Abstract
Here, we report the novel observation that natural killer T (NKT) cells contribute to the cutaneous wound repair process. Using an excisional wound model in wild-type versus NKT cell-deficient mice, this report shows that when NKT cells are absent, initial wound closure is markedly accelerated. We report here for the first time that NKT cells are a significant constituent of early wound inflammation and that they regulate the local production of a key subset of neutrophil and monocyte/macrophage chemokines, as well as TGF-b1 content and collagen deposition. Together, our findings support the concept that NKT cells regulate the early inflammatory and fibroproliferative phases of nonpathologic healing wounds, positioning the NKT cell as an attractive potential therapeutic target for modulation of impaired wound healing.
Keywords: wound healing, NKT cells, chemokines, inflammation
INTRODUCTION
Natural killer T (NKT) cells are innate lymphocytes known to influence peripheral immunity [1], modulating T cell and antigen-presenting cell (APC) functions during infection [2, 3], tolerance [4], autoimmunity [5, 6], and cancer [7, 8]. Such diverse and seemingly contradictory roles arise from the NKT cells’ response to antigen presenting cell (APC)-derived signals upon activation with glycolipid antigen presented in the context of CD1d. When exposed to IL-10 or TGF-β-producing APCs, NKT cells assume an IL-4/IL-10/TGF-β producing phenotype [9]. Conversely, in the presence of APC-derived IL-12, NKT cells acquire a predominantly IFN-γ producing phenotype [10]. In this way, NKT cells direct the outcome of cellular immunity. depending on local signals.
Recently, we reported that following burn injury, NKT cells acquire an IL-4 secreting phenotype, which contributes to the systemic immune paralysis seen after burn injury [11]. To date, there are no reports of NKT cell involvement at sites of local injury such as in cutaneous wounds. Of the innate lymphocytes, only γδ T cells are known to play a role in cutaneous wound healing [12]. Wound repair begins with inflammation and the sequential infiltration of neutrophils, followed by macrophages and, finally, fibroblasts and conventional lymphocytes. Local production of chemoattractants controls the kinetics and chronology of these inflammatory infiltrates [13]. NKT cells can both produce and respond to certain CXC chemokines, such as MIP-2 and KC, which are classically associated with the early inflammatory phase of wound healing [14, 15].
Here we demonstrate a novel role for NKT cells in the wound repair process. Specifically, we identified CD1d-restricted NKT cells as constituents of the early wound inflammatory infiltrate and as regulators of the local production of pro-inflammatory and fibroproliferative mediators. Using a murine excisional punch wound model, we observed that compared with wild-type mice, NKT cell-deficient mice had accelerated early wound closure, which correlated with augmented local production of collagen as well as a select subset of chemokines and TGFβ1 during this same early time period. Since thioglycolate-elicited macrophages from NKT cell-deficient mice displayed augmented production of the same chemokine subset seen elevated in the wounds NKT cell deficient mice, it appeared that NKT cells regulated inflammatory cell function. Together, our findings support a novel and intriguing role for CD1d-restricted NKT cells as critical regulators of early wound inflammation and collagen production.
MATERIALS AND METHODS
Animals
Eight to 12-wk-old male BALB/c and BALB/c CD1d knockout (ko) mice used in these studies were obtained from Harlan Laboratory (Madison, WI). Invariant NKT cell-deficient BALB/c-Jα281ko mice were provided by M. Taniguchi (RIKEN, Yokohama, Japan). All animals were housed on a 12/12-h light/dark cycle and provided with food and water ad libitum. All mice were treated humanely and in accordance with the guidelines set forth by the Loyola University Institutional Animal Care and Use Committee and the National Institutes of Health.
Excisional Injury Model and Tissue Collection
The excisional punch wound model was used as described previously [16]. Briefly, BALB/c (wild type) or NKT cell deficient (CD1dko or Jα281ko) mice were anesthetized by intraperitoneal injection of ketamine (Abbott Laboratories, North Chicago, IL) and xylazine (Phoenix Pharmaceutical, St. Joseph, MO). The fur on their dorsal surface was then shaved with animal clippers, and six full-thickness dermal excisional wounds were produced on the dorsum of each mouse with a three or five mm dermal biopsy punch (Acu Punch; Acuderm, Ft. Lauderdale, FL). At 12 h, and d 1, 2, 3, and 5 post-wounding, mice were killed, their pelts removed, and the wounds excised with a larger biopsy punch. Some wounds were snap frozen in liquid nitrogen and stored at –80°C for molecular analysis, while other wounds were processed immediately for flow cytometry (see below).
Determination of Wound Closure
Animals were wounded as described above. At da 1, 3, 5, and 7 post-injury, groups of animals were killed and their pelts removed. After removing all subcutaneous tissue, each wound was placed flat and photographed from a fixed distance of 20 cm with a ruler placed within each photograph. Photoshop 7.0 (Adobe Systems Inc., San Jose, CA) was used to determine the number of pixels in the open wound area with the “magic wand” tool and a tolerance setting of 60. Wound areas at each time point were compared wih fresh 3 mm wounds (0% closed at time zero) to calculate the percent wound closure.
Preparation of Dermal Cell Suspensions by Tissue Dispersion
Previously described protocols for tissue dispersion of skin and other organs [17–19) were modified to obtain dermal cell suspensions adequate for flow cytometric analysis. Animals were wounded as described above. At 12 h, 1, 3, and 5 d post-wounding, animals were sacrificed and their pelts removed. Three wounds (cephalad, middle, and caudal) were pooled and weighed as one sample. After finely mincing these wounds, each sample was placed in a solution containing 1 mg/mL dispase II (Roche, Mannheim, Germany), 5% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, NY), and 10 mg/mL gentamicin sulfate (Mediatech, Inc., Herndon, VA) diluted in Hank's balanced salt solution (Invitrogen). Wound samples were incubated in 15.5 mL of this solution per gram of tissue on a shaker at 4°C for 18 h. Any remaining tissue was then removed from this solution, weighed, and incubated in an enzyme solution (20 mL/g of tissue) containing 2% heat-inactivated fetal bovine serum, hyaluronidase I from bovine testes (1 mg/mL) (Sigma-Aldrich, St. Louis, MO), collagenase type IA from Clostridium histolyticum (1 mg/mL) (Sigma-Aldrich), DNase I from bovine pancreas (1.2 mg/mL) (Roche), and gentamicin sulfate (5 mg/mL) (Mediatech, Inc) diluted in RPMI 1640 (Invitrogen). Following this incubation, this solution was combined with the dispase solution and filtered through a 70-μm nylon cell strainer (BD Biosciences, San Jose, CA) to obtain single cell suspensions. These cells were then washed twice using a solution of 3% heat-inactivated fetal bovine serum in sterile PBS before transferring to a solution of 1% bovine serum albumin (Sigma, St. Louis, MO) and 0.1% sodium azide (Sigma) in 1 × PBS for cell staining and flow cytometric analysis.
Flow Cytometric Analyses of Wound Neutrophil, Macrophage, and NKT Cell Infiltrates
Wound cell suspensions were prepared as described above. Nonspecific staining was blocked with anti-CD16/CD32 (FcBlock) (clone no. 93; eBioscience, LaJolla, CA). After blocking, cells were stained with a combination of allophycocyanine (APC)-conjugated anti-CD3ε, fluorescein isothiocyanate (FITC)-conjugated Ly49c (clone no. 14B11, eBioscience), and glycolipid loaded dimeric CD1d: Ig Fusion Protein (BD Pharmingen, San Jose, CA) counter-stained with a secondary phycoerythrin (PE)-conjugated anti-IgG1 (clone no. A85-1; BD Pharmingen).
A separate set of cells were stained with a cocktail of anti-APC-F4/80 (clone no. BM8, eBioscience) and FITC-conjugated anti-GR-1 (clone no. RB6-8C5; eBioscience). Flow cytometric determinations were made using a BD FACS Canto flow cytometer (BD Biosciences) and FlowJo version 8.5.2 (Tree Star, Inc., Ashland, OR).
Measurement of Wound Chemokine and Cytokine Content
Individual wounds were homogenized in 1 × PBS containing protease inhibitor cocktail (Roche). Homogenates were then sonicated briefly on ice and centrifuged at 5000 rpm for 10 min and filtered through a 1.2-μm pore filter. Levels of MIP-2, KC, MIP-1α, MIP-1β, MCP-1, and TGF-β1 were determined with commercially available ELISA kits (R and D Systems, Minneapolis, MN) according to the manufacturer's specifications. Only the endogenously activated fraction of TGF-β1 was measured (i.e., samples were not acidified to activate all latent TGF-β1). ELISA plates were read using a SpectraMAX Plus 384 plate reader (Amersham Biosciences, Piscataway, NJ), and analyses of ELISA data were done with SoftMax Pro 3.1.2 (Molecular Devices Corp., Sunnyvale, CA).
Myeloperoxidase Assay
Animals were wounded and samples collected as described above. MPO levels were determined using the Myeloperoxidase Assay Kit (CytoStore, Calgary, Alberta, Canada) according to the manufacturer's instructions.
Quantitative Real-time Polymerase Chain Reaction (PCR) of Type I Collagen
Total RNA was isolated from one 3 mm wound per animal using the Rneasy Fibrous Tissue Mini Kit (Qiagen Inc.,Valencia, CA) following the manufacturer's instructions. After RNA quantitation with the Experion RNA StdSens Analysis Kit and Automated Electrophoresis System (Bio-Rad Laboratories, Inc., Hercules, CA), 500 ng of RNA per sample was subsequently reverse transcribed using the high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). These cDNAs were quantified using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE), and 40 ng of each cDNA combined with probes, primers, and Taq-Man universal PCR master mix for real-time RNA quantitation using the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems). Each cDNA sample was combined with two different standard probe/primer sets: α1-procallegen type I (Col1a2) and glyceraldehyde oxidoreductase dehydrogenase 3-phosophate (GAPDH) (Applied Biosystems) which were run on the same Micro-Amp Optical 96-well reaction plate (Applied Biosystems) according to the manufacturer's protocol. The values generated for collagen gene expression in each sample were adjusted by the corresponding amount of GAPDH gene expression.
Analysis of Wound Collagen Content
The hydroxyproline content of skin from unwounded animals and d 1 and 3 wounds was determined by the previously described protocol [20]. Briefly, snap frozen tissue was hydrolyzed in 2 mL of 6 N HCL at 110°C for 16 h. The reaction was neutralized with 2.5 N NaOH and diluted 40-fold with MilliQ water (Millipore, Billerica, MA). One milliliter of 3.15 M perchloric acid was added, and the solution was incubated for 5 min at room temperature. One milliliter of 20% p-dimethylaminobenzaldehyde was subsequently added, and the mixture was incubated for 20 min in a 60°C water bath. After cooling the samples in cold tap water, the amount of hydroxyproline was determined by comparison to a standard curve measured spectrophotometrically at an absorbance of 540 nm, subtracting the absorbance of the plate (557 nm).
Statistical Analysis
Statistical determinations were made by Student's t-tests. Statistical significance was determined when P < 0.05.
RESULTS
NKT Cell Deficient Mice Exhibit Accelerated Wound Closure
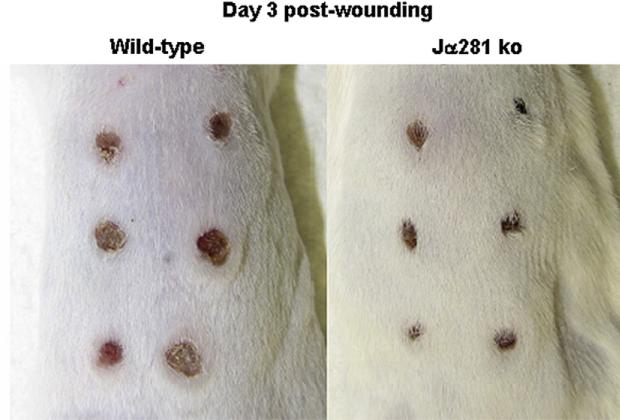
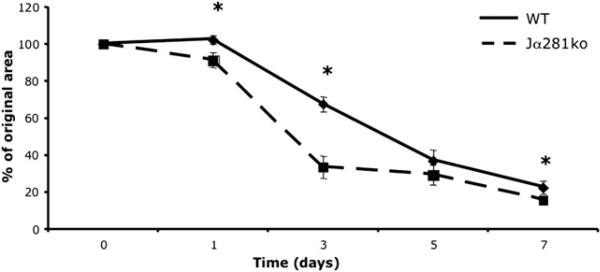
Given their role in regulating peripheral immunity after burn injury [11, 21], we hypothesized that NKT cells might regulate local inflammation during wound repair. To study the role of NKT cells in cutaneous wound repair, we utilized an excisional punch wound model as previously described [22] to compare wound closure and other inflammatory and wound healing parameters in wild-type versus NKT cell-deficient mice (Jα281ko a). Strikingly, we observed that at day three, cutaneous wounds in invariant NKT cell-deficient Jα281ko mice were markedly smaller than wounds in wild-type mice (Fig. 1). Through examination of open wound areas, we observed that differences in the degree of wound closure occurred early after wounding, as d 1e punch wounds in Jα281ko mice were 52.3% closed (versus day 0) while d 1 wounds from WT mice were only 32.6% closed (Fig. 2). Compared with WT mice, wound closure in Jα281ko mice continued at a significantly faster rate through day three (33.5% versus 67.6%, respectively). After d 3, open wound areas from WT and Jα281ko mice approached each other, but wounds from Jα281ko mice were still smaller than WT wounds at 7 d post wounding (Fig. 2). Complete wound closure occurred in both groups by d 10. Similar results were obtained in BALB/c-CD1dko mice that lack both CD1d-restricted NKT cells and CD1d molecules (data not shown). Since cutaneous punch wounds in rodents heal in an asymmetrical fashion, we also compared wound diameters along the longest axis of the wounds and likewise, observed significantly accelerated wound closure in NKT cell-deficient mice (Supplemental Fig. 1). Specifically, the average diameters along the longest axes of wounds from Jα281ko animals were significantly smaller than wild type wounds through 7 d post-injury, with the greatest differences seen at d 1 and 3, when Jα281ko wounds were 23.1% and 26.5% smaller, respectively (Supplemental Fig. 1). Thus, our observation that NKT cell deficient animals exhibit accelerated early wound closure at early time points was confirmed in two different NKT cell deficient mouse strains, using two different methods of wound closure measurement. Although wild type wound closure eventually catches up to the NKT cell deficient animals at later time points, an early acceleration in wound closure might have consequences in the setting of wound infection or delayed wound healing defects such as diabetes.
FIG. 1.
Comparison of excisional wound closure in wild type versus NKT cell deficient mice. Wild type BALB/c and BALB/c-Jα281ko mice were each given six 3 mm dorsal punch excisional wounds. Three days later, wounds were photographed; n = 3–6 per group. Similar results were obtained from two separate experiments for WT versus Jα281ko and two separate experiments for WT versus CD1dko. (Color version of figure is available online.)
FIG. 2.
Wound closure wild-type versus NKT cell-deficient Jα281ko mice. Wild type BALB/c and BALB/c-Jα281ko mice were each given six 3 mm dorsal punch excisional punch wounds. At d 1, 3, 5, and 7 post-wounding, pelts were removed and the individual wounds photographed. The percent open wound area was calculated from the number of pixels in the open wound area. *P < 0.05, n = 6 per group. Experiments were done three times.
NKT Cells Infiltrate Cutaneous Wounds at Early Time Points
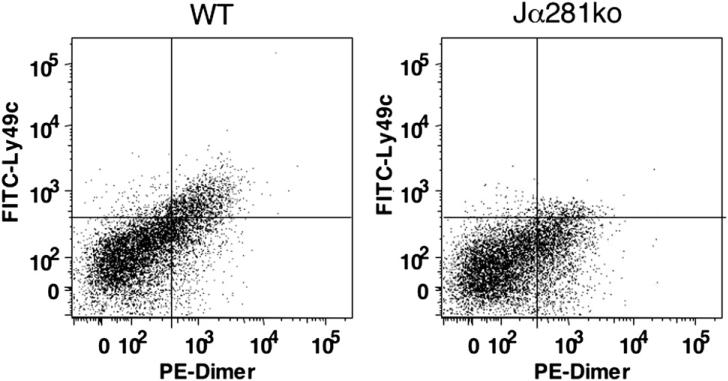
Both conventional natural killer (NK) cells and lymphocytes are known to home to cutaneous wounds during the later stages of wound repair, mainly at d 3 to 7 [23]. Since NKT cells respond to a number of chemokines, including the CXC chemokine MIP-2 [14], we hypothesized that NKT cells might also infiltrate the wound site. Given that NKT cells comprise only 1% of mouse splenocytes and circulating peripheral blood mononuclear cells (PBMCs) [1], we further reasoned that a wound-associated NKT cell would be present at low frequencies compared with the more abundant infiltrating cells (neutrophils, macrophages, fibroblasts) making identification via immunohistochemistry impractical. Suitable reagents for specifically identifying NKT cells in tissue sections, fixed or frozen, are as yet not available or not fully validated. Therefore, we developed a protocol for tissue dispersion to identify wound-associated NKT cells by flow cytometry. Similar methods have accurately and precisely identified NKT cells in other organs [19, 24], where NKT cells are few in number compared with other, more copious infiltrating cells. Using this approach, we observed that while normal skin was devoid of NKT cells, CD3+Ly49c+CD1d dimmer+ cells were indeed present in wild type cutaneous wounds. This method did not detect NKT cells in wounds from any time point in our knockout animals (Fig. 3).
FIG. 3.
Day 1 wound NKT cell infiltrates in wild type versus Jα281ko mice. Day 1 wound dermal cell suspensions were immunostained with APC CD3, PE IgG:CD1d dimeric fusion protein, and FITC Ly49c and analyzed by flow cytometry. After gating on the CD3+ population, NKT cells were identified as those positive for Ly49c and intermediate staining of CD1d dimer. One representative sample per group is shown. Data are shown as percentage of live cells that are CD3 + IgG:CD1d dimeric fusion protein+ Ly49c+. The cumulative data from this experiment are shown in Fig. 4.
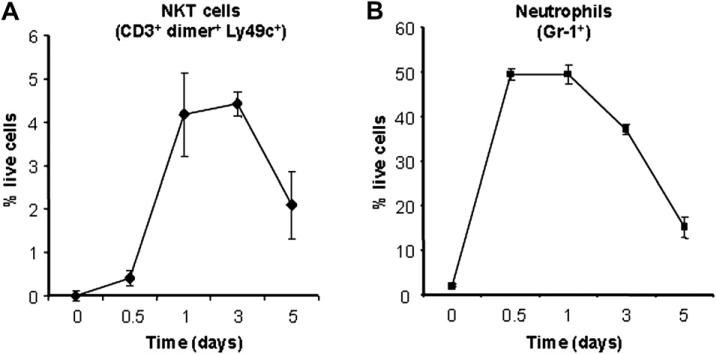
NKT cells were detected in wounds as early as 12 h after injury, constituting 0.41% ± 0.96% of live cells. However, by 24 h post-wounding, the number of NKT cells increased 10-fold to 4.18% ± 0.96% of live cells (Fig. 4) and remained so through d 3 (4.42% ± 0.28% of live cells) (Fig. 4). By d 5, NKT cell numbers diminished to 2.09% ± 0.77% of live cells. There were no NKT cells found in wounds beyond 5 d (data not shown). From these observations, we concluded that NKT cells were a significant constituent of early wound infiltrating cells and that they accumulate in wounds early during the inflammatory phase with kinetics that coincide with the infiltration of neutrophils [25]. Additionally, we observed that their numbers decline as the inflammatory phase of wound repair wanes (i.e., after d 3).
FIG. 4.
Wound NKT cell versus neutrophil content over time. Wild type BALB/c mice were given 3 mm excisional punch wounds. At 12 h and 1, 3, and 5 d post-wounding, animals were sacrificed, their wounds collected, and dermal cell suspensions created. NKT cells were identified by flow cytometric analysis of dermal cell suspensions as those staining positively for CD3, Ly49c, and glycolipid-loaded IgG:CD1d dimeric fusion protein (A). Neutrophils were identified via flow cytometry for Gr-1+ cells (B). Dermal cell suspensions created from the dorsal skin of uninjured animals were also analyzed (d 0). Data are presented as the percentage of live cells ± SEM; n = 4 animals per group.
Local Production of Select Neutrophil and Monocyte/Macrophage Chemokines is Transiently Enhanced in Wounds from NKT Cell Knockout Animals
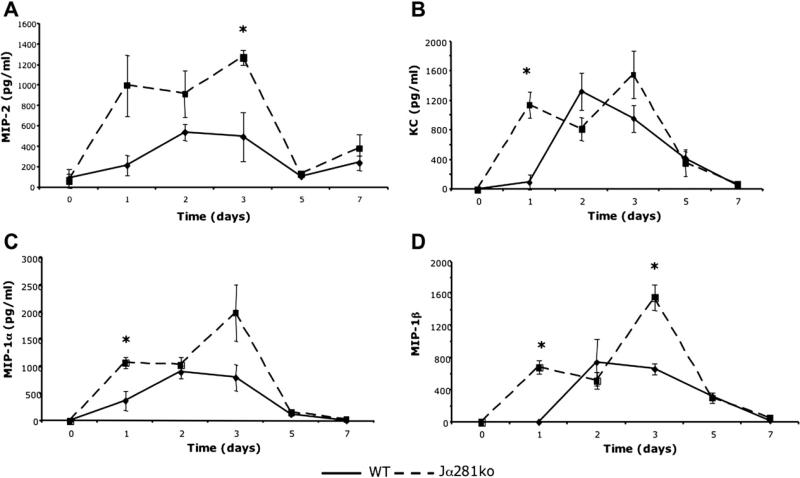
Because NKT cells infiltrated wounds early during the inflammatory phase, and given their previously known immune regulatory functions, we screened the gene expression profiles of wild-type, Jα281ko, and CD1dko wounds for a subset of key pro-inflammatory mediators predominant in this phase of wound repair. Interestingly, we observed that compared with wounds from wild-type mice, those from both Jα281ko and CD1dko mice displayed up-regulation of a select subset of chemokines including MIP-1α, MIP-1β, and MIP-2 (data not shown). To determine if protein levels of these same mediators correlated with enhanced gene expression, we conducted ELISA for MIP-2, KC, MIP-1α, and MIP-1β on Jα281ko and wild-type wound protein extracts. KC, like MIP-2, is a neutrophil chemokine and a functional homolog of human IL-8. In agreement with our gene expression studies, local production of the same chemokine subset was transiently enhanced during the inflammatory phase in knockout versus wild-type wounds (Fig. 5). One day post-injury, Jα281ko wounds contain over 1000-fold more KC than wild type wounds (Fig. 5b). Three days post-injury, Jα281ko wounds contained 5- to 7-fold greater levels MIP-2 and KC compared with wild-type wounds (Fig. 5a and b). Additionally, at 1 d post-wounding, we observed 3- and 8-fold higher levels of MIP-1α and MIP-1β, respectively (Fig. 5c and d). There were no significant differences in wound chemokine content after 3 d post-wounding (Fig. 5). Therefore, in the absence of NKT cells, local production of selected neutrophil (MIP-2, KC), and monocyte/macrophage (MIP-1α, MIP-1β) chemokines were transiently enhanced during the inflammatory phase of wound repair.
FIG. 5.
Wound chemokine content in WT versus Jα281ko mice. WT BALB/c and BALB/c-Jα281ko mice were given 3 mm excisional punch wounds and wound biopsies were collected 1, 2, 3, 5, and 7 d later. Skin biopsies from uninjured animals were used as time 0 controls. Wounds were homogenized in protease inhibitor cocktail and chemokine content was determined by ELISA. Data are represented as mean pg chemokine per mL of homogenate; n = 3–4 mice per group; *P < 0.05.
Neutrophil and Monocyte/Macrophage Infiltrates
To determine whether differential influx of inflammatory cells occurred as a result of the augmented chemokine production seen in wounds from NKT cell-deficient mice, we examined wound cell suspensions by flow cytometry to compare both the relative frequencies and absolute numbers of neutrophils and monocyte/macrophages in wounds from wild-type versus NKT cell-deficient mice. Interestingly, neither the kinetics nor magnitude of neutrophil or monocyte/macrophage infiltration differed between wild type and Jα281ko wounds at any of the time points measured (see Supplemental Tables 1 and 2). As expected, maximal neutrophil infiltration occurred at 24 h post-injury, while peak macrophage infiltration occurred 3 d after injury, which is in line with previous reports [25–27]. Given that these chemokines are also highly chemotactic for T cells, we also analyzed the overall numbers of CD3+ cells between wounds from wild type and knockout animals but found no differences in either the kinetics or magnitude of overall T cell infiltration (data not shown). To verify these flow cytometry data, we also determined neutrophil content by myeloperoxidase (MPO) assay and again found that wounds from wild type and Jα281ko animals contained equivalent neutrophil content (Supplemental Fig. 2). Therefore, the transient enhancement of select neutrophil and monocyte/macrophage chemokines seen in the absence of NKT cells did not result in greater or accelerated recruitment of these cell types to the wound.
Wound TGF-β1 Content is Enhanced in NKT Cell Knockout Animals
Equivalent inflammatory cell infiltrates in wild type and NKT cell knockout animals helps explain why knockout animals do not exhibit delayed wound closure, but it does not explain why their wound closure is actually accelerated. To address this question, we measured wound TGF-β1 content, since this growth factor is considered to be a major signal that facilitates wound closure via stimulation of fibroblast proliferation, collagen deposition, and induction of α smooth-muscle actin [28, 29].
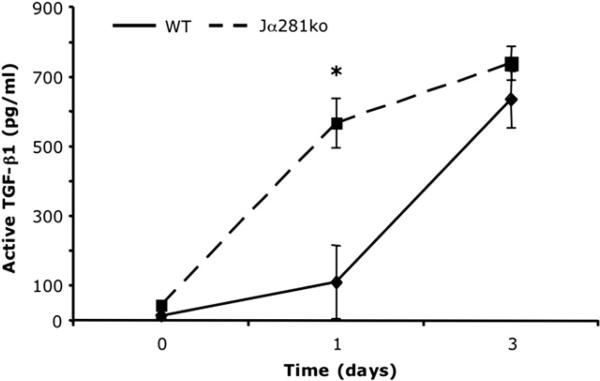
The active fraction of TGF-β1 was measured in wound protein extracts from wild type and Jα281ko animals. One day post-injury, wounds from Jα281ko animals contained greater than 5-fold more active TGF-β1 compared with wild type wounds (Fig. 6). By 3 d post-injury, the active TGF-β1 content in wild type and Jα281ko animals reached equivalence (Fig. 6). Like the chemokines (Fig. 5), the amount of biologically active TGF-β1 was transiently enhanced early after wounding when NKT cells were absent.
FIG. 6.
Wound TGF-β1 content in WT versus Jα281ko mice. WT BALB/c and BALB/c-Jα281ko mice were given 3 mm excisional punch wounds, and wound biopsies were collected 1 and 3 d later. Skin biopsies from uninjured animals were used as time 0 controls. Wounds were homogenized in protease inhibitor cocktail. The active fraction of TGF-β1 was determined by ELISA. Data are represented as mean pg TGF-β1 per mL of homogenate; n = 4 mice per group; *P < 0.05.
Wounds from NKT Cell Deficient Animals Display Augmented Collagen Deposition
Since TGF-β1 is one of the primary determinants for new matrix deposition within a healing wound [28], we speculated that local collagen gene expression might be enhanced in wounds from Jα281ko animals. To test this hypothesis, we examined the expression of the α2 procollagen(I) (COL1A2) gene in wild type and Jα281ko wounds using real-time PCR.
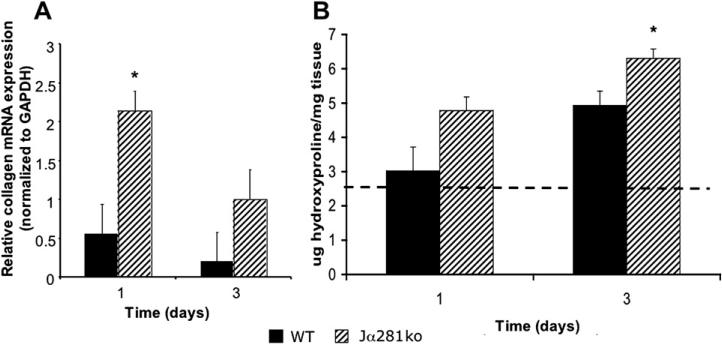
Using quantitative real-time polymerase chain reaction (PCR), we found that wounds from NKT cell deficient mice had nearly 4-fold more procollagen gene expression than wild type wounds at 1 d post-wounding (Fig. 7A). There were no statistical differences in procollagen gene expression between wild type and NKT cell deficient mice at d 3 or in normal skin from uninjured animals (d 0) (Fig. 7A).
FIG. 7.
Wound α2(I)-procollagen gene expression and collagen content in WT versus Jα281ko mice. WT BALB/c and BALB/c-Jα281ko mice were given 3 mm punch wounds and at d 1 and 3, wound mRNA expression for α2(I)-procollagen and GAPDH was quantitated by real-time polymerase chain reaction (PCR). Relative procollagen mRNA expression was determined by normalization of each sample to GAPDH expression (A). Wounds from the same animals were hydrolyzed in 6 N HCL and subjected to the hydroxyproline assay to quantitate wound collagen content (B); n = 4 mice per group; *P < 0.05.
Because there are numerous post-translational controls over collagen production and secretion, we acknowledged that procollagen gene expression alone may not correlate with collagen deposition. We therefore quantitated wound collagen content by hydroxyproline assay (Fig. 7B). Day three wounds from Jα281ko mice contained 27.9% more collagen than wild type wounds at the same time point (Fig. 7B). There was no statistical difference between d 1 wild type versus Jα281ko wound collagen content (Fig. 7B). The appearance of increased d 3 wound collagen content is consistent with our initial observations of accelerated wound closure (Figs. 1 and 2, Suppl. Fig. 1) as the inflammatory phase is still resolving 3 d post-wounding.
DISCUSSION
This is the first report of a role for NKT cells in cutaneous wound repair. Using the excisional punch wound model, we demonstrated that early wound closure was accelerated in the absence of NKT cells. Importantly, we also made the novel observation that NKT cells themselves are a constituent of the early wound inflammatory infiltrate, and their kinetics of accumulation coincide with neutrophil influx. We propose that under normal, nonpathologic wound repair processes that occur within the wound environment, NKT cells exert a regulatory role, since their absence led to increased local production of a select subset of neutrophil and monocyte/macrophage chemokines. Specifically, the dermal production of MIP-2, KC, MIP-1α, and MIP-1β was transiently enhanced when NKT cells were absent. This transient enhancement occurred during the first 3 d after wounding and correlated with the kinetics of NKT cell infiltration.
Despite the elevation of select neutrophil and monocyte/macrophage chemoattractants, wounds from NKT-deficient animals did not contain greater numbers of neutrophils or macrophages. Additionally, while inflammatory infiltrates were identical, wounds from NKT cell deficient animals had 5-fold greater levels of the fibrogenic cytokine TGF-β1 at 1 d post-wounding, which correlated with markedly enhanced procollagen gene expression in wounds from NKT cell deficient animals at the same time point (d 1). Day three wounds from NKT cell deficient animals contained more collagen than wild type wounds. Therefore, wounds from NKT cell deficient animals display augmented matrix deposition, consistent with an accelerated rate of closure.
Our current findings alter, and in some cases challenge, conventional dogma regarding the functionality of both the chemokines within the wound environment and NKT cells in general. Within the wound, chemokines are typically associated with inflammation, chemotaxis of inflammatory cells, and local immunity [30]. At first glance, these functions might seem inconsistent with accelerated wound closure as we observed among NKT cell deficient mice. NKT cells are traditionally associated with regulation of peripheral immunity and host defense. There are numerous reports that pair single NKT cell derived cytokines (either IL-4 or IFN-γ) with target cell(s) activation and cytokine production. In the studies reported here, we demonstrated alterations in the local production of a select chemokine subset when NKT cells were absent, suggesting that their regulatory functions can be more global than single cell, single mediator pairings. We hypothesize that the NKT cell regulates neutrophil and macrophage functions. The exact nature of the NKT cell–inflammatory cell interaction also requires further study, but the NKT cell literature provides examples of such an interaction [19, 24].
While the traditionally cited function of chemokines is leukocyte chemoattraction to sites of inflammation, it is known that chemotactic responses reach a threshold for recruitment despite increasing concentrations of the chemokine [30, 31]. Thus, it is reasonable to suggest that a nonpathologic healing wound may already be at or near such a threshold and the increased levels of chemokines seen in the wounds of NKT cell deficient mice do not further elicit cell recruitment, but instead activate additional cells within the wound (i.e., keratinocytes and fibroblasts) to accelerate the epithelial closure and fibroproliferative aspects of wound healing. It is also important to emphasize the transient nature of the chemokine elevations, which may not have offered time for additional cell recruitment. The seemingly paradoxical finding of accelerated wound closure despite enhanced pro-inflammatory signals becomes explicable when one considers the myriad of functions the chemokines exhibit within the wound environment. Beyond leukocyte recruitment, chemokines have known roles in angiogenesis, fibrosis, and re-epithelialization [32–35]. These alternative functions of chemokines might explain how augmented pro-inflammatory signals are not inconsistent with accelerated wound closure, especially given the transient nature of their elevation and the lack of additional inflammatory cell recruitment.
NKT cells are known to act locally in varied circumstances as others have described NKT cells infiltrating a variety of organs during states of inflammation [19, 24] including the skin, where NKT cells appear to instigate psoriatic plaques in susceptible models or individuals [36–38]. It is not surprising, therefore, that NKT cells would infiltrate the skin during early wound healing, another instance of cutaneous inflammation, albeit more transient. Here, we provide the first in vivo demonstration of NKT cells infiltrating the skin and, in contrast to these studies in psoriasis, NKT cells appear to negatively regulate the inflammatory process instead of propagating or perpetuating it. This difference is presumably due to differential cytokine secretion profiles resulting from APC-derived signals upon glycolipid antigen presentation. Our laboratory is currently investigating the wound-associated NKT cell phenotype further.
Other innate lymphocytes have been implicated in regulating cutaneous inflammation such as psoriasis. The γδ TCR-bearing dendritic epidermal T cell (DETC) regulates epidermal homeostasis in normal skin via its secretion of insulin-like growth factor 1 (IGF-1) [39], and epidermal renewal via its secretion of FGF-7 and 10 during wound repair [12]. Although there is evidence of an NKT–DETC interaction in mouse models of airway hyper-responsiveness [40], there is no known interaction between these two innate lymphocytes in the context of psoriasis or cutaneous injury.
In summary, this is the first description of NKT cells’ involvement in cutaneous wound repair. These studies describe the kinetics of NKT cell infiltration into cutaneous wounds during the first 3 d of wound repair. During this same period, the NKT cell also regulates the local production of selected chemokines, TGF-β1, and collagen deposition. NKT cells’ effect on the overall rate of wound closure also occurs during the first 3 d. During this early phase, their influence on wound repair is focused, transient, but dramatic. This specificity positions the NKT cell as an attractive therapeutic target for preventing nonhealing chronic wounds where the inflammatory phase becomes exaggerated or delayed.
Supplementary Material
ACKNOWLEDGMENTS
Jα281ko mice were generously provided by Dr. Masaru Taniguchi, Yokohama, Japan. We are grateful to Ms. Patricia Simms for assistance with flow cytometry, and to Dr. John Callaci and Ryan Himes for their assistance with real-time PCR. The studies presented here were supported by NIH R01 AI056108, NIH R21 AI073987, NIH T32 GM08750, and the Ralph and Marion C. Falk Medical Research Trust.
Footnotes
SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jss.2009.09.030
REFERENCES
- 1.Joyce S. CD1d and natural T cells: How their properties jump-start the immune system. Cell Molec Life Sci. 2001;58:442. doi: 10.1007/PL00000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawakami K, Yamamoto N, Kinjo Y, et al. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwenhuis EE, Matsumoto T, Exley MA, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nature Med. 2002;8:588. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 4.Faunce DE, Stein-Streilein J. NKT cell-derived RANTES recruits APCs and CD8 + T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol. 2002;169:31. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24 JαQ T cells in type 1 diabetes. Nature. 1998;1998(391):177. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 6.Van Kaer L. α-galactosylceramide therapy for autoimmune diseases: Prospects and obstacles. Nat Rev. 2005;5:31. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 7.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 8.Moodycliffe AM, Nghiem D, Clydesdale G, et al. Immune suppression and skin cancer development: Regulation by NKT cells. Nat Immunol. 2000;1:521. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda KH, Faunce DE, Taniguchi M, et al. NKT cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey DI, Konenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faunce DE, Gamelli RL, Kovacs EJ. A role for CD1d-NKT cells in injury-associated T cell suppression. J Leukoc Biol. 2003;73:747. doi: 10.1189/jlb.1102540. [DOI] [PubMed] [Google Scholar]
- 12.Jameson JL, Ugarte K, Chen N, et al. A role for skin γ δ T cells in wound repair. Science. 2002;296:747. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 13.Baum CL, Arpey CJ. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 14.Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: Shared and differential chemokine receptor expression among Va24 + VB11 + NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 16.Dovi JV, He L-K, DiPietro LA. Accelerated wound closure in neutrophil-delpeted mice. J Leukoc Biol. 2003;73:448. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 17.Wilson L, Fathke C, Isik F. Tissue dispersion and flow cytometry for the cellular analysis of wound healing. BioTechniques. 2002;32:548. doi: 10.2144/02323st07. [DOI] [PubMed] [Google Scholar]
- 18.Angel CE, George E, Brooks AE, et al. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Huang L, Sung SJ, et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 20.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JL, Tulley JM, Kovacs EJ, et al. Injury-induced suppression of effector T cell immunity requires CD1d-positive APCs and CD1d-restricted NKT cells. J Immunol. 2006;177:92. doi: 10.4049/jimmunol.177.1.92. [DOI] [PubMed] [Google Scholar]
- 22.DiPietro LA, Polverini PJ, Rahbe SM, et al. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol. 1995;146:868. [PMC free article] [PubMed] [Google Scholar]
- 23.Agaiby AD, Dyson M. Immuno-inflammatory cell dynamics during cutaneous wound healing. J Anat. 1999;195:531. doi: 10.1046/j.1469-7580.1999.19540531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SJ, Kim S, Park WS, et al. IL-4 secreting NKT cells prevent hypersensitivity pneumonitis by suppressing IFN-γ producing neutrophils. J Immunol. 2006;177:5258. doi: 10.4049/jimmunol.177.8.5258. [DOI] [PubMed] [Google Scholar]
- 25.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair. J Clin Invest. 1972;51:2009. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibovich SJ, Ross R. The role of the macrophage in wound repair. Am J Pathol. 1975;78:71. [PMC free article] [PubMed] [Google Scholar]
- 27.Singer AJ, Clark RAF. Cutaneous wound healing. N Eng J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 28.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1983;83:4167. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasek JT, Gabbiani G, Hinz B, et al. Myofibroblasts and mechanoregulation of connective tissue remodelling. Nat Rev. 2002;3:349. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 30.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513. [PubMed] [Google Scholar]
- 31.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032. [PubMed] [Google Scholar]
- 32.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1. [PubMed] [Google Scholar]
- 33.Devalaraja RM, Nanney LB, Du J, et al. Delayed wound healing in CXCR2 knock out mice. J Invest Dermatol. 2000;115:234. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor B1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T, Eckes B, Mauch C, et al. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1a loop. J Immunol. 2000;164:6174. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 36.Nickoloff BJ, Bonish BD, Wrone-Smith T, et al. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: Detection of T cells expressing receptors typically present on natural killer cells including CD94, CD158, CD161. Arch Dermatol. 1999;135:546. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- 37.Nickoloff BJ, Bonish BD, Huang BB, et al. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 38.Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4 + T cells induces psoriasis. Am J Pathol. 1999;155:145. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharp LL, Jameson JM, Cauvi G, et al. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 40.Jin N, Miyahara N, Roark CL, et al. Airway hyperresponsiveness through synergy of γ δ T cells and NKT cells. J Immunol. 2007;179:2961. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.