Abstract
Although protein folding is a simple outcome of the underlying thermodynamics, arriving at a quantitative and predictive understanding of how proteins fold nevertheless poses huge challenges. Therefore, both advanced experimental and computational methods are continuously being developed and refined to probe and reveal the atomistic details of protein folding dynamics and mechanisms. Herein, we provide a concise review of recent developments in spectroscopic studies of protein folding, with a focus on new triggering and probing methods. In particular, we describe several laser-based techniques for triggering protein folding/unfolding on the picosecond and/or nanosecond timescales and various linear and nonlinear spectroscopic techniques for interrogating protein conformations, conformational transitions, and dynamics.
Keywords: 2D-IR, conformational trigger, conformational probe, FCS, fluorescence, FRET, infrared, isotope editing, kinetics, protein folding, Raman, temperature-jump
Introduction
Despite extensive study over the past few decades, the protein folding problem1 continues to inspire new research directions. This is due not only to the sheer complexity of the process of protein folding, which makes it extremely difficult to achieve a mechanistic, quantitative, and predictive understanding of how proteins fold, but also to the fact that it provides an excellent test-bed for the development and validation of new computational and experimental methods for biology, biochemistry, and biophysics. Herein, we present a concise review of recent advances in the application of spectroscopic methods to investigations of protein folding, with a focus on fluorescence and vibrational spectroscopies, as well as conformational triggering methods. Although dynamic information can sometimes be revealed or inferred by static spectroscopic measurements, for most ensemble kinetic experiments, a suitable triggering event is required to start the “reaction” of interest; in addition, one or more physical observables or probes are used to follow the progression of this reaction. For protein folding studies, it is apparent that the choice of the triggering method determines the time resolution of the observable kinetics, whereas the conformational probes used dictate the structural resolution of the experiment. Below, we will first discuss available protein folding and conformational triggering methods, with a focus on those that offer nanosecond or faster time resolutions, and then will review spectroscopic methods that offer varying degrees of structural resolution and sensitivity when applied to probe protein folding/unfolding equilibria and kinetics.
Triggering Methods
The free energy of a given protein in solution depends on the temperature, pressure, solvent composition, and other external constraints if they exist. Thus, the thermodynamic equilibrium between its folded and unfolded states can be manipulated by simply changing one of these factors. As such, all current protein folding triggering methods rely on this principle, whereby the system in question is rapidly (compared with the kinetic events of interest) shifted to a nonequilibrium state. The subsequent relaxation kinetics towards the new thermodynamic equilibrium, which could be probed by a wide variety of techniques, can be dominated by either folding, unfolding, or both, depending on the final condition of the protein solution. For example, a folding or unfolding process could be initiated by rapid mixing of two solutions via either the stopped-flow or continuous-flow techniques.2, 3 Although the majority of previous folding kinetic studies used such mixing-based triggering methods, a comprehensive review of these subjects is beyond the scope of this paper. Below we only discuss triggering methods that rely on the application of short (picosecond–nanosecond) laser pulses, which may involve direct excitation of chemical groups in the protein, resulting in chemical changes that leave the protein backbone in a nonequilibrium state (e.g., by removal of a conformational constraint), or photoinduced changes of physical and/or solution properties (e.g., temperature or pH) via excitation of solvent or solute molecules.
Laser-induced temperature-jump
The ultrafast (picosecond or nanosecond) temperature-jump (T-jump) method relies on the fact that in condensed phases vibrational relaxations typically take place on the picosecond timescale,4 thus allowing a burst of photons to rapidly heat up the solution of interest through excitation of solvent or solute5, 6 vibrations. For aqueous solutions, a rapid T-jump can be achieved by exciting water's near-infrared (IR) overtone transitions in the vicinity of either 1.5 μm (for H2O solution) or 2 μm (for D2O solution) using either nanosecond (ns) or picosecond (ps) laser pulses.7–12 The amplitude of the T-jump depends on the pump energy and excitation volume and can be determined from solvent absorbance using a calibration curve.7 Because the thermal energy initially concentrated in the laser excitation volume will gradually dissipate into the surroundings, the useful time window of this technique in which the temperature remains approximately constant ranges from a few to tens of milliseconds (ms), depending on the optical setup and sample holder. Because the T-jump technique does not require any specific protein modifications, it is, therefore, the most commonly used triggering method in the study of ultrafast protein folding events.13–23
Azobenzene isomerization
The reversible cis/trans photoisomerization of azobenzenes has also been demonstrated to have the capability to control peptide and protein conformations and thus has found utility in folding kinetic studies.24–29 A thorough review on the use of azobenzene and derivatives as photoswitches in biomolecules can be found elsewhere.30 Simple azobenzene compounds photoisomerize on the ps timescale and, therefore are suitable for studying the early events in protein folding. Additionally the azobenzene cis/trans isomers exhibit well separated spectral features, allowing for independent excitation of either isomer and thus reversible switching of the trigger. As shown first by Kumita et al.,26 a reversible change in helicity of up to 36% can be obtained on photoisomerization of the azobenzene cross-linker in an alanine-based peptide. More recent work has been done to understand how such linkers affect peptide backbone conformations,31, 32 and to probe the dynamics and mechanism of the helix–coil transition,33–35 among other applications.36–38
Photodissociation of a cage compound or disulfide bond
Another method of conformational switching is to use a relatively large molecular moiety (i.e., a cage) to force the protein of interest to unfold under native conditions; on cleavage of the cage by excitation with an appropriate laser pulse, the protein will fold to its native conformation. Thus, several photocages, such as benzoinyl cages, have been used to trigger protein folding kinetics.39–43 Photocaged molecules can also be used in a cyclization scheme to produce a photocleavable intramolecular linker. In some cases, it is desirable to impose a well defined structural constraint on the protein molecule, enabling the folding/unfolding reaction to start from a unique position on the folding energy landscape on removal of the conformational constraint. For example, this approach has been applied to the study of a mutant of the small α-helical villin headpiece subdomain (i.e., cVHP-34 M12C), in which an internal cysteine residue and a 3′-(carboxymethoxy)benzoin (CMB) molecule, which is covalently attached to the N-terminus of the polypeptide, have been linked to restrict the conformation in the unfolded state. On illumination with a 355 nm laser pulse, this linker molecule is irreversibly cleaved with a yield of 0.6–0.7; the loop is therefore broken to yield the linear protein that is free to refold. The time resolution of this method is determined by the rate of photolysis of the linker, which is faster than one nanosecond.44 However, it is worth mentioning that if the photocage has to diffuse out of its initial position for the protein to fold, the time resolution of such cage cleaving methods would be determined by the rate of this diffusion process.
One convenient structural constraint commonly found in proteins is the disulfide bond formed between two thiol-containing amino acid sidechains. Lu et al.45 have shown that a disulfide cross-linker can be transiently broken by an ultraviolet (UV) laser pulse, making it potentially useful as a phototriggering method in protein folding studies. However, in practice this method is less useful as the resultant thiyl radicals undergo rapid geminate recombination, which greatly reduces the population of protein molecules that will eventually undergo a conformational transition. Recently, Hamm and coworkers46, 47 have shown that for small peptides this problem can be alleviated to some extent due to the increased strain exerted on the disulfide bond by cross-linking two cysteines that are close in sequence. A more promising method, which is currently under investigation in our laboratory,48 however, is to use a photoinduced electron transfer (PET) reaction to permanently break the disulfide cross-linker of interest. Cleavage of disulfide bonds via light-induced electron transfer from adjacent tryptophan residues has been observed in various proteins49–54 suggesting that it could be engineered to control protein/peptide conformations using light.
Other phototriggering methods
Besides the methods discussed above, other avenues for generating high free energy conformations in proteins, such as electron transfer55, 56 and ligand dissociation,57, 58 have also found important applications, some of which have been thoroughly reviewed in the past.59, 60 More recently, Tucker et al.61 have shown that disulfonyl-tetrazine could be used as an ultrafast phototrigger of protein conformations because its photodissociation, which yields N2, occurs on the ps timescale with a reasonably high yield.
Probing Methods
The greatest challenge in the study of the molecular mechanism of protein folding is that none of the existing experimental techniques are capable of capturing conformational snapshots of the protein in question along its “folding coordinate” with both high spatial and temporal resolutions. As such, a wide variety of spectroscopic techniques have been developed and used, some of which are continuously being refined, to probe the fine detail of certain regions or features of the underlying folding free energy landscape. Although all of these techniques are worthy of discussion, below we will focus on linear and nonlinear methods based on the application of fluorescence and vibrational spectroscopies. The spectroscopic properties of a particular sample are determined by its polarization, 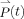 , induced by the electric field of an incoming light beam, E(t), which can be expressed as,62
, induced by the electric field of an incoming light beam, E(t), which can be expressed as,62
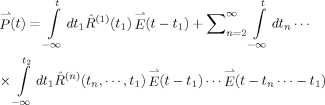 |
(1) |
where 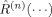 is the nth order response function of the system. If the electric field is sufficiently weak so that only the first term (i.e.,
is the nth order response function of the system. If the electric field is sufficiently weak so that only the first term (i.e., 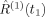 ) in Eq. (1) needs to be considered to describe the underlying light-matter interaction, various linear spectroscopies, such as absorption spectroscopy and fluorescence excitation, result. In contrast, nonlinear spectroscopic techniques require consideration of higher order terms (i.e., n > 1) and typically involve application of ultrafast laser pulses.
) in Eq. (1) needs to be considered to describe the underlying light-matter interaction, various linear spectroscopies, such as absorption spectroscopy and fluorescence excitation, result. In contrast, nonlinear spectroscopic techniques require consideration of higher order terms (i.e., n > 1) and typically involve application of ultrafast laser pulses.
Native fluorescence
Three aromatic amino acids, that is, tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe), are fluorescent and thus could be used to probe protein conformational changes. In practice, however, only Trp fluorescence is extensively used in protein folding studies due to its sensitivity to the environment and its larger quantum yield. Because many reviews63–67 on Trp fluorescence and its application to protein conformational and folding studies exist in the literature, below we focus the remainder of our discussion on fluorescent methods utilizing nonnative fluorophores.
Nonnative fluorescence
Recently, we have shown that the unnatural amino acid, p-cyanophenylalanine (PheCN), is a very useful fluorescent probe of protein folding and binding.68 The absorption spectrum of PheCN exhibits two broad bands, one centered around 240 nm and the other one centered around 280 nm, whereas its fluorescence spectrum peaks at ∼295 nm and is insensitive to excitation wavelength and solvent.69 However, the fluorescence quantum yield (QF) of PheCN is sensitive to its immediate environment (e.g., in water QF = 0.11, whereas in acetonitrile QF = 0.03), making it an ideal fluorescent probe of protein folding and binding transitions.70, 71 For example, using this probe, Tang et al.72 have investigated the binding/insertion/dimerization kinetics of a transmembrane peptide. As shown (Fig. 1), their results showed that when placed at the same position PheCN is a more sensitive fluorescent reporter of the insertion and dimerization processes than Trp.
Figure 1.
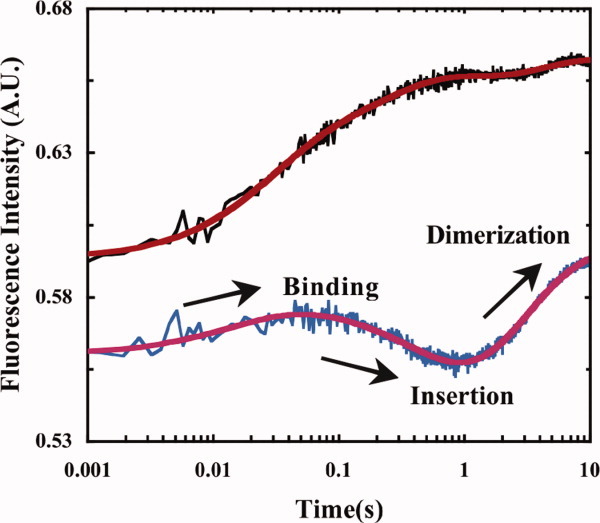
Stopped-flow kinetics of a transmembrane peptide on association with a model membrane probed via Trp (black) and PheCN (blue) fluorescence, with the latter exhibiting greater sensitivity to the key kinetic events. (Adapted from Tang J, et al., (2009) J Am Chem Soc 131:3816–3817, with permission from American Chemical Society.)72
Moreover, Serrano et al.69 have shown that the fluorescence of PheCN exhibits single-exponential decay kinetics with a lifetime (τF) of 5–8 ns, depending on the solvent. In particular, they found that for a series of protic solvents τF increases with increasing hydrogen bonding strength of the solvent, due to a decrease in the nonradiative rate of the excited state. These findings further indicate that when combined with time-resolved lifetime measurements (see below), PheCN fluorescence could be used to probe the conformational distribution of proteins under native or nonnative conditions.
Fluorescence resonance energy transfer
Modulation of the fluorescence intensity of a fluorophore of interest by another molecular moiety (or quencher) through various mechanisms73 has been widely used as a means to measure the proximity of the fluorophore to the quencher. For protein molecules, such modulations have been used to report on conformations and conformational changes as a function of various physical variables (e.g., time, temperature, or denaturant concentration). When fluorescence quenching arises solely from fluorescence resonance energy transfer (FRET), distance information may be obtained. According to Förster theory,74 the mean FRET efficiency of a fluctuating biopolymer, 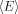 , can be expressed as:
, can be expressed as:
| (2) |
where r is the separation distance between the donor and acceptor, P(r) is the equilibrium distribution function of the donor–acceptor separation distances, and R0 is the Förster radius75 defined as:
| (3) |
where NA, η, and QD are the Avogadro constant, the refractive index of the solvent, the quantum yield of the donor, respectively, whereas κ describes the relative orientation between the transition dipole moments of the donor and acceptor. J is the overlap integral of the donor fluorescence spectrum and acceptor absorption spectrum.
FRET measurements can be used to determine folding/unfolding thermodynamics. For example, for a simple two-state system, it can be easily shown that the mean energy transfer efficiency as a function of a denaturation variable, x (e.g., temperature, urea concentration, etc.), is
| (4) |
where 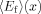 and
and 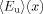 are the denaturant dependent transfer efficiency for the folded and unfolded state, respectively, and Keq is the equilibrium constant for unfolding. However, in practice caution must be taken when applying FRET to extract any quantitative information from the experimental data as the R0 of the FRET pair used may show a strong dependence on x and, in many cases, the accurate determination of is rather difficult.76
are the denaturant dependent transfer efficiency for the folded and unfolded state, respectively, and Keq is the equilibrium constant for unfolding. However, in practice caution must be taken when applying FRET to extract any quantitative information from the experimental data as the R0 of the FRET pair used may show a strong dependence on x and, in many cases, the accurate determination of is rather difficult.76
Recently, we suggested a simpler approach77 to determine folding/unfolding thermodynamics from FRET denaturation data based on the ratio of the integrated areas of the donor and acceptor fluorescence spectra in the case where the donor quantum efficiency is invariant as a function of unfolding, that is,
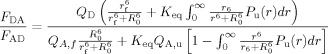 |
(5) |
where r is the donor-to-acceptor separation distance, QD is the quantum efficiency of the donor, and QA,f and QA,u are the quantum efficiencies of the acceptor in the folded and unfolded states, respectively. The above expression can be further simplified if it is assumed that P(r) = δ(r) holds for both the folded and unfolded state ensembles, yielding two distance parameters rf and ru. Provided that the acceptor can be selectively excited in the presence of the donor, the normalized fluorescence quantum yield of the acceptor as a function of the denaturant (e.g., urea) can be determined:
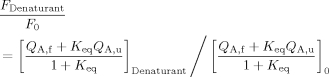 |
(6) |
where F0 is the integrated fluorescence intensity of the acceptor in the absence of denaturant. The quantum yield of the acceptor and the folding free energy are furthermore assumed to be linear functions of denaturant concentration:
| (7) |
| (8) |
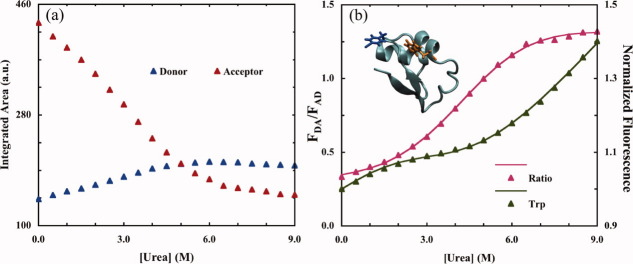
The six fitting parameters (i.e., ΔG°(0), m, 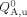 , rf, ru, and q) in Eqs. (5–(8) are used to globally fit the measured curves of FDA/FAD and FDenaturant/F0 as a function of urea concentration x. As shown (Fig. 2), using this approach Glasscock et al.77 showed that the free energy of unfolding as well as the donor-to-acceptor separation distance in the folded state of the LysM domain were in good agreement with those found by alternative spectroscopic methods,77, 78 even under the simplifying assumption that P(r) is a delta function in the unfolded state.
, rf, ru, and q) in Eqs. (5–(8) are used to globally fit the measured curves of FDA/FAD and FDenaturant/F0 as a function of urea concentration x. As shown (Fig. 2), using this approach Glasscock et al.77 showed that the free energy of unfolding as well as the donor-to-acceptor separation distance in the folded state of the LysM domain were in good agreement with those found by alternative spectroscopic methods,77, 78 even under the simplifying assumption that P(r) is a delta function in the unfolded state.
Figure 2.
(a) PheCN-Trp FRET data obtained with the LysM domain (pdb code: 1E0G) and (b) the corresponding FDA to FAD ratios at different urea concentrations. The locations of the PheCN (blue) and Trp (orange) are shown in the inset of (b). Also shown in (b) is the normalized fluorescence intensity of Trp, measured on direct excitation at 290 nm. Smooth lines are global fits to these data according to the methods discussed in the text, yielding the following parameters: rf = 13.0 Å, ru = 16.4 Å, and ΔG0(0) = 1.4 kcal/mol. The latter is comparable to that (1.2 kcal/mol) determined via CD spectroscopy.77 (Adapted from Glasscock JM, et al., (2008) Biochemistry 47:11070–12076, with permission from American Chemical Society.)
Another interesting application of FRET is its use in extracting chain dynamics of biopolymers. For such studies, however, it is most useful to choose a donor with a long fluorescence lifetime, as backbone motions that move the FRET pair closer or farther apart modulate the energy transfer rate, kT, according to:
| (9) |
where τD is the donor fluorescence lifetime in the absence of acceptor. Thus, long-range intrachain diffusive motions lead to complex, nonsingle-exponential decay of the donor fluorescence. The time dependent probability of finding a donor in its excited state at a distance r from the acceptor can be described by the following equation79, 80:
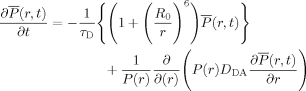 |
(10) |
with 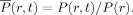 The first term in Eq. (10) represents the distance dependence of the FRET signal, whereas the second term accounts for intrachain diffusive motions with a diffusion coefficient DAD. To probe both the equilibrium distance distribution, P(r), and DAD for flexible biopolymers, one can measure the donor fluorescence decay while varying one or more properties such as τD or the solvent viscosity. For example, by using the naphthalene/dansyl and pyrene/dansyl FRET pairs, Kiefhaber and coworkers81 used the latter approach to probe the end-to-end distance distribution and chain dynamics of poly(Gly–Ser) chains. The fluorescence lifetimes of naphthalene (∼36.9 ns) and pyrene (∼225.5 ns) dyes in water are significantly different, but their Förster radii are very similar, with 23.3 and 20.5 Å for the naphthalene/dansyl and pyrene/dansyl FRET pairs, respectively. Thus, by fitting the experimental data to Eq. (10) and using a model for P(r), information about both the distance distribution and DAD were determined.
The first term in Eq. (10) represents the distance dependence of the FRET signal, whereas the second term accounts for intrachain diffusive motions with a diffusion coefficient DAD. To probe both the equilibrium distance distribution, P(r), and DAD for flexible biopolymers, one can measure the donor fluorescence decay while varying one or more properties such as τD or the solvent viscosity. For example, by using the naphthalene/dansyl and pyrene/dansyl FRET pairs, Kiefhaber and coworkers81 used the latter approach to probe the end-to-end distance distribution and chain dynamics of poly(Gly–Ser) chains. The fluorescence lifetimes of naphthalene (∼36.9 ns) and pyrene (∼225.5 ns) dyes in water are significantly different, but their Förster radii are very similar, with 23.3 and 20.5 Å for the naphthalene/dansyl and pyrene/dansyl FRET pairs, respectively. Thus, by fitting the experimental data to Eq. (10) and using a model for P(r), information about both the distance distribution and DAD were determined.
Although FRET pairs based on fluorescent dyes have been extensively used in biological and biophysical studies, applying them to studies involving natively folded proteins could be problematic as they tend to be large, thus greatly destabilizing and possibly altering their folded states. Thus, we and others have developed a range of FRET pairs that are amino acid-like in nature, and thus minimally perturb the energetic and structural properties of the protein and peptide systems in question.68, 71, 82–85 As shown (Table I), these FRET pairs are mostly analogs of amino acid sidechains, making them ideally suited for probing conformational distributions and transitions of peptides and small proteins, as well as conformational changes occurring over a relatively short distance. Moreover, and perhaps more importantly, these FRET pairs could be used as a starting point for constructing multi-FRET systems, allowing more structural information to be extracted from a single FRET experiment. For example, Rogers et al.84 have shown that PheCN, Trp, and 7-azatryptophan (7AW) can be used together to form a two-step FRET system and have utilized it to investigate the denaturant induced unfolding of the WW domain.
Table I.
Unnatural Amino Acid-Based FRET Pairs
| Donor | Acceptor | R0 (Å) | Ref. |
|---|---|---|---|
| p-Cyanophenylalanine | Tryptophan | 16.0 | 68 |
| p-Cyanophenylalanine | Tyrosine | 12.0 | 71 |
| p-Cyanophenylalanine | 5-Hydroxytryptophan | 18.5 | 84 |
| p-Cyanophenylalanine | 5-Cyanotryptophan | n.d. | 82 |
| p-Ethynylphenylalanine | Tryptophan | n.d. | 83 |
| p-Cyanophenylalanine | 7-Azatryptophan | 18.5 | 84 |
| p-Cyanophenylalanine | Thioamide | 15.6 | 85 |
Triplet state and fluorescence quenching
Similar to FRET, other types of quenching mechanisms that lead to shortening of either the excited singlet or triplet state lifetimes have also found very important applications in protein folding studies. Unlike FRET, however, such quenching processes occur only over a very short distance, that is, typically only on formation of van der Waals contacts between the chromophore and quencher.86, 87 For example, for a simple loop closure process (i.e., open (O) to closed (C) state), the kinetics of quenching can be described by the following scheme:
where kTT is the quenching rate in the closed state, whereas kop and kcl are the intrinsic opening and closing rates of the loop. It can be shown that if the rate of quenching is much larger than the rate of opening (i.e., 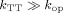 ) then the observed rate constant corresponds to the rate of closing. Several research groups have utilized such quenching methods,88–90 especially quenchers of the triplet state of Trp (Table II), to investigate peptide loop closure rates,89–91 native state conformational fluctuations,92 and global cooperative folding/unfolding transitions.93
) then the observed rate constant corresponds to the rate of closing. Several research groups have utilized such quenching methods,88–90 especially quenchers of the triplet state of Trp (Table II), to investigate peptide loop closure rates,89–91 native state conformational fluctuations,92 and global cooperative folding/unfolding transitions.93
Table II.
Tryptophan Triplet State Quenchers
Recently, fluorescence correlation spectroscopy (FCS) has become increasingly used as a tool to investigate the dynamics of protein folding and conformational changes.94–102 FCS is based on correlating the fluorescence intensity fluctuations of the molecular species in question induced by either a fluorescence quenching event or by molecular diffusion in and out of a small confocal laser excitation volume. For example, Sauer and coworkers103, 104 have shown that the fluorescence of oxazine dyes can be effectively quenched by Trp through the mechanism of photoinduced electron transfer (PET), making them ideal for use as probes of peptide and protein dynamics by means of FCS105, 106 More recently, Rogers et al.107 have applied the PET-FCS technique, wherein the fluorescence of the dye Atto 655 is effectively quenched by nearby Trp residues, to reveal the conformational dynamics of a truncated version (M2TM) of the Influenza A M2 proton channel in model membranes and found that the conformational transition between the N-terminally open and C-terminally open states of the M2TM channel occurs on a timescale of about 500 μs.
Linear IR spectroscopy
Vibrational transitions in condensed phase occur on fs and/or ps timescales, thus IR spectroscopy can be used to investigate ultrafast conformational events of biological molecules. For proteins and peptides, IR bands arising from backbone vibrations are particularly useful as they show varying degrees of sensitivity to conformations.108 Due to having the largest intensity, however, the amide I band, which originates mostly from the stretching vibrations of backbone carbonyls, is the most widely used as a probe of protein folding.109–116 The conformational sensitivity of the backbone amide vibrational transitions arises from anharmonic interactions between the different vibrators located on different amide groups.117, 118 The underlying theoretical framework is often represented in terms of an N-dimensional Hamiltonian written in the basis of the N number of free floating vibrators located at the positions of the corresponding backbone amide groups:
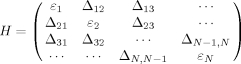 |
(11) |
where εi is the unperturbed frequency of the ith oscillator, and Δij is the interaction energy between the ith and jth vibrators. Although experimentally determining Δij is not straightforward, it is generally assumed that long range electrostatic interactions play a dominant role, while through-bond contributions may be important for nearest neighbor terms.119, 120 Because both εi and Δij are sensitive to various environmental and structural factors, the resulting IR band shows a dependence on the protein conformation and hydration status.121, 122 For example, in D2O the amide I′ band of solvated α-helices is centered at ∼1630 cm−1,11 whereas that of antiparallel β-sheets consists of two bands at ∼1620 cm−1 (strong) and ∼1680 cm−1 (weak),117 respectively.
In conjunction with laser-induced T-jump techniques, time-resolved IR measurements have significantly enhanced our understanding of the folding dynamics of protein secondary structures in isolation123, 124 and in various mini-proteins.125–127 For example, Dyer and coworkers7 are among the first to show that, following a T-jump, the conformation relaxation of short alanine-based α-helical peptides, probed by IR spectroscopy, occurs on the ns timescale. In addition, application of the T-jump IR technique to the study of β-hairpin folding dynamics has allowed us to substantiate the idea that turn formation is the rate limiting step in β-hairpin folding128–130 and helix–turn–helix formation.131, 132 More recently, we have shown, using the mini-protein Trp-cage133, 134 as an example (Fig. 3), that T-jump IR measurements carried out using different probing frequencies within the amide I′ band of the protein of interest can reveal important information regarding the individual folding rates of the constituent structural elements.127
Figure 3.
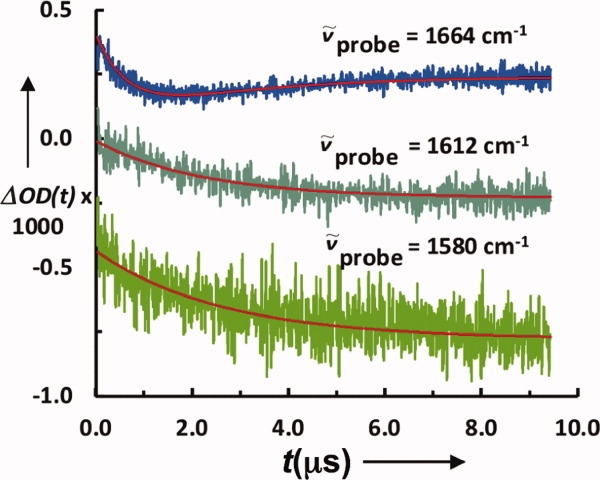
Conformational relaxation kinetics of a Trp-cage in response to a T-jump obtained with different probing frequencies, showing that folding intermediates or kinetic heterogeneity could be revealed by recording transient IR kinetics over the entire amide I′ band region. (Adapted from Culik RM, et al., (2011) Angew Chem Int Ed 50:10884–10887, with permission from John Wiley and Sons.)127
Strategies to enhance the structural resolution of IR spectroscopy
Although the aforementioned couplings among different amide vibrators provide the intrinsic sensitivity of the amide I vibration to protein conformation, it also causes the transition to broaden. In addition, the amide I frequencies of different secondary structures are not well separated. As a result, the amide I band of proteins is often broad, featureless, and congested with overlapping peaks. Thus, to gain site-specific structural information, certain strategies need to be used. One such strategy is to alter the reduced mass and hence the vibrational frequency of the vibrator of interest using isotopic substitution.135–138 For example, assuming that the backbone C=O stretching vibration is harmonic, simply changing 12C to 13C would lead to a decrease in its vibration frequency by approximately 40 cm−1, making it possible to separate the 13C=O vibration from a background arising from unlabeled (i.e., 12C=O) vibrators (Fig. 4). Therefore, the technique of isotope editing has been widely used in protein folding and aggregation studies to gain a better structural resolution when used in conjunction with IR spectroscopy.113, 139
Figure 4.
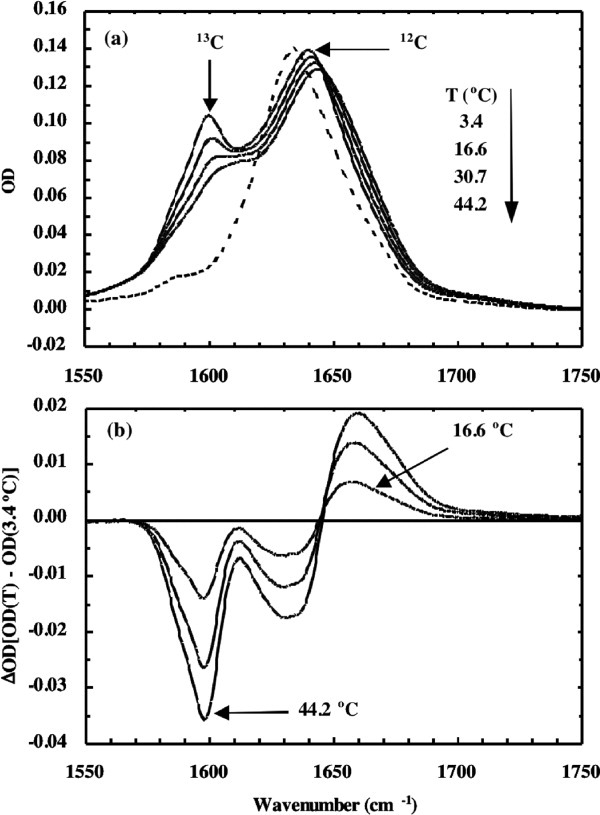
(a) FTIR spectra of a 13C labeled (solid lines) in D2O solution measured at different temperatures and (b) the corresponding difference FTIR spectra. Also shown in (a) is the FTIR spectrum (dashed line) of the unlabeled peptide. (Reprinted from Huang CY, et al., (2001) J Am Chem Soc 123:12111–12112, with permission from American Chemical Society.)139
In addition to backbone vibrations, many sidechain vibrations are also expected to be useful IR markers of protein conformations. However, sidechain vibrations are much less used in protein folding studies due in part to their smaller molar extinction coefficients. Exceptions include the carboxylate asymmetric stretching vibrations of the Glu and Asp sidechains, which possess strong absorptions appearing near 1567 and 1584 cm−1, respectively.114 On protonation of these sidechains at low pH, the corresponding vibrations shift to near 1700 cm−1. Thus, these sidechain absorptions could be used as sensitive IR probes of local pH as well as salt bridge and ion pair formations. Moreover, we and others have recently demonstrated a novel method to enhance the structural resolution in IR studies by using unnatural amino acids as site-specific environmental and conformational reporters. Interested readers can find more details of this topic in a recent review.140
UV resonance Raman
Protein vibrational transitions can also be probed via Raman spectroscopy. It has been shown that excitation of an electronic transition of the polypeptide using deep UV photons, for example the π → π* transition of the amide backbone or the 1Lb transition of Trp residues, allows resonantly enhanced Raman spectra to be collected.141–145 In comparison to IR measurements, which often suffer from interference of solvent vibrational bands in the amide II and III regions, the UV resonance Raman (UVRR) technique can greatly reduce solvent interferences, thus allowing for more easy recording of protein vibrational bands below 1600 cm−1, for example, the amide III band and the Cα—H stretching vibration. The ability to reliably record protein vibrational spectrum in the lower wavenumber region makes UVRR a very useful tool of reporting on protein conformations. For example, Asher et al.146 have proposed that the amide III peak frequency shows a roughly sinusoidal dependence on the backbone Psi angle, making a more quantitative interpretation of the vibrational spectrum possible. In addition, UVRR has also been used in conjunction with the laser-induced T-jump technique to study protein and peptide folding kinetics.147–151 Further details on the application of UVRR to protein folding studies can be found in a recent review by Asher and coworkers.145
Nonlinear IR methods
Probing the nonlinear response of a protein sample induced by a series of ultrashort laser pulses can provide additional dynamic and structural information that cannot be directly acquired via linear spectroscopic measurements. In particular, 2-dimensional infrared (2D-IR) spectroscopy has proven to be very useful in generating new insights into the ultrafast conformational and solvation dynamics of proteins and peptides152–154 and is expected to play a growing role in studying the protein folding problem.
Because a detailed description of the 2D-IR technique can be found elsewhere,155 below we offer only a brief description. As shown (Fig. 5), one common method for generating 2D-IR spectra makes use of four ultrashort (<100 fs), phase locked, IR laser pulses that may have different polarizations and are delayed in time with respect to each other. The first pulse (k1) is used to promote the sample vibration into a superposition of the ground and excited states (or to produce a coherence), which is intersected by a second pulse (k2) at a certain time delay, τ, generating a transient population grating that is then allowed to interact with a third pulse (k3) after a delay time T, stimulating emission of a photon echo signal.156 The magnitude and phase of the photon echo are then determined through interference with a fourth pulse, the local oscillator (LO), along the detection time axis, t. Finally, Fourier analysis of this signal along the two time axes, τ and t, produces the 2D-IR spectrum, which is a function of two frequencies, ωτ and ωt, and the mixing time T. One of the advantages of 2D-IR spectroscopy is that it allows one to spread the spectrum of interest into two dimensions, thus effectively increasing the spectral resolution by revealing features that overlap, cancel, or simply do not exist in linear IR spectra.157 As shown (Fig. 6), a 2D-IR spectrum can also reveal cross correlations between frequencies that go undetected in linear IR spectroscopy, thus providing additional structural information.156 Moreover, by following the evolution of the spectral line-widths in 2D spectra as a function of T, one can measure ultrafast (ps) dynamics due to solvent or peptide motions.158 Additionally, 2D-IR signals show a fourth-power dependence on the underlying vibrational transition dipole moment (i.e., proportional to μ4), allowing for enhanced discrimination of overlapping IR bands.159
Figure 5.
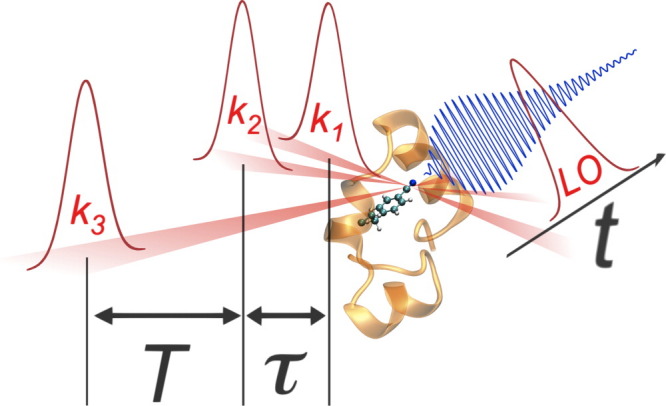
Schematic representation of the 2D IR photon echo experiment. Three ultrashort IR pulses (k1, k2 and k3) interact in succession with a protein sample to produce a photon echo signal (depicted in blue), which is analyzed along the time axis t by overlapping with a reference pulse (LO).
Figure 6.
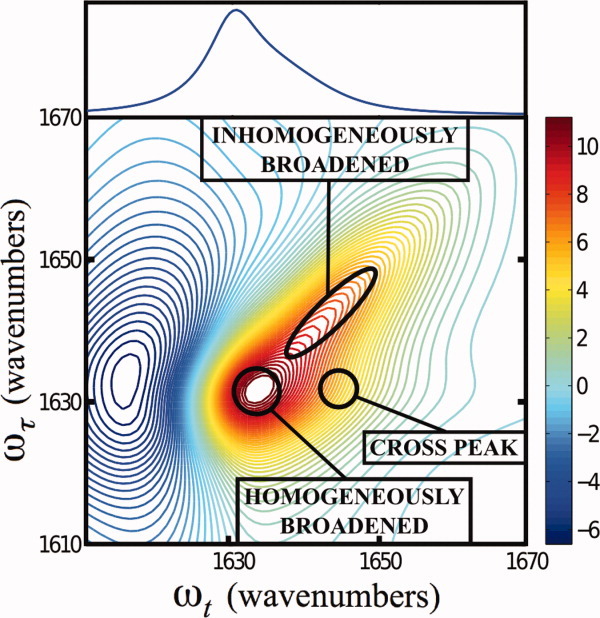
A representation of the 2D-IR photon echo spectrum (bottom) for two interacting anharmonic vibrators, demonstrating characteristic features that distinguish it from the linear IR spectrum (top) such as the appearance of well-defined homogeneously or inhomogeneously broadened peaks, as well as cross peaks arising from coupling.
Taken together, these added spectral and dynamic details contained in the nonlinear response of a protein makes 2D-IR spectroscopy a more sensitive structural tool for studying protein folding, conformational transitions, and local hydration status, among many other applications. For example, Hochstrasser and coworkers160 have used the 2D-IR technique to study the conformational heterogeneity of HP35 via the C≡N (nitrile) stretching vibration of PheCN incorporated into the hydrophobic core of the protein. It was found that the 2D-IR spectra could only be described by two nearly overlapping distributions of vibrators that do not interconvert on a timescale similar to the range of T (0–3 ps). Because the nitrile stretching vibration is sensitive to hydration, this finding provided strong evidence that in the native potential well of HP35 two or more slowly interconverting subpopulations exist. A similar conclusion was also arrived at by a triplet–triplet state quenching study.92
Although much progress is left to be made in the implementation of nonlinear spectroscopic methods such as 2D-IR spectroscopy to protein folding studies, several previous works stand out so far as showing their potential usefulness. For example, a large amount of effort has been made to characterize the 2D-IR spectral features of different secondary structural motifs,161–165 laying the foundation for interpreting 2D-IR data of more complex protein systems. In addition, a small number of protein folding kinetic studies46, 166–168 have demonstrated that when combined with other resolution enhancement strategies, such as isotope-editing, 2D-IR spectroscopy is capable of revealing details regarding the folding/unfolding dynamics of the protein system of interest that are inaccessible from linear spectroscopy methods. With the development of other light-induced triggering methods,64 and continuing refinement of the experimental methods,169 we expect that multidimensional nonlinear spectroscopies will serve as critical tools in providing new mechanistic insights into protein folding dynamics.
Summary
The problem of protein folding has inspired a vast variety of studies by scientists from different disciplines in the past and continues to stimulate the development of new experimental methods that can measure and report, with as much structural detail as possible, on how and on what range of timescales specific and/or nonspecific conformational transitions occur. In particular, the development and application of various ultrafast triggering methods, such as the laser-induced T-jump technique, have made it possible to directly characterize the folding kinetics and mechanism of protein secondary structures and proteins that fold on the nanosecond and microsecond timescales. In addition, and perhaps more importantly, the recent advances in vibrational and optical spectroscopies hold great promise for disentangling and even “imaging” the complex network of molecular interactions involved in protein folding. Herein, we provide a concise summary of several recent developments in spectroscopic studies of protein folding, with the hope that it will provide useful information to those interested in applying some of the aforementioned methods to protein conformational studies. Due to page limit, however, we are unable to discuss several other deserving spectroscopic methods, such as those based on single-molecule fluorescence and/or FRET measurements. Finally, it is worth noting that many of those spectroscopic methods discussed are not limited to protein folding studies, they can also be applied to investigate other biophysical and biological questions, such as aggregation,170–173 membrane protein conformation and dynamics,174–178 peptide–peptide interactions, RNA and DNA dynamics,179–182 and structure determination.154
References
- 1.Anfinsen CB. Principles that govern folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 2.Chan CK, Hu Y, Takahashi S, Rousseau DL, Eaton WA, Hofrichter J. Submillisecond protein folding kinetics studied by ultrarapid mixing. Proc Natl Acad Sci USA. 1997;94:1779–1784. doi: 10.1073/pnas.94.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roder H, Maki K, Cheng H. Early events in protein folding explored by rapid mixing methods. Chem Rev. 2006;106:1836–1861. doi: 10.1021/cr040430y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitzan A. Chemical dynamics in condensed phases: relaxation, transfer, and reactions in condensed molecular systems. New York: Oxford University Press Inc; 2006. [Google Scholar]
- 5.Beitz JV, Flynn GW, Turner DH, Sutin N. Stimulated Raman effect—a new source of laser temperature-jump heating. J Am Chem Soc. 1970;92:4130–7296. [Google Scholar]
- 6.Phillips CM, Mizutani Y, Hochstrasser RM. Ultrafast thermally-induced unfolding of RNase-A. Proc Natl Acad Sci USA. 1995;92:7292–7296. doi: 10.1073/pnas.92.16.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams S, Causgrove TP, Gilmanshin R, Fang KS, Callender RH, Woodruff WH, Dyer RB. Fast events in protein folding: helix melting and formation in a small peptide. Biochemistry. 1996;35:691–697. doi: 10.1021/bi952217p. [DOI] [PubMed] [Google Scholar]
- 8.Ballew RM, Sabelko J, Gruebele M. Direct observation of fast protein folding: the initial collapse of apomyoglobin. Proc Natl Acad Sci USA. 1996;93:5759–5764. doi: 10.1073/pnas.93.12.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PA, Eaton WA, Hofrichter J. Laser temperature jump study of the helix reversible arrow coil kinetics of an alanine peptide interpreted with a ‘kinetic zipper’ model. Biochemistry. 1997;36:9200–9210. doi: 10.1021/bi9704764. [DOI] [PubMed] [Google Scholar]
- 10.Gruebele M, Sabelko J, Ballew R, Ervin J. Laser temperature jump induced protein refolding. Acc Chem Res. 1998;31:699–707. [Google Scholar]
- 11.Dyer RB, Gai F, Woodruff WH. Infrared studies of fast events in protein folding. Acc Chem Res. 1998;31:709–716. [Google Scholar]
- 12.Mohammed OF, Jas GS, Lin MM, Zewail AH. Primary peptide folding dynamics observed with ultrafast temperature jump. Angew Chem Int Ed. 2009;48:562856–562832. doi: 10.1002/anie.200900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzler R, Klafter J, Jortner J, Volk M. Multiple time scales for dispersive kinetics in early events of peptide folding. Chem Phys Lett. 1998;293:477–484. [Google Scholar]
- 14.Wang J, El-Sayed MA. Temperature jump-induced secondary structural change of the membrane protein bacteriorhodopsin in the premelting temperature region: a nanosecond time-resolved Fourier transform infrared study. Biophys J. 1999;76:2777–2783. doi: 10.1016/S0006-3495(99)77431-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansari A, Kuznetsov SV, Shen Y. Configurational diffusion down a folding funnel describes the dynamics of DNA hairpins. Proc Natl Acad Sci USA. 2001;98:7771–7776. doi: 10.1073/pnas.131477798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu L, Pabit SA, Roitberg AE, Hagen SJ. Smaller and faster: the 20-residue trp-cage protein folds in 4 μs. J Am Chem Soc. 2002;124:12952–12953. doi: 10.1021/ja0279141. [DOI] [PubMed] [Google Scholar]
- 17.Mayor U, Guydosh NR, Johnson CM, Grossmann JG, Sato S, Jas GS, Freund SMV, Alonso DOV, Daggett V, Fersht AR. The complete folding pathway of a protein from nanoseconds to microseconds. Nature. 2003;421:863–867. doi: 10.1038/nature01428. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson N, Fersht AR. Early events in protein folding. Curr Opin Struct Biol. 2003;13:75–81. doi: 10.1016/s0959-440x(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 19.Sadqi M, Lapidus LJ, Munoz V. How fast is protein hydrophobic collapse? Proc Natl Acad Sci USA. 2003;100:12117–12122. doi: 10.1073/pnas.2033863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz V, Ghirlando R, Blanco FJ, Jas GS, Hofrichter J, Eaton WA. Folding and aggregation kinetics of a β-hairpin. Biochemistry. 2006;45:7023–7035. doi: 10.1021/bi052556a. [DOI] [PubMed] [Google Scholar]
- 21.Callender R, Dyer RB. Advances in time-resolved approaches to characterize the dynamical nature of enzymatic catalysis. Chem Rev. 2006;106:3031–3042. doi: 10.1021/cr050284b. [DOI] [PubMed] [Google Scholar]
- 22.Khuc MT, Mendonca L, Sharma S, Solinas X, Volk M, Hache F. Measurement of circular dichroism dynamics in a nanosecond temperature-jump experiment. Rev Sci Instrum. 2011;82:054302. doi: 10.1063/1.3592331. [DOI] [PubMed] [Google Scholar]
- 23.Buchner GS, Murphy RD, Buchete N, Kubelka J. Dynamics of protein folding: probing the kinetic network of folding–unfolding transitions with experiment and theory. Biochim Biophys Acta. 2011;1814:1001–1020. doi: 10.1016/j.bbapap.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Lednev IK, Ye TQ, Hester RE, Moore JN. Femtosecond time-resolved UV-visible absorption spectroscopy of trans-azobenzene in solution. J Phys Chem. 1996;100:13338–13341. [Google Scholar]
- 25.Nagele T, Hoche R, Zinth W, Wachtveitl J. Femtosecond photoisomerization of cis-azobenzene. Chem Phys Lett. 1997;272:489–495. [Google Scholar]
- 26.Kumita JR, Smart OS, Woolley GA. Photo-control of helix content in a short peptide. Proc Natl Acad Sci USA. 2000;97:3803–3808. doi: 10.1073/pnas.97.8.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flint DG, Kumita JR, Smart OS, Woolley GA. Using an azobenzene cross-linker to either increase or decrease peptide helix content upon trans-to-cis photoisomerization. Chem Biol. 2002;9:391–397. doi: 10.1016/s1074-5521(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 28.Renner C, Kusebauch U, Loweneck M, Milbradt AG, Moroder L. Azobenzene as photoresponsive conformational switch in cyclic peptides. J Peptide Res. 2005;65:4–14. doi: 10.1111/j.1399-3011.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 29.Wachtveitl J, Sporlein S, Satzger H, Fonrobert B, Renner C, Behrendt R, Oesterhelt D, Moroder L, Zinth W. Ultrafast conformational dynamics in cyclic azobenzene peptides of increased flexibility. Biophys J. 2004;86:2350–2362. doi: 10.1016/S0006-3495(04)74292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beharry AA, Woolley GA. Azobenzene photoswitches for biomolecules. Chem Soc Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 31.Hamm P, Helbing J, Bredenbeck J. Two-dimensional infrared spectroscopy of photoswitchable peptides. Annu Rev Phys Chem. 2008;59:291–317. doi: 10.1146/annurev.physchem.59.032607.093757. [DOI] [PubMed] [Google Scholar]
- 32.Backus EHG, Bloem R, Donaldson PM, Ihalainen JA, Pfister R, Paoli B, Caflisch A, Hamm P. 2D-IR study of a photoswitchable isotope-labeled α-helix. J Phys Chem B. 2010;114:3735–3740. doi: 10.1021/jp911849n. [DOI] [PubMed] [Google Scholar]
- 33.Sporlein S, Carstens H, Satzger H, Renner C, Behrendt R, Moroder L, Tavan P, Zinth W, Wachtveitl J. Ultrafast spectroscopy reveals subnanosecond peptide conformational dynamics and validates molecular dynamics simulation. Proc Natl Acad Sci USA. 2002;99:7998–8002. doi: 10.1073/pnas.122238799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bredenbeck J, Helbing J, Kumita JR, Woolley GA, Hamm P. α-Helix formation in a photoswitchable peptide tracked from picoseconds to microseconds by time-resolved IR spectroscopy. Proc Natl Acad Sci USA. 2005;102:2379–2384. doi: 10.1073/pnas.0406948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ihalainen JA, Paoli B, Muff S, Backus EHG, Bredenbeck J, Woolley GA, Caflisch A, Hamm P. α-Helix folding in the presence of structural constraints. Proc Natl Acad Sci USA. 2008;105:9588–9593. doi: 10.1073/pnas.0712099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie S, Natansohn A, Rochon P. Recent developments in aromatic azo polymers research. Chem Mater. 1993;5:403–411. [Google Scholar]
- 37.Tamai N, Miyasaka H. Ultrafast dynamics of photochromic systems. Chem Rev. 2000;100:1875–1890. doi: 10.1021/cr9800816. [DOI] [PubMed] [Google Scholar]
- 38.Natansohn A, Rochon P. Photoinduced motions in azo-containing polymers. Chem Rev. 2002;102:4139–4175. doi: 10.1021/cr970155y. [DOI] [PubMed] [Google Scholar]
- 39.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 40.Haines LA, Rajagopal K, Ozbas B, Salick DA, Pochan DJ, Schneider JP. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc. 2005;127:17025–17029. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirota S, Fujimoto Y, Choi J, Baden N, Katagiri N, Akiyama M, Hulsker R, Ubbink M, Okajima T, Takabe T, Funasaki N, Watanabe Y, Terazima M. Conformational changes during apoplastocyanin folding observed by photocleavable modification and transient grating. J Am Chem Soc. 2006;128:7551–7558. doi: 10.1021/ja058788e. [DOI] [PubMed] [Google Scholar]
- 42.Sankaranarayanan J, Muthukrishnan S, Gudmundsdottir AD. Photoremovable protecting groups based on photoenolization. Adv Phys Org Chem. 2009;43 43:39–77. [Google Scholar]
- 43.Chen H, Hsu JC, Man Hoang Viet, Li MS, Hu C, Liu C, Luh FY, Chen SS, Chang ES, Wang AH, Hsu M, Fann W, Chen RP. Studying submicrosecond protein folding kinetics using a photolabile caging strategy and time-resolved photoacoustic calorimetry. Proteins. 2010;78:2973–2983. doi: 10.1002/prot.22823. [DOI] [PubMed] [Google Scholar]
- 44.Hansen KC, Rock RS, Larsen RW, Chan SI. A method for photoinitating protein folding in a nondenaturing environment. J Am Chem Soc. 2000;122:11567–11568. [Google Scholar]
- 45.Lu HSM, Volk M, Kholodenko Y, Gooding E, Hochstrasser RM, DeGrado WF. Aminothiotyrosine disulfide, an optical trigger for initiation of protein folding. J Am Chem Soc. 1997;119:7173–180. [Google Scholar]
- 46.Kolano C, Helbing J, Kozinski M, Sander W, Hamm P. Watching hydrogen-bond dynamics in a β-turn by transient two-dimensional infrared spectroscopy. Nature. 2006;444:469–472. doi: 10.1038/nature05352. [DOI] [PubMed] [Google Scholar]
- 47.Kolano C, Helbing J, Bucher G, Sander W, Hamm P. Intramolecular disulfide bridges as a phototrigger to monitor the dynamics of small cyclic peptides. J Phys Chem B. 2007;111:11297–11302. doi: 10.1021/jp074184g. [DOI] [PubMed] [Google Scholar]
- 48.Waegele MM. 2011. On the folding and conformation of peptides and the development of novel methods for their study. Publicly accessible Penn Dissertations.
- 49.Prompers JJ, Hilbers CW, Pepermans HAM. Tryptophan mediated photoreduction of disulfide bond causes unusual fluorescence behavior of Fusarium solani pisi cutinase. FEBS Lett. 1999;456:409–416. doi: 10.1016/s0014-5793(99)00990-4. [DOI] [PubMed] [Google Scholar]
- 50.Neves-Petersen MT, Gryczynski Z, Lakowicz J, Fojan P, Pedersen S, Petersen E, Petersen SB. High probability of disrupting a disulphide bridge mediated by an endogenous excited tryptophan residue. Protein Sci. 2002;11:588–600. doi: 10.1110/ps.06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanhooren A, Devreese B, Vanhee K, Van Beeumen J, Hanssens I. Photoexcitation of tryptophan groups induces reduction of two disulfide bonds in goat α-lactalbumin. Biochemistry. 2002;41:11035–11043. doi: 10.1021/bi0258851. [DOI] [PubMed] [Google Scholar]
- 52.Kehoe JJ, Remondetto GE, Subirade M, Morris ER, Brodkorb A. Tryptophan-mediated denaturation of β-lactoglobulin A by UV irradiation. J Agric Food Chem. 2008;56:4720–4725. doi: 10.1021/jf0733158. [DOI] [PubMed] [Google Scholar]
- 53.Wu LZ, Sheng YB, Xie JB, Wang W. Photoexcitation of tryptophan groups induced reduction of disulfide bonds in hen egg white lysozyme. J Mol Struct. 2008;882:101–106. [Google Scholar]
- 54.Neves-Petersen MT, Klitgaard S, Pascher T, Skovsen E, Polivka T, Yartsev A, Sundstrom V, Petersen SB. Flash photolysis of cutinase: identification and decay kinetics of transient intermediates formed upon UV excitation of aromatic. Biophys J. 2009;97:211–226. doi: 10.1016/j.bpj.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mines GA, Pascher T, Lee SC, Winkler JR, Gray HB. Cytochrome c folding triggered by electron transfer. Chem Biol. 1996;3:491–497. doi: 10.1016/s1074-5521(96)90097-6. [DOI] [PubMed] [Google Scholar]
- 56.Gray HB, Winkler JR. Electron transfer in proteins. Annu Rev Biochem. 1996;65:537–561. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- 57.Ansari A, Berendzen J, Bowne SF, Frauenfelder H, Iben IE, Sauke TB, Shyamsunder E, Young RD. Protein states and protein quakes. Proc Natl Acad Sci USA. 1985;82:5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones CM, Henry ER, Hu Y, Chan CK, Luck SD, Bhuyan A, Roder H, Hofrichter J, Eaton WA. Fast events in protein-folding initiated by nanosecond laser photolysis. Proc Natl Acad Sci USA. 1993;90:11860–11864. doi: 10.1073/pnas.90.24.11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen E, Goldbeck RA, Kliger DS. Nanosecond time-resolved spectroscopy of biomolecular processes. Annu Rev Biophys Biomol Struct. 1997;26:327–355. doi: 10.1146/annurev.biophys.26.1.327. [DOI] [PubMed] [Google Scholar]
- 60.Eaton WA, Munoz V, Hagen JS, Jas GS, Lapidus LJ, Henry ER, Hofrichter J. Fast kinetics and mechanisms in protein folding. Annu Rev Biophys Biomol Struct. 2000;29:327–359. doi: 10.1146/annurev.biophys.29.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tucker MJ, Courter JR, Chen J, Atasoylu O, Smith, Amos B, III, Hochstrasser RM. Tetrazine phototriggers: probes for peptide dynamics. Angew Chem Int Ed. 2010;49:3612–3616. doi: 10.1002/anie.201000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukamel S. Principles of nonlinear optical spectroscopy. New York: Oxford Press; 1995. [Google Scholar]
- 63.Chen Y, Barkley MD. Toward understanding tryptophan fluorescence in proteins. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 64.Gruebele M. The fast protein folding problem. Annu Rev Phys Chem. 1999;50:485–516. doi: 10.1146/annurev.physchem.50.1.485. [DOI] [PubMed] [Google Scholar]
- 65.Engelborghs Y. The analysis of time resolved protein fluorescence in multi-tryptophan proteins. Spectrochim Acta Part A. 2001;57:2255–2270. doi: 10.1016/s1386-1425(01)00485-1. [DOI] [PubMed] [Google Scholar]
- 66.Royer CA. Probing protein folding and conformational transitions with fluorescence. Chem Rev. 2006;106:1769–1784. doi: 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- 67.Zhong D. Hydration dynamics and coupled water–protein fluctuations probed by intrinsic tryptophan. Adv Chem Phys. 2009;143:83–149. [Google Scholar]
- 68.Tucker MJ, Oyola R, Gai F. A novel fluorescent probe for protein binding and folding studies: p-cyanophenylalanine. Biopolymers. 2006;83:571–576. doi: 10.1002/bip.20587. [DOI] [PubMed] [Google Scholar]
- 69.Serrano AL, Troxler T, Tucker MJ, Gai F. Photophysics of a fluorescent non-natural amino acid: p-cyanophenylalanine. Chem Phys Lett. 2010;487:303–306. doi: 10.1016/j.cplett.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: a combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J Mol Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taskent-Sezgin H, Marek P, Thomas R, Goldberg D, Chung J, Carrico I, Raleigh DP. Modulation of p-cyanophenylalanine fluorescence by amino acid side chains and rational design of fluorescence probes of α-helix formation. Biochemistry. 2010;49:6290–6295. doi: 10.1021/bi100932p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang J, Yin H, Qiu J, Tucker MJ, DeGrado WF, Gai F. Using two fluorescent probes to dissect the binding, insertion, and dimerization kinetics of a model membrane peptide. J Am Chem Soc. 2009;131:3816–3817. doi: 10.1021/ja809007f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noomnarm U, Clegg RM. Fluorescence lifetimes: fundamentals and interpretations. Photosynthesis Res. 2009;101:181–194. doi: 10.1007/s11120-009-9457-8. [DOI] [PubMed] [Google Scholar]
- 74.Haas E. The study of protein folding and dynamics by determination of intramolecular distance distributions and their fluctuations using ensemble and single-molecule FRET measurements. Chemphyschem. 2005;6:858–970. doi: 10.1002/cphc.200400617. [DOI] [PubMed] [Google Scholar]
- 75.Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer; 1999. [Google Scholar]
- 76.Berney C, Danuser G. FRET or no FRET: a quantitative comparison. Biophys J. 2003;84:3992–4010. doi: 10.1016/S0006-3495(03)75126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glasscock JM, Zhu Y, Chowdhury P, Tang J, Gai F. Using an amino acid fluorescence resonance energy transfer pair to probe protein unfolding: application to the villin headpiece subdomain and the LysM domain. Biochemistry. 2008;47:11070–12076. doi: 10.1021/bi8012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nickson AA, Stoll KE, Clarke J. Folding of a LysM domain: entropy–enthalpy compensation in the transition state of an ideal two-state folder. J Mol Biol. 2008;380:557–569. doi: 10.1016/j.jmb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haas E, Katchalski-Katzir E, Steinberg I. Brownian-motion of ends of oligopeptide chains in solution as estimated by energy-transfer between chain ends. Biopolymers. 1978;17:11–31. [Google Scholar]
- 80.Beechem JM, Haas E. Simultaneous determination of intramolecular distance distributions and conformational dynamics by global analysis of energy-transfer measurements. Biophys J. 1989;55:1225–1236. doi: 10.1016/S0006-3495(89)82918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moglich A, Joder K, Kiefhaber T. End-to-end distance distributions and intrachain diffusion constants in unfolded polypeptide chains indicate intramolecular hydrogen bond formation. Proc Natl Acad Sci USA. 2006;103:12394–12399. doi: 10.1073/pnas.0604748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waegele MM, Tucker MJ, Gai F. 5-Cyanotryptophan as an infrared probe of local hydration status of proteins. Chem Phys Lett. 2009;478:249–253. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyake-Stoner SJ, Miller AM, Hammill JT, Peeler JC, Hess KR, Mehl RA, Brewer SH. Probing protein folding using site-specifically encoded unnatural amino acids as FRET donors with tryptophan. Biochemistry. 2009;48:5953–5962. doi: 10.1021/bi900426d. [DOI] [PubMed] [Google Scholar]
- 84.Rogers JMG, Lippert LG, Gai F. Non-natural amino acid fluorophores for one- and two-step fluorescence resonance energy transfer applications. Anal Biochem. 2010;399:182–189. doi: 10.1016/j.ab.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg JM, Batjargal S, Peterson EJ. Thioamides as fluorescence quenching probes: minimalist chromophores to monitor protein dynamics. J Am Chem Soc. 2010;132:14718–14720. doi: 10.1021/ja1044924. [DOI] [PubMed] [Google Scholar]
- 86.Siegel S, Judeikis HS. Relative interaction radii for quenching of triplet-state molecules. J Chem Phys. 1968;48:1613–1619. [Google Scholar]
- 87.Stone AJ. The theory of intermolecular forces (international series of monographs on chemistry) New York: Oxford University Press Inc; 1997. [Google Scholar]
- 88.Gonnelli M, Strambini GB. Intramolecular quenching of tryptophan phosphorescence in short peptides and proteins. Photochem Photobio. 2009;81:614–622. doi: 10.1562/2004-11-09-RA-367. [DOI] [PubMed] [Google Scholar]
- 89.Lapidus LJ, Eaton WA, Hofrichter J. Measuring the rate of intramolecular contact formation in polypeptides. Proc Natl Acad Sci USA. 2000;97:7220–7225. doi: 10.1073/pnas.97.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krieger F, Fierz B, Bieri O, Drewello M, Kiefhaber T. Dynamics of unfolded polypeptide chains as model for the earliest steps in protein folding. J Mol Biol. 2003;332:265–274. doi: 10.1016/s0022-2836(03)00892-1. [DOI] [PubMed] [Google Scholar]
- 91.Huang F, Nau WM. A conformational flexibility scale for amino acids in peptides. Angew Chem Int Ed. 2003;42:2269–2272. doi: 10.1002/anie.200250684. [DOI] [PubMed] [Google Scholar]
- 92.Reiner A, Henklein P, Kiefhaber T. An unlocking/relocking barrier in conformational fluctuations of villin headpiece subdomain. Proc Natl Acad Sci USA. 2010;107:4955–4960. doi: 10.1073/pnas.0910001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cellmer T, Buscaglia M, Henry ER, Hofrichter J, Eaton WA. Making connections between ultrafast protein folding kinetics and molecular dynamics simulations. Proc Natl Acad Sci USA. 2011;108:6103–6108. doi: 10.1073/pnas.1019552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magde D, Elson E, Webb WW. Thermodynamic fluctuations in a reacting system—measurement by fluorescence correlation spectroscopy. Phys Rev Lett. 1972;29:705–708. [Google Scholar]
- 95.Rigler R, Mets U, Widengren J, Kask P. Fluorescence correlation spectroscopy with high count rate and low-background—analysis of translational diffusion. Eur Biophys J Biophys Lett. 1993;22:169–175. [Google Scholar]
- 96.Purkayastha P, Klemke J, Lavender S, Oyola R, Cooperman BS, Gai F. α1-Antitrypsin polymerization: a fluorescence correlation spectroscopic study. Biochemistry. 2005;44:2642–2649. doi: 10.1021/bi048662e. [DOI] [PubMed] [Google Scholar]
- 97.Werner JH, Joggerst R, Dyer RB, Goodwin PM. A two-dimensional view of the folding energy landscape of cytochrome c. Proc Natl Acad Sci USA. 2006;103:11130–11135. doi: 10.1073/pnas.0604712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chowdhury P, Wang W, Lavender S, Bunagan MR, Klemke JW, Tang J, Saven JG, Cooperman BS, Gai F. Fluorescence correlation spectroscopic study of serpin depolymerization by computationally designed peptides. J Mol Biol. 2007;369:462–473. doi: 10.1016/j.jmb.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo L, Chowdhury P, Glasscock JM, Gai F. Denaturant-induced expansion and compaction of a multi-domain protein: IgG. J Mol Biol. 2008;384:1029–1036. doi: 10.1016/j.jmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherman E, Itkin A, Kuttner YY, Rhoades E, Amir D, Haas E, Haran G. Using fluorescence correlation spectroscopy to study conformational changes in denatured proteins. Biophys J. 2008;94:4819–4827. doi: 10.1529/biophysj.107.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neuweiler H, Johnson CM, Fersht AR. Direct observation of ultrafast folding and denatured state dynamics in single protein molecules. Proc Natl Acad Sci USA. 2009;106:18569–18574. doi: 10.1073/pnas.0910860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sherman E, Haran G. Fluorescence correlation spectroscopy of fast chain dynamics within denatured protein L. Chemphyschem. 2011;12:696–703. doi: 10.1002/cphc.201000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buschmann V, Weston KD, Sauer M. Spectroscopic study and evaluation of red-absorbing fluorescent dyes. Bioconjug Chem. 2003;14:195–204. doi: 10.1021/bc025600x. [DOI] [PubMed] [Google Scholar]
- 104.Doose S, Neuweiler H, Sauer M. A close look at fluorescence quenching of organic dyes by tryptophan. Chemphyschem. 2005;6:2277–2285. doi: 10.1002/cphc.200500191. [DOI] [PubMed] [Google Scholar]
- 105.Neuweiler H, Doose S, Sauer M. A microscopic view of miniprotein folding: enhanced folding efficiency through formation of an intermediate. Proc Natl Acad Sci USA. 2005;102:16650–16655. doi: 10.1073/pnas.0507351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Doose S, Neuweiler H, Sauer M. Fluorescence quenching by photoinduced electron transfer: a reporter for conformational dynamics of macromolecules. ChemPhysChem. 2009;10:1389–1398. doi: 10.1002/cphc.200900238. [DOI] [PubMed] [Google Scholar]
- 107.Rogers JMG, Poishchuk AL, Guo L, Wang J, DeGrado WF, Gai F. Photoinduced electron transfer and fluorophore motion as a probe of the conformational dynamics of membrane proteins: application to the influenza A M2 proton channel. Langmuir. 2011;27:3815–3821. doi: 10.1021/la200480d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silva RAGD, Yasui SC, Kubelka J, Formaggio F, Crisma M, Toniolo C, Keiderling TA. Discriminating 310- from α-helices: vibrational and electronic CD and IR absorption study of related aib-containing oligopeptides. Biopolymers. 2002;65:229–243. doi: 10.1002/bip.10241. [DOI] [PubMed] [Google Scholar]
- 109.Kennedy DF, Crisma M, Toniolo C, Chapman D. Studies of peptides forming 310-helices and α-helices and β-bend ribbon structures in organic solution and in model biomembranes by Fourier-transform infrared-spectroscopy. Biochemistry. 1991;30:6541–6548. doi: 10.1021/bi00240a026. [DOI] [PubMed] [Google Scholar]
- 110.Reinstadler D, Fabian H, Backmann J, Naumann D. Refolding of thermally and urea-denatured ribonuclease A monitored by time-resolved FTIR spectroscopy. Biochemistry. 1996;35:15822–15830. doi: 10.1021/bi961810j. [DOI] [PubMed] [Google Scholar]
- 111.From NB, Bowler BE. Urea denaturation of staphylococcal nuclease monitored by Fourier transform infrared spectroscopy. Biochemistry. 1998;37:1623–1631. doi: 10.1021/bi970620l. [DOI] [PubMed] [Google Scholar]
- 112.Goormaghtigh E, Raussens V, Ruysschaert JM. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim Biophys Acta Rev Biomembr. 1999;1422:105–185. doi: 10.1016/s0304-4157(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 113.Silva RAGD, Kubelka J, Bour P, Decatur SM, Keiderling TA. Site-specific conformational determination in thermal unfolding studies of helical peptides using vibrational circular dichroism with isotopic substitution. Proc Natl Acad Sci USA. 2000;97:8318–8323. doi: 10.1073/pnas.140161997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barth A, Zscherp C. What vibrations tell us about proteins. Q Rev Biophys. 2002;35:369–430. doi: 10.1017/s0033583502003815. [DOI] [PubMed] [Google Scholar]
- 115.Keiderling TA. Protein and peptide secondary structure and conformational determination with vibrational circular dichroism. Curr Opin Chem Biol. 2002;6:682–688. doi: 10.1016/s1367-5931(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 116.Mukherjee S, Chowdhury P, Gai F. Tuning the cooperativity of the helix–coil transition by aqueous reverse micelles. J Phys Chem B. 2006;110:11615–11619. doi: 10.1021/jp062362k. [DOI] [PubMed] [Google Scholar]
- 117.Miyazawa T. Perturbation treatment of the characteristic vibrations of polypeptide chains in various configurations. J Chem Phys. 1960;32:1647–1652. [Google Scholar]
- 118.Krimm S, Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- 119.Hamm P, Lim M, DeGrado WF, Hochstrasser RM. The two-dimensional IR nonlinear spectroscopy of a cyclic penta-peptide in relation to its three-dimensional structure. Proc Natl Acad Sci USA. 1999;96:2036–2041. doi: 10.1073/pnas.96.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mukamel S, Abramavicius D. Many-body approaches for simulating coherent nonlinear spectroscopies of electronic and vibrational excitons. Chem Rev. 2004;104:2073–2098. doi: 10.1021/cr020681b. [DOI] [PubMed] [Google Scholar]
- 121.Montalvo G, Waegele MM, Shandler S, Gai F, DeGrado WF. Infrared signature and folding dynamics of a helical β-peptide. J Am Chem Soc. 2010;132:5616–5618. doi: 10.1021/ja100459a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mukherjee S, Chowdhury P, Gai F. Infrared study of the effect of hydration on the amide I band and aggregation properties of helical peptides. J Phys Chem B. 2007;111:4596–4602. doi: 10.1021/jp0689060. [DOI] [PubMed] [Google Scholar]
- 123.Huang CY, Getahun Z, Zhu YJ, Klemke JW, DeGrado WF, Gai F. Helix formation via conformation diffusion search. Proc Natl Acad Sci USA. 2002;99:2788–2793. doi: 10.1073/pnas.052700099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Y, Oyola R, Gai F. Infrared study of the stability and folding kinetics of a 15-residue β-hairpin. J Am Chem Soc. 2003;125:15388–15394. doi: 10.1021/ja037053b. [DOI] [PubMed] [Google Scholar]
- 125.Bunagan MR, Gao J, Kelly JW, Gai F. Probing the folding transition state structure of the villin headpiece subdomain via side chain and backbone mutagenesis. J Am Chem Soc. 2009;131:7470–7476. doi: 10.1021/ja901860f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bunagan MR, Yang X, Saven JG, Gai F. Ultrafast folding of a computationally designed trp-cage mutant: Trp2-cage. J Phys Chem B. 2006;110:3759–3763. doi: 10.1021/jp055288z. [DOI] [PubMed] [Google Scholar]
- 127.Culik RM, Serrano AL, Bunagan MR, Gai F. Achieving secondary structural resolution in kinetic measurements of protein folding: a case study of the folding mechanism of trp-cage. Angew Chem Int Ed. 2011;50:10884–10887. doi: 10.1002/anie.201104085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du D, Zhu YJ, Huang CY, Gai F. Understanding the key factors that control the rate of β-hairpin folding. Proc Natl Acad Sci USA. 2004;101:15915–15920. doi: 10.1073/pnas.0405904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du D, Tucker MJ, Gai F. Understanding the mechanism of β-hairpin folding via phi-value analysis. Biochemistry. 2006;45:2668–2678. doi: 10.1021/bi052039s. [DOI] [PubMed] [Google Scholar]
- 130.Xu Y, Wang T, Gai F. Strange temperature dependence of the folding rate of a 16-residue β-hairpin. Chem Phys. 2006;323:21–27. [Google Scholar]
- 131.Du D, Gai F. Understanding the folding mechanism of an alpha-helical hairpin. Biochemistry. 2006;45:13131–13139. doi: 10.1021/bi0615745. [DOI] [PubMed] [Google Scholar]
- 132.Waegele MM, Gai F. Infrared study of the folding mechanism of a helical hairpin: porcine PYY. Biochemistry. 2010;49:7659–7664. doi: 10.1021/bi100851c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Neidigh JW, Fesinmeyer RM, Andersen NH. Designing a 20-residue protein. Nat Struct Biol. 2002;9:425–430. doi: 10.1038/nsb798. [DOI] [PubMed] [Google Scholar]
- 134.Barua B, Lin JC, Williams VD, Kummler P, Neidigh JW, Andersen NH. The trp-cage: optimizing the stability of a globular miniprotein. Protein Eng Des Sel. 2008;21:171–185. doi: 10.1093/protein/gzm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilson EB, Decius JC, Cross PC. Molecular vibrations: the theory of infrared and Raman vibrational spectra. New York: Dover; 1980. [Google Scholar]
- 136.Mirkin NG, Krimm S. Ab initio vibrational analysis of isotopic derivatives of aqueous hydrogen-bonded trans-N-methylacetamide. J Mol Struct. 1996;377:219–234. [Google Scholar]
- 137.Decatur SM, Antonic J. Isotope-edited infrared spectroscopy of helical peptides. J Am Chem Soc. 1999;121:11914–11915. [Google Scholar]
- 138.Brauner JW, Dugan C, Mendelsohn R. C-13 isotope labeling of hydrophobic peptides. Origin of the anomalous intensity distribution in the infrared amide I spectral region of β-sheet structures. J Am Chem Soc. 2000;122:677–683. [Google Scholar]
- 139.Huang CY, Getahun Z, Wang T, DeGrado WF, Gai F. Time-resolved infrared study of the helix–coil transition using C-13-labeled helical peptides. J Am Chem Soc. 2001;123:12111–12112. doi: 10.1021/ja016631q. [DOI] [PubMed] [Google Scholar]
- 140.Waegele MM, Culik RM, Gai F. Site-specific spectroscopic reporters of the local electric field, hydration, structure, and dynamics of biomolecules. J Phys Chem Lett. 2011;2:2598–2609. doi: 10.1021/jz201161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Purrello R, Jordan T, Spiro TG. UVRR spectroscopy of the peptide-bond. 1. Amide-S, a nonhelical structure marker, is a Cα—H bending mode. J Am Chem Soc. 1991;113:6359–6368. [Google Scholar]
- 142.Wang Y, Purrello R, Georgiou S, Spiro TG. UVRR spectroscopy of the peptide-bond. 2. Carbonyl H-bond effects on the ground-state and excited-state structures of N-methylacetamide. J Am Chem Soc. 1991;113:6368–6377. [Google Scholar]
- 143.Huang CY, Balakrishnan G, Spiro TG. Protein secondary structure from deep-UV resonance Raman spectroscopy. J Raman Spectrosc. 2006;37:277–282. [Google Scholar]
- 144.Chi Z, Chen XG, Holtz JSW, Asher SA. UV resonance Raman-selective amide vibrational enhancement: quantitative methodology for determining protein secondary structure. Biochemistry. 1998;37:2854–2864. doi: 10.1021/bi971160z. [DOI] [PubMed] [Google Scholar]
- 145.Oladepo SA, Xiong K, Hong Z, Asher SA. Elucidating peptide and protein structure and dynamics: UV resonance Raman spectroscopy. J Phys Chem Lett. 2011;2:334–344. doi: 10.1021/jz101619f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Asher SA, Ianoul A, Mix G, Boyden MN, Karnoup A, Diem M, Schweitzer-Stenner R. Dihedral psi angle dependence of the amide III vibration: a uniquely sensitive UV resonance Raman secondary structural probe. J Am Chem Soc. 2001;123:11775–11781. doi: 10.1021/ja0039738. [DOI] [PubMed] [Google Scholar]
- 147.Lednev IK, Karnoup AS, Sparrow MC, Asher SA. α-Helix peptide folding and unfolding activation barriers: a nanosecond UV resonance Raman study. J Am Chem Soc. 1999;121:8074–8086. [Google Scholar]
- 148.Lednev IK, Karnoup AS, Sparrow MC, Asher SA. Transient UV Raman spectroscopy finds no crossing barrier between the peptide α-helix and fully random coil conformation. J Am Chem Soc. 2001;123:2388–2392. doi: 10.1021/ja003381p. [DOI] [PubMed] [Google Scholar]
- 149.Mikhonin AV, Asher SA. Uncoupled peptide bond vibrations in α-helical and polyproline II conformations of polyalanine peptides. J Phys Chem B. 2005;109:3047–3052. doi: 10.1021/jp0460442. [DOI] [PubMed] [Google Scholar]
- 150.Huang CY, Balakrishnan G, Spiro TG. Early events in apomyoglobin unfolding probed by laser T-jump/UV resonance Raman spectroscopy. Biochemistry. 2005;44:15734–15742. doi: 10.1021/bi051578u. [DOI] [PubMed] [Google Scholar]
- 151.JiJi RD, Balakrishnan G, Hu Y, Spiro TG. Intermediacy of poly(l-proline) II and β-strand conformations in poly(l-lysine) β-sheet formation probed by temperature-jump/UV resonance Raman spectroscopy. Biochemistry. 2006;45:34–41. doi: 10.1021/bi051507v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zanni MT, Gnanakaran S, Stenger J, Hochstrasser RM. Heterodyned two-dimensional infrared spectroscopy of solvent-dependent conformations of acetylproline-NH2. J Phys Chem B. 2001;105:6520–6535. [Google Scholar]
- 153.Gnanakaran S, Hochstrasser RM. Conformational preferences and vibrational frequency distributions of short peptides in relation to multidimensional infrared spectroscopy. J Am Chem Soc. 2001;123:12886–12898. doi: 10.1021/ja011088z. [DOI] [PubMed] [Google Scholar]
- 154.Remorino A, Korendovych IV, Wu Y, DeGrado WF, Hochstrasser RM. Residue-specific vibrational echoes yield 3D structures of a transmembrane helix dimer. Science. 2011;332:1206–1209. doi: 10.1126/science.1202997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim YS, Hochstrasser RM. Applications of 2D IR spectroscopy to peptides, proteins, and hydrogen-bond dynamics. J Phys Chem B. 2009;113:8231–8251. doi: 10.1021/jp8113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hochstrasser RM. Two-dimensional spectroscopy at infrared and optical frequencies. Proc Natl Acad Sci USA. 2007;104:14190–14196. doi: 10.1073/pnas.0704079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhuang W, Sgourakis NG, Li Z, Garcia AE, Mukamel S. Discriminating early stage A β 42 monomer structures using chirality-induced 2DIR spectroscopy in a simulation study. Proc Natl Acad Sci USA. 2010;107:15687–15692. doi: 10.1073/pnas.1002131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ganim Z, Chung HS, Smith AW, Deflores LP, Jones KC, Tokmakoff A. Amide I two-dimensional infrared spectroscopy of proteins. Acc Chem Res. 2008;41:432–441. doi: 10.1021/ar700188n. [DOI] [PubMed] [Google Scholar]
- 159.Moran AM, Park SM, Dreyer J, Mukamel S. Linear and nonlinear infrared signatures of local α- and 310-helical structures in alanine polypeptides. J Chem Phys. 2003;118:3651–3659. [Google Scholar]
- 160.Urbanek DC, Vorobyev DY, Serrano AL, Gai F, Hochstrasser RM. The two-dimensional vibrational echo of a nitrile probe of the villin HP35 protein. J Phys Chem Lett. 2010;1:3311–3315. doi: 10.1021/jz101367d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maekawa H, Toniolo C, Moretto A, Broxterman QB, Ge NH. Different spectral signatures of octapeptide 310- and α-helices revealed by two-dimensional infrared spectroscopy. J Phys Chem B. 2006;110:5834–5837. doi: 10.1021/jp057472q. [DOI] [PubMed] [Google Scholar]
- 162.Sul S, Karaiskaj D, Jiang Y, Ge NH. Conformations of N-acetyl-l-prolinamide by two-dimensional infrared spectroscopy. J Phys Chem B. 2006;110:19891–19905. doi: 10.1021/jp062039h. [DOI] [PubMed] [Google Scholar]
- 163.Zhuang W, Hayashi T, Mukamel S. Coherent multidimensional vibrational spectroscopy of biomolecules: concepts, simulations, and challenges. Angew Chem Int Edit. 2009;48:3750–3781. doi: 10.1002/anie.200802644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maekawa H, De Poli M, Moretto A, Toniolo C, Ge N. Toward detecting the formation of a single helical turn by 2D IR cross peaks between the Amide-I and -II modes. J Phys Chem B. 2009;113:11775–11786. doi: 10.1021/jp9045879. [DOI] [PubMed] [Google Scholar]
- 165.Jeon J, Yang S, Choi J, Cho M. Computational vibrational spectroscopy of peptides and proteins in one and two dimensions. Acc Chem Res. 2009;42:1280–1289. doi: 10.1021/ar900014e. [DOI] [PubMed] [Google Scholar]
- 166.Chung HS, Khalil M, Smith AW, Tokmakoff A. Transient two-dimensional IR spectrometer for probing nanosecond temperature-jump kinetics. Rev Sci Instrum. 2007;78:063101. doi: 10.1063/1.2743168. [DOI] [PubMed] [Google Scholar]
- 167.Smith AW, Tokmakoff A. Probing local structural events in β-hairpin unfolding with transient nonlinear infrared spectroscopy. Angew Chem Int Edit. 2007;46:7984–7987. doi: 10.1002/anie.200701172. [DOI] [PubMed] [Google Scholar]
- 168.Smith AW, Lessing J, Ganim Z, Peng CS, Tokmakoff A, Roy S, Jansen TLC, Knoester J. Melting of a β-hairpin peptide using isotope-edited 2D IR spectroscopy and simulations. J Phys Chem B. 2010;114:10913–10924. doi: 10.1021/jp104017h. [DOI] [PubMed] [Google Scholar]
- 169.Shim SH, Strasfeld DB, Ling YL, Zanni MT. Automated 2D IR spectroscopy using a mid-IR pulse shaper and application of this technology to the human islet amyloid polypeptide. Proc Natl Acad Sci USA. 2007;104:14197–202. doi: 10.1073/pnas.0700804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mukherjee S, Chowdhury P, Gai F. Effect of dehydration on the aggregation kinetics of two amyloid peptides. J Phys Chem B. 2009;113:531–535. doi: 10.1021/jp809817s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shim SH, Gupta R, Ling YL, Strasfeld DB, Raleigh DP, Zanni MT. Two-dimensional IR spectroscopy and isotope labeling defines the pathway of amyloid formation with residue-specific resolution. Proc Natl Acad Sci USA. 2009;106:6614–6619. doi: 10.1073/pnas.0805957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim YS, Liu L, Axelsen PH, Hochstrasser RM. 2D IR provides evidence for mobile water molecules in β-amyloid fibrils. Proc Natl Acad Sci USA. 2009;106:17751–17756. doi: 10.1073/pnas.0909888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Londergan CH, Wang J, Axelsen PH, Hochstrasser RM. Two-dimensional infrared spectroscopy displays signatures of structural ordering in peptide aggregates. Biophys J. 2006;90:4672–4685. doi: 10.1529/biophysj.105.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mukherjee P, Kass I, Arkin IT, Zanni MT. Picosecond dynamics of a membrane protein revealed by 2D IR. Proc Natl Acad Sci USA. 2006;103:3528–3533. doi: 10.1073/pnas.0508833103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guo L, Chowdhury P, Fang JY, Gai F. Heterogeneous and anomalous diffusion inside lipid tubules. J Phys Chem B. 2007;111:14244–1429. doi: 10.1021/jp076562n. [DOI] [PubMed] [Google Scholar]
- 176.Guo L, Gai F. Heterogeneous diffusion of a membrane-bound pHLIP peptide. Biophys J. 2010;98:2914–2922. doi: 10.1016/j.bpj.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith-Dupont KB, Guo L, Gai F. Diffusion as a probe of the heterogeneity of antimicrobial peptide-membrane interactions. Biochemistry. 2010;49:4672–468. doi: 10.1021/bi100426p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ghosh A, Qiu J, DeGrado WF, Hochstrasser RM. Tidal surge in the M2 proton channel, sensed by 2D IR spectroscopy. Proc Natl Acad Sci USA. 2011;108:6115–6120. doi: 10.1073/pnas.1103027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ansari A, Kuznetsov SV. Is hairpin formation in single-stranded polynucleotide diffusion-controlled? J Phys Chem B. 2005;109:12982–12989. doi: 10.1021/jp044838a. [DOI] [PubMed] [Google Scholar]
- 180.Brauns EB, Dyer RB. Time-resolved infrared spectroscopy of RNA folding. Biophys J. 2005;89:3523–3530. doi: 10.1529/biophysj.105.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma H, Proctor DJ, Kierzek E, Kierzek R, Bevilacqua PC, Gruebele M. Exploring the energy landscape of a small RNA hairpin. J Am Chem Soc. 2006;128:1523–1530. doi: 10.1021/ja0553856. [DOI] [PubMed] [Google Scholar]
- 182.Kuznetsov SV, Sugimura S, Vivas P, Crothers DM, Ansari A. Direct observation of DNA bending/unbending kinetics in complex with DNA-bending protein IHF. Proc Natl Acad Sci USA. 2006;103:18515–18520. doi: 10.1073/pnas.0608394103. [DOI] [PMC free article] [PubMed] [Google Scholar]