed, recently, it has been successfully used in isolated pancreatic acini where the exocytosis of large secretory granules containing syncollin-pHluorin has been studied under both physiological and pathological conditions (21).
Table 1.
Current light microscopy-based techniques used to study regulated exocytosis
| Technique | Imaged Step and advantages | Limitations | Model systems |
|---|---|---|---|
| TIRF | Docking Tethering Kinetics of the fusion pore Small vesicles |
Only vesicles at the PM No info on pre- or post-fusion steps Only adherent cells |
In vitro |
| Spinning disk Confocal | Dynamics of vesicles during exocytosis Morphology of vesicles Modality of fusion |
Large exocytic vesicles Low depth |
In vitro
Ex vivo |
| Confocal | Dynamics of vesicles during exocytosis Morphology of vesicles Modality of fusion |
Large exocytic vesicles Slow exocytic events (sec-min) Low depth |
In vitro
Ex vivo In vivo |
| Two-photon | Dynamics of vesicles during exocytosis Morphology of vesicles Modality of fusion Deep tissue |
Large exocytic vesicles Slow exocytic events (sec-min) |
In vitro
Ex vivo In vivo |
Both confocal and two-photon laser scanning microscopy have also been widely used to study regulated exocytosis. However, due to their temporal and spatial resolution they are better suited to study larger vesicles that exocytose with slow kinetics. In addition, both techniques have been used in thick tissues such as ex-vivo model systems derived from exocrine organs. Several studies were carried out by bathing acinar preparations in small fluorescent dyes, such as low molecular weight dextrans or sulphorhodamine-B, which accumulate in the extracellular space and upon the opening of the fusion pore access the secretory granules (9, 10, 22-25). This approach enabled measuring the kinetics of the pore expansion, the diffusion of proteins and lipids from the plasma membrane into the secretory granules (26-28). Furthermore, both techniques have permitted to image simultaneously regulated exocytosis and other processes such as Ca++ signaling that elicits and regulates most of the exocytic processes (9, 29). Finally, expression of fluorescently tagged proteins have been instrumental to identify several factors implicated in exocytosis such as SNAREs (22, 26), Rabs (30), and component of the cytoskeleton such as F-actin (31) and myosin motors (4, 32-34). Overall, these complementary techniques have enabled acquiring detailed information of different exocytic events before, during and after the fusion steps.
Regulated exocytosis in live animals: insights from intravital microscopy
Light microscopy has clearly contributed to advance our knowledge of the machinery controlling regulated exocytosis. Nonetheless, although light microscopy techniques have improved to the extent of breaking the diffraction limit of light (35), their application has been primarily restricted to in vitro (i.e. cell cultures) or ex vivo preparations. These systems offer several advantages, such as a tight control of the experimental conditions, which results in high reproducibility, and in the possibility of performing genetic and pharmacological manipulations. However, often they do not fully reproduce the conditions found in the native tissue of a live multicellular organism. For example, cell cultures are excellent tools to study endocrine and neuroendocrine secretion whereas they exhibit some limitations as models for regulated exocytosis in exocrine glands. Indeed, acinar cells isolated from explanted salivary glands rapidly de-differentiate and within few hours they lose their polarity and all the secretory granules (36). For these reasons, the field of exocrine secretion has relied extensively on preparations derived from freshly explanted organs. However, these preparations are based on the use of mechanical and enzymatic procedures that may have adverse effects on the response to exocytic stimuli. Moreover, although some aspects of the architecture of intact organs are maintained, other structural components are missing, such as extracellular matrix, supporting cells, and ductal structures. More importantly, they lack the contribution from signaling molecules provided by the vasculature and the nervous system. Particularly, the latter has a profound influence on the response to excitatory stimuli, since denervation in live animals results in alterations in both the morphology and the exocytic capacity of the secretory apparatus, as shown in several studies (37).
Hence, the ideal approach to study regulated exocytosis would apply light microscopy techniques to live animals. This can be achieved by using intravital microscopy (IVM). The term IVM encompasses a series of techniques based on light microscopy that enables imaging biological processes in living organisms (38-41). IVM has been initially used in the early 1920's but only in the last two decades have been successfully developed and applied to various fields such as neuroscience (40), immunology (42) and tumor biology (38, 39). IVM has made it possible to study the dynamics of tissue response under various physiological and pathological conditions (43), the behavior of single cell during the immune response (42), the invasive and metastatic activity of tumor cells (44, 45), and the dynamics of structures in the submicron range, such as dendritic spines (46). The first attempts to image intracellular organelles have been performed in the kidney of live rats and mice, where the internalization of fluorescently tagged molecules, such as dextran or folate, were imaged in the proximal tubuli (47, 48). However, due to motion artifacts originating from heartbeat and respiration, the dynamics of the endocytic events were only imaged for short periods of time. To overcome this issue a series of strategies that permit imaging the behavior of subcellular structures in live animals for long periods of time were developed (41, 49, 50). The salivary glands of live rodents were used as a model organ, and two-photon IVM was used to analyze the endocytosis of fluorescent molecules and their trafficking through the endo-lysosomal system (49). Notably, these organs provide the opportunity to study dynamic cellular processes at the molecular level since they are amenable to both pharmacological and genetic manipulations in situ (50).
Recently, confocal IVM has been used to study the regulation and the modality of the exocytosis of large secretory granules in salivary glands in vivo, and to unravel the role of the actin cytoskeleton in the dynamics of this process (6). These questions were addressed by using a combination of different approaches such as: 1) transgenic mouse models, 2) the ability to transfect genes into the acinar cells, and 3) the ability to selectively deliver fluorescent probes and pharmacological agents into the salivary glands. Mice expressing cytoplasmic GFP and/or a membrane-targeted peptide fused with the fluorescent protein Tomato (m-tomato) has enabled imaging and analyzing the kinetics of secretory granules exocytosis leading to two major findings. First, in salivary glands in vivo exocytosis of large secretory granules is triggered exquisitely by beta-adrenergic stimulation (Fig. 2A). This finding that is also supported by data from another group (51), is at odd with most of the data in the literature, which were generated in ex-vivo salivary glands models. Indeed, several studies suggested that protein secretion and exocytosis of secretory granules are regulated by the synergistic action of both muscarinic and beta-adrenergic receptors (36, 52, 53). Second, by using three independent experimental approaches in vivo, secretory granules were observed to fuse with the plasma membrane and gradually collapse after the opening of the fusion pore (Fig. 2A). This process occurred without any evidence of compound exocytosis (6), as previously described in several ex-vivo systems (24, 25, 53-55).
Figure 2. Exocytosis of large secretory granules in the salivary glands of live animals.
A -Mice expressing GFP (upper panels), m-Tomato (center panels) or rats expressing GFP-lifeact (lower panels) were anesthetized, the submandibular Salivary glands were surgically exposed and imaged by confocal IVM as described in (6). Isoproterenol, an agonist of the beta-adrenergic receptor, was injected subcutaneously to stimulate exocytosis. The Secretory granules (arrows) gradually collapse with the apical plasma membrane (red asterisk). F-actin is recruited onto the membrane of the Secretory granules (lower panels). Bars 5 μm (left panels) and 2 μm (time sequences). B – Diagram summarizing the exocytosis of the Secretory granules in Salivary glands. After fusion with the plasma membrane, F-actin (blue) and myosin IIA and IIB (yellow) are recruited onto the membranes of the Secretory granules. The contractile activity of the actomyosin complex facilitates the gradual collapse of the Secretory granules.
The ability to transiently express in vivo a fluorescently tagged probe for F-actin (lifeact, (20)), either alone or in combination with a marker for the plasma membrane has been instrumental to investigate the mechanism driving the gradual collapse of the secretory granules and the role of the actin cytoskeleton in regulating this process. Upon stimulation of exocytosis, F-actin is recruited onto those secretory granules close to the apical plasma membrane, as previously reported by others (55, 56). However, the recruitment occurs only after their fusion with the plasma membrane and the opening of the fusion pore. In addition, the disruption of the actin cytoskeleton, by delivery of pharmacological agents such as cytochalasin D or Latrunculin A, results in the impairment of the secretory granules collapse and in an almost two-fold increase in their size. This effect is due to two main factors: the initial increase in the hydrostatic pressure generated by fluid secretion that occurs in parallel to exocytosis (6, 57), and the fact the secretory granules in the cytoplasm fuse with the large secretory granules that are fused at the plasma membrane in a process reminiscent of compound exocytosis. Finally, two of the isoforms of myosin II (IIA and IIB) are recruited onto the secretory granules after fusion with the plasma membrane. Their contractile activity affects the gradual collapse of the granules and counteracts the expansion produced by the hydrostatic pressure. These data obtained by IVM have unraveled a complex scenario in which a functional contractile actomyosin complex assembles onto the secretory granules during exocytosis to perform at least three functions: 1) to counteract the effect of the hydrostatic pressure as discussed in detail elsewhere (57), 2) to prevent the homotypic fusion of the granules at the apical plasma membrane and 3) to facilitate the gradual collapse of the secretory granules (Fig. 2B).
In summary, IVM has enabled investigating both the regulation and modality of regulated exocytosis in salivary glands and dissecting some of the molecular components involved in this process. It is important to emphasize that IVM has unraveled discrepancies between in vitro and in vivo model systems, and revealed novel aspects associated with exocytosis, such as the contribution of the hydrostatic pressure, that can only be observed in live animals. Although the reasons for discrepancies between in vivo and in vitro model systems have not been investigated thoroughly, it is reasonable to assume that the procedures used to isolate the acinar structures and the lack of signaling molecules may alter some aspects of the glands physiology. For example, the formation of micropores at the plasma membrane has been reported to activate membrane-repair mechanisms that use Ca++ dependent pathways to mobilize intracellular membranes (58). This may lead to the generation of a pool of pre-docked secretory granules that would be more sensitive to muscarinic stimulation. On the other hand, the lack of the extracellular matrix and other signaling molecules may affect the dynamics of the actin cytoskeleton, whose organization is linked to signals coming from the extracellular space (59), providing some suggestions on why compound exocytosis is observed in ex-vivo models. As for the hydrostatic pressure, its effect on exocytosis and the homeostasis of the apical plasma membrane, was not observed in ex vivo models, where the ductal systems are either absent or only partially preserved (57). This underscores how IVM provides the opportunity to observe novel phenomena that are unique to in vivo settings.
Technical challenges of intravital microscopy
Although most of the experimental details have been described elsewhere (6, 49, 60), we feel that the reader would benefit from a brief description of the major technical challenges that have to be addressed in order to successfully use IVM to study regulated exocytosis or any other membrane trafficking event. As mentioned previously, one of the major challenges is the minimization of the motion artifacts due to the respiration and the heartbeat. In this respect, the salivary glands represent an ideal organ for IVM, since in rodents they are located in the neck region, where motion artifacts are lower when compared to other areas of the body, such as the abdomen. Nonetheless, two strategies were used to further reduce the motion artifacts: one based on a custom made holder, which accommodate the exteriorized glands while completely connected to the vasculature and the central nervous system (49, 60), and the other based on the use of specific tools, which properly position the glands on the microscope stage and immobilize the animal body (6, 60). Another crucial factor is the temperature of the body and the exteriorized organs. This has to be maintained within of 2-3°C to the normal body temperature (mice, 37°C and rats, 38-39°C) to avoid a reduction in the number of exocytic events and a more general halting of the movements of other subcellular organelles. Moreover, particular care has to be taken in externalizing the glands without compromising their integrity and in positioning them in order to avoid reduction in blood flow. Indeed, reduction in blood supply almost immediately results in alteration of the morphology of the plasma membrane such as blebbing or vacuolization.
At the moment, the major limitations of IVM are the ability to image very fast processes, such as the regulated exocytosis of synaptic vesicles, and to reach specific area of the tissue that are well below the maximal depth currently reachable by light microscopy, such as the adrenal medulla in the adrenal glands. The first issue can be solved with the new generation of fast-scanning microscopes, whereas the second issue could be overcome by perfecting surgical procedures, or with the use of specific probes such as the GRIN lenses (61), chronic ports of observations (62, 63), or implanted prisms (64).
Conclusions and Perspectives
Regulated exocytosis is a very complex multi-step process that occurs in different modalities depending on the secretory systems. The use of in vitro and ex vivo experimental systems in combination with light microscopy provides the basis of our current knowledge on the kinetics of single exocytic events and is instrumental to unraveling the molecular machinery controlling regulated exocytosis. However, since the architecture of the secretory cells and their surrounding environment strongly influence the modality and the machineries controlling regulated exocytosis, it is important to choose and maintain experimental settings that are as close as possible to the physiological environment. Studies in the salivary glands of live rodents show that there are some significant differences between in vivo and ex vivo models in terms of both regulation and modality of regulated exocytosis. Although it is not known to what extent these differences extend to other organs or to other cellular processes, it is important to be aware of them. IVM can be used to investigate the underlying reasons for these discrepancies and to confirm data or hypothesis generated in in vitro models. However major efforts should be devoted towards using IVM as one of the main methods to study regulated exocytosis thus complementing other experimental approaches. The same tools developed for the salivary glands can be easily applied to other exocrine glands, such as pancreas, lacrimal and mammary glands. Furthermore, novel tools are now available to address additional questions using IVM. Among them, mice expressing fluorescently tagged lifeact (65), myosin IIa and IIb (66), GLUT4 (67), membrane markers (68), and relatively novel procedures to perform protein ablation in vivo via siRNA or shRNA (69).
In conclusion, we believe that IVM has already reached the maturity to be fully exploited to study not only regulated exocytosis and other membrane trafficking events but to address fundamental questions in cell biology.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research (NIDCR). We apologize to those whose work could not be cited due to space limitations.
References
- 1.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83(2):581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 2.Monck JR, Fernandez JM. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8(4):524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 3.Martin TF. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641(2-3):157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 4.Martina JA, Wu XS, Catalfamo M, Sakamoto T, Yi C, Hammer JA., 3rd Imaging of lytic granule exocytosis in CD8(+) cytotoxic T lymphocytes reveals a modified form of full fusion. Cell Immunol. 2011;271(2):267–279. doi: 10.1016/j.cellimm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretorylysosomes. Science. 2004;305(5680):55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 6.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci U S A. 2011;108(33):13552–13557. doi: 10.1073/pnas.1016778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17(4):1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzoli SO, Jahn R. Kiss-and-run, collapse and ‘readily retrievable’ vesicles. Traffic. 2007;8(9):1137–1144. doi: 10.1111/j.1600-0854.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 9.Kasai H, Kishimoto T, Nemoto T, Hatakeyama H, Liu TT, Takahashi N. Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev. 2006;58(7):850–877. doi: 10.1016/j.addr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic. 2006;7(2):109–116. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 11.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 12.Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763(11):1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Burchfield JG, Lopez JA, Mele K, Vallotton P, Hughes WE. Exocytotic vesicle behaviour assessed by total internal reflection fluorescence microscopy. Traffic. 2010;11(4):429–439. doi: 10.1111/j.1600-0854.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 14.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169(3):481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Rubin BR, Orme CM, Karpikov A, Yu C, Bogan JS, Toomre DK. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J Cell Biol. 2011;193(4):643–653. doi: 10.1083/jcb.201008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiegand UK, Don-Wauchope A, Matskevich I, Duncan RR, Greaves J, Shipston MJ, Apps DK, Chow RH. Exocytosis studies in a chromaffin cell-free system: imaging of single-vesicle exocytosis in a chromaffin cell-free system using total internal reflection fluorescence microscopy. Ann N Y Acad Sci. 2002;971:257–261. doi: 10.1111/j.1749-6632.2002.tb04472.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Castle D. Regulation of fusion pore closure and compound exocytosis in neuroendocrine PC12 cells by SCAMP1. Traffic. 2011;12(5):600–614. doi: 10.1111/j.1600-0854.2011.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohara-Imaizumi M, Nagamatsu S. Insulin exocytotic mechanism by imaging technique. J Biochem. 2006;140(1):1–5. doi: 10.1093/jb/mvj127. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011;194(4):613–629. doi: 10.1083/jcb.201011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez NA, Liang T, Gaisano HY. Live pancreatic acinar imaging of exocytosis using syncollin-pHluorin. Am J Physiol Cell Physiol. 2011;300(6):C1513–1523. doi: 10.1152/ajpcell.00433.2010. [DOI] [PubMed] [Google Scholar]
- 22.Behrendorff N, Dolai S, Hong W, Gaisano HY, Thorn P. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem. 2011;286(34):29627–29634. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Iino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3(3):253–258. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 24.Segawa A, Riva A. Dynamics of salivary secretion studied by confocal laser and scanning electron microscopy. Eur J Morphol. 1996;34(3):215–219. doi: 10.1076/ejom.34.3.215.13023. [DOI] [PubMed] [Google Scholar]
- 25.Segawa A, Terakawa S, Yamashina S, Hopkins CR. Exocytosis in living salivary glands: direct visualization by video-enhanced microscopy and confocal laser microscopy. Eur J Cell Biol. 1991;54(2):322–330. [PubMed] [Google Scholar]
- 26.Pickett JA, Thorn P, Edwardson JM. The plasma membrane Q-SNARE syntaxin 2 enters the zymogen granule membrane during exocytosis in the pancreatic acinar cell. J Biol Chem. 2005;280(2):1506–1511. doi: 10.1074/jbc.M411967200. [DOI] [PubMed] [Google Scholar]
- 27.Thorn P, Fogarty KE, Parker I. Zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity. Proc Natl Acad Sci U S A. 2004;101(17):6774–6779. doi: 10.1073/pnas.0400336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorn P, Parker I. Two phases of zymogen granule lifetime in mouse pancreas: ghost granules linger after exocytosis of contents. J Physiol. 2005;563(Pt 2):433–442. doi: 10.1113/jphysiol.2004.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yule DI. Pancreatic acinar cells: molecular insight from studies of signal-transduction using transgenic animals. Int J Biochem Cell Biol. 2011;42(11):1757–1761. doi: 10.1016/j.biocel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang L, Ngo J, Schechter JE, Karvar S, Tolmachova T, Seabra MC, Hume AN, Hamm-Alvarez SF. Rab27b regulates exocytosis of secretory vesicles in acinar epithelial cells from the lacrimal gland. Am J Physiol Cell Physiol. 2011;301(2):C507–521. doi: 10.1152/ajpcell.00355.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5(8):727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 32.Bhat P, Thorn P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell. 2009;20(6):1795–1803. doi: 10.1091/mbc.E08-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118(Pt 20):4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larina O, Bhat P, Pickett JA, Launikonis BS, Shah A, Kruger WA, Edwardson JM, Thorn P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell. 2007;18(9):3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippincott-Schwartz J. Bridging structure and process in developmental biology through new imaging technologies. Dev Cell. 2011;21(1):5–10. doi: 10.1016/j.devcel.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84(6):500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Amornphimoltham P, Masedunskas A, Weigert R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv Drug Deliv Rev. 2011;63(1-2):119–128. doi: 10.1016/j.addr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011;124(Pt 3):299–310. doi: 10.1242/jcs.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50(6):823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol. 2010;133(5):481–491. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5(5):e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20(1):54–62. doi: 10.1016/j.copbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 46.Pan F, Gan WB. Two-photon imaging of dendritic spine development in the mouse cortex. Dev Neurobiol. 2008;68(6):771–778. doi: 10.1002/dneu.20630. [DOI] [PubMed] [Google Scholar]
- 47.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283(3):C905–916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 48.Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol. 2004;287(2):C517–526. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 49.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9(10):1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sramkova M, Masedunskas A, Parente L, Molinolo A, Weigert R. Expression of plasmid DNA in the salivary gland epithelium: novel approaches to study dynamic cellular processes in live animals. Am J Physiol Cell Physiol. 2009;297(6):C1347–1357. doi: 10.1152/ajpcell.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peter B, Van Waarde MA, Vissink A, s-Gravenmade EJ, Konings AW. Degranulation of rat salivary glands following treatment with receptor-selective agonists. Clin Exp Pharmacol Physiol. 1995;22(5):330–336. doi: 10.1111/j.1440-1681.1995.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 52.Castle JD. Protein secretion by rat parotid acinar cells. Pathways and regulation. Ann N Y Acad Sci. 1998;842:115–124. doi: 10.1111/j.1749-6632.1998.tb09639.x. [DOI] [PubMed] [Google Scholar]
- 53.Warner JD, Peters CG, Saunders R, Won JH, Betzenhauser MJ, Gunning WT, 3rd, Yule DI, Giovannucci DR. Visualizing form and function in organotypic slices of the adult mouse parotid gland. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G629–640. doi: 10.1152/ajpgi.90217.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanno T. Compound exocytosis of secretory granules containing salivary chromogranin A in granular duct cells in rat submandibular gland: the last study in collaboration with the late Professor Noboru Yanaihara at Yanaihara Institute. Regul Pept. 2004;123(1-3):3–7. doi: 10.1016/j.regpep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Segawa A, Yamashina S. Roles of microfilaments in exocytosis: a new hypothesis. Cell Struct Funct. 1989;14(5):531–544. doi: 10.1247/csf.14.531. [DOI] [PubMed] [Google Scholar]
- 56.Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A. 2000;97(3):1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masedunskas A, Sramkova M, Weigert R. Homeostasis of the apical plasma membrane during regulated exocytosis in the salivary glands of live rodents. Bioarchitecture. doi: 10.4161/bioa.18405. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180(5):905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masdunskas A, Weigert R. Intravital microscopy to image membrane trafficking in live rats. In: Douglas J. Taatjes, Jurgen Roth M., editors. Cell Imaging Techniques: Methods and Protocols. Second Edition. Humana Press; In press. [Google Scholar]
- 61.Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol. 2004;91(4):1908–1912. doi: 10.1152/jn.01007.2003. [DOI] [PubMed] [Google Scholar]
- 62.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130(6):1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 63.Barretto RP, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat Med. 2011;17(2):223–228. doi: 10.1038/nm.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chia TH, Levene MJ. Microprisms for in vivo multilayer cortical imaging. J Neurophysiol. 2009;102(2):1310–1314. doi: 10.1152/jn.91208.2008. [DOI] [PubMed] [Google Scholar]
- 65.Riedl J, Flynn KC, Raducanu A, Gartner F, Beck G, Bosl M, Bradke F, Massberg S, Aszodi A, Sixt M, Wedlich-Soldner R. Lifeact mice for studying F-actin dynamics. Nat Methods. 2010;7(3):168–169. doi: 10.1038/nmeth0310-168. [DOI] [PubMed] [Google Scholar]
- 66.Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282(30):22102–22111. doi: 10.1074/jbc.M702731200. [DOI] [PubMed] [Google Scholar]
- 67.Lauritzen HP, Ploug T, Prats C, Tavare JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes. 2006;55(5):1300–1306. doi: 10.2337/db05-1216. [DOI] [PubMed] [Google Scholar]
- 68.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 69.Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(-) reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1729–1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]