Abstract
The isolation of thionein (T) from tissues has not been reported heretofore. T contains 20 cysteinyl residues that react with 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide to form fluorescent adducts. In metallothionein (MT) the cysteinyl residues, which are bound to zinc, do not react. However, they do react in the presence of a chelating agent such as EDTA. The resultant difference in chemical reactivity provides a means to measure T in the absence of EDTA, (MT + T) in its presence, and, of course, MT by difference. The 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide derivative of T can be isolated from tissue homogenates by HPLC and quantified fluorimetrically with a detection limit in the femtomolar range and a linear response over 3 orders of magnitude. Analysis of liver, kidney, and brain of rats reveals almost as much T as MT. Moreover, in contrast to earlier views, MT in tissue extracts appears to be less stable than T. The existence of T in tissues under normal physiological conditions has important implications for its function both in zinc metabolism and the redox balance of the cell.
Chemical modification is one of the major avenues of approach to explore the function of proteins and enzyme active sites (1). The use of borohydride reduction of lysyl residues in enzyme-substrate Schiff-base complexes (2) or the use of quasi-substrates for the covalent trapping of catalytic groups in seryl enzymes (3) have become classic examples. The designation “active site” is now commonly used to refer to enzymes in which highly specialized regions are hyperreactive as compared with other parts of the enzyme molecule (4). In metallothionein (MT), the 20 cysteinyl residues that interact with seven zinc atoms in cluster networks are good candidates for such a role and the metal-binding cysteinyl clusters in thionein (T) would seem to represent such a branch of active site chemistry that could then become the basis of unique group labeling by covalent chemical modification. In this instant, the presence or absence of a metal can serve as an approach to the differential labeling of T in the presence of MT. In this regard, the fluorescent compound 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide (ABD-F) was used to modify the free sulfhydryl group of cysteine in peptides (5). A similar reagent, SBD-F (7-fluorobenz-2-oxa-1,3-diazole-4-sulfonate), labels MT, but only in the presence of a chelating agent (6), and we surmised that the protective effect of zinc in MT toward ABD-F would allow differential covalent labeling of T and MT in the presence and absence of a chelating agent. This turns out to be the case.
Indeed, labeling MT/T with the fluorescent probe ABD-F in the absence and presence of EDTA and in combination with chromatographic separation of the products does allow the determination of both MT + T and T and the calculation of the amount of MT as the difference between them. This differential labeling has established that the amount of T in rat liver, kidney, and brain is of the same order of magnitude as that of MT. There is no previous record of the isolation of T from tissues. Hence, the discovery that relatively and unexpectedly large amounts of T and MT concomitantly exist in cells opens avenues of approach and opportunity to study the biological activity and role of this protein in zinc and redox metabolism.
Materials and Methods
Materials.
Zinc-reconstituted MT and T (7) were prepared from rabbit liver cadmium MT-2 (Sigma). Rabbit liver T was stored at liquid nitrogen temperatures (8). Ammonium 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonate (SBD-F), ABD-F, and Tris-(2-carboxyethyl)phosphine (TCEP) were from Molecular Probes; sodium borate and EDTA were from Sigma. Prestained, broad range protein standards were from Bio-Rad.
Instrumentation.
A Hypersil C4 HPLC column (150 × 4.6 mm i.d.) and a precolumn were from Alltech Associates. Fluorescence was measured either with a Waters 470 scanning fluorescence detector or a FluoroMax-2 fluorimeter (Instrument SA, Edison, NJ) at room temperature. millenium32 chromatography software (Waters) was used for data acquisition and processing from the chromatographic system. The samples were maintained at 0°C by a cooling unit for the automatic injector.
Sample Preparation.
Freshly collected rat tissues were cut with scissors and homogenized on ice with 4 vol of buffer (0.2 M mannitol/0.05 M sucrose/10 mM potassium chloride/10 mM Hepes-Na+, pH 7.5). The nature and composition of this buffer do not affect the outcome of the analysis, nor does the buffer introduce any zinc into the sample. Homogenization was performed with a Potter-Elvehjem homogenizer by using 10 strokes with a rotating Teflon pestle with a clearance of less than 0.1 mm. Homogenates were spun at 10,000 × g for 5 min at 4°C. Acetonitrile was added to the supernatant to a final concentration of 40% (vol/vol). There was some acetonitrile-induced precipitation but this did not cause a loss of either MT (9) or T. After incubation for 10 min at room temperature, the supernatant was collected by centrifugation at 18,000 × g for 10 min at 4°C and derivatized with ABD-F. For this, a 100-μl sample was added to a mixture of 15 μl of 46 mM ABD-F in dimethyl sulfoxide, 15 μl of 300 mM TCEP, 3 μl of 0.5 M EDTA, and 0.2 M potassium borate-Cl− buffer, pH 7.5 (300 μl final volume). After incubation for 5 min at 50°C, the reaction mixture was cooled on ice and then split into two samples. One-half was subjected to ultrafiltration at 4°C with a Microcon YM-3 centrifugal concentrator (Millipore) at 12,000 × g and the filtrate was collected. Both samples then were analyzed by HPLC. At room temperature, a small, but significant, decrease of the fluorescence of the ABD-F derivative of T (ABD-T) with time was noted. Because of this effect, when performing multiple analyses, samples were kept in an automatic injector at 0°C, limiting systematic errors to less than 5%.
Gel Electrophoresis.
Tris-Tricine gradient gels and SDS running buffer were from Bio-Rad. For SDS/PAGE, 10 μl of 300 mM TCEP was added to a 15-μl sample, and the mixture was incubated for 10 min at room temperature. Then 25 μl of sample buffer [0.2 M Tris⋅HCl, pH 6.8/40% (vol/vol) glycerol/2% (wt/vol) SDS/0.04% (wt/vol) Coomassie brilliant blue G-250] was added, and the sample was incubated for at least 10 min and then loaded on the gel.
Results
Differential Covalent Labeling of MT and T and Isolation of Products.
In the presence of a chelating agent, ABD-F reacts with MT much faster than SBD-F (7-fluorobenz-2-oxa-1,3-diazole-4-sulfonate), leaving thiol groups less susceptible to oxidation and allowing more complete derivatization. At neutral pH and 50°C and with EDTA, the reaction is completed almost instantaneously for all three MT isoforms, i.e., MT-1, MT-2, and MT-3, yielding a derivative (ABD-T) that fluoresces strongly at 512 nm when excited at 384 nm. Thiol groups are more stable toward oxidation at neutral than at alkaline pH, making it possible to render the modification selective for sulfhydryls. ABD-F also reacts with amines, but the reaction is rather slow near neutral pH, and amine adducts emit at 590 nm when excited at 450 nm, quite different from where thiol adducts emit. Based on these emission properties we conclude that ABD-F preferentially modifies the thiol groups of MT. EDTA is only one type of chelating agent that is effective in this reaction. When testing different chelating reagents, the rates of the reaction follow the order 1,10-phenanthroline (stability constant logβ3 = 17.5) > N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (log K = 15.6) > EDTA (16.4) > nitrilotriacetic acid (10.7) > EGTA (12.9). Thus, it appears that the rates are not simply a function of the zinc binding constant of the chelating agent, but rather, that they are limited by ligand-substitution reactions (10).
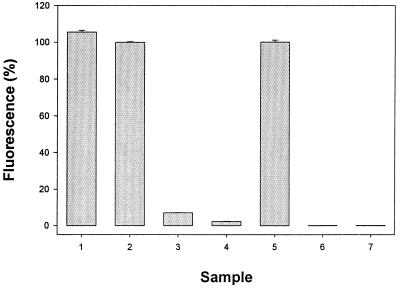
ABD-F derivatizes T maximally either in the absence or presence of EDTA (Fig. 1, bars 1 and 2) as determined by HPLC (see below). MT, on the other hand, undergoes almost no derivatization in the absence of EDTA. The amount of ABD-T formed is quite low (Fig. 1, bar 3) and is decreased even further on addition of zinc (Fig. 1, bar 4). In the presence of EDTA, however, MT also derivatizes maximally (Fig. 1, bar 5). The fluorescence spectrum of the T derivative is identical to that of MT, indicating that the modification leads to the same product in both cases. These data corroborate the finding that a chelating agent is essential for the modification of MT and that zinc MT is much less reactive than T. Thus, differential labeling in the absence of a chelating agent such as EDTA can be used to measure T directly.
Figure 1.
Fluorescence of the ABD-F derivatives of MT and T under different reaction conditions. Final concentrations: 0.6 μM T or zinc MT, 0.2 M potassium borate-Cl−, pH 7.5, 2.7 mM ABD-F. Reaction mixtures were incubated for 5 min at 50°C, and then analyzed by HPLC as described in the legend of Fig. 2. Samples: 1, T + TCEP; 2, T + TCEP + EDTA; 3, MT + TCEP; 4, MT + TCEP + Zn; 5, MT + TCEP + EDTA; 6, MT + EDTA; 7, T + EDTA. Concentrations of TCEP, EDTA, and Zn(NO3)2: 18 mM, 6 mM, and 3 μM, respectively. Data are given as percentages relative to sample 5 (n = 3).
Trialkylphosphines have been used for the specific, rapid reduction of disulfides over a wide range of pH values. They lack reactivity with residues other than cystine and are compatible with many alkylating agents (11, 12). Here, the water-soluble phosphine TCEP (13, 14) was used in all experiments. If oxidized forms of MT or T exist, these will be reduced readily by TCEP. Miyairi et al. (6) reported that tributylphosphine is essential for the modification of MT with 7-fluorobenz-2-oxa-1,3-diazole-4-sulfonate (SBD-F) in the presence of EDTA. Indeed, neither MT nor T reacts with ABD-F in the absence of TCEP (Fig. 1, bars 6 and 7), despite the fact that T retains its full complement of reduced sulfhydryl groups over this period. This finding suggests to us that the essentiality of TCEP in the derivatization of either MT or T is not due to its action as a reducing agent, but rather as a catalyst of the reaction between MT/T and ABD-F. Although the mechanism of the reaction is not known, tributylphosphines are indeed used as Lewis base catalysts in organic chemistry (15).
Thus, in the presence of EDTA, both MT and T react with ABD-F, and, hence their total concentrations can be determined. Importantly, in the absence of EDTA, only T reacts with ABD-F. Hence, only T is determined. The difference between the measurements is therefore the amount of MT.
The Presence of T in Rat Tissues.
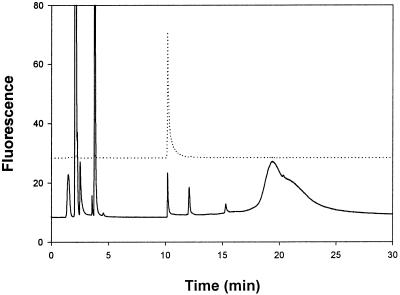
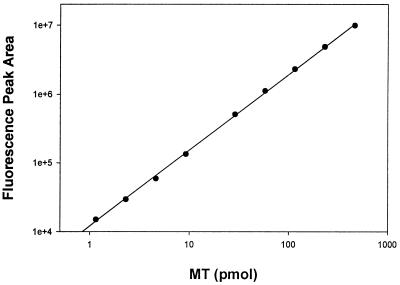
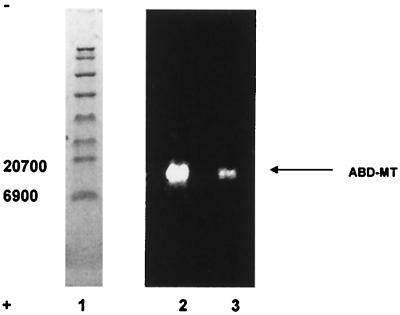
Tissue homogenates were treated with ABD-F in the presence of TCEP and EDTA. Reversed-phase chromatography on a C4 column with a 2-propanol gradient provided sufficient resolution to separate ABD-T from other labeled compounds. In the chromatograms, the ABD-F derivative prepared from either zinc MT-2 (Fig. 2, upper chromatogram) or MT + T in a rat liver sample (Fig. 2, lower chromatogram) elutes at 10.2 min as a sharp fluorescent peak. The peak area is proportional to the amount of MT injected from 1 to 1,000 pmol and the detection limit is below 100 fmol MT + T (Fig. 3). This exquisite sensitivity surpasses even that of RIAs. To determine whether or not the fluorescent material is solely ABD-T, fractions were pooled and subjected to SDS/PAGE (Fig. 4). Only one fluorescent band, which comigrates with ABD-T, is seen at 14.3 kDa. The pooled fraction was analyzed by fluorescence spectroscopy. It emits at 512 nm when excited at 384 nm and exhibits a spectrum identical with that of ABD-T. Hence this chromatographic method separates ABD-T from all other labeled compounds in the liver sample.
Figure 2.
Separation of ABD-T by reversed-phase HPLC. Samples were treated with acetonitrile and then derivatized with ABD-F in the presence of TCEP and EDTA as described in Materials and Methods. Chromatography was performed with a Hypersil C4 column. Mobile phase: A, 5 mM Tris⋅HCl, pH 7.5; B, 50% 2-propanol in A. Flow rate: 1 ml/min. The column was washed with 100% A for 2 min, followed by a linear gradient from 0 to 75% B for 15 min and another wash with 75% B. Excitation and emission wavelengths were 384 nm and 510 nm, respectively. The fluorescence detector gain was set at ×100 scale expansion. Injection volume was 50 μl. The dotted line is the chromatogram of the ABD-F derivative of purified MT (23 pmol). The solid line is the chromatogram of the derivatized rat liver sample (2 mg total protein).
Figure 3.
Relationship between fluorescence peak area and amount of ABD-T injected into the HPLC system. Different concentrations of zinc MT-2 were derivatized and then subjected to HPLC analysis as described in the legend of Fig. 2. Errors are too small to be displayed in the plot (n = 2).
Figure 4.
SDS/PAGE of ABD-T fractions from rat liver and ABD-T. The sample was prepared as described in Materials and Methods, subjected to HPLC as described in the legend of Fig. 2. The ABD-T peak fraction was pooled, and then analyzed on a 10–20% Tris-Tricine SDS gel with fluorescence detected on a UV transilluminator. Lane 1, prestained protein standard. Lane 2, ABD-T fraction from rat liver. Lane 3, ABD-F modified, purified rabbit zinc MT-2.
In rat liver, kidney, and brain, a large proportion of total MT is in the form of T (Table 1). The total MT content (MT + T) of liver is comparable to that measured by RIA (16, 17). Thus, RIAs must measure total MT. Values for total MT in brain and kidney are higher than those reported previously, however. The reason for this is that the fluorimetric assay measures all MT isoforms, whereas RIAs are specific for MT-1 and MT-2, and in some cases for MT-1 only. Thus, the presence of MT-3 in brain (18) and in kidney (19) makes a significant contribution to the total amount of MT measured by HPLC. In testis, the HPLC assay detects only about 10% of the total MT found by RIA.
Table 1.
MT/T in different rat organs
| Organ | Total MT (T + MT), nmol/g tissue | T/total MT, % | Total MT (T + MT), nmol/g tissue (16) | Total MT (T + MT), nmol/g tissue (17) |
|---|---|---|---|---|
| Liver | 4 ± 2 | 27 ± 5 | 5.2 ± 0.5 | 2.0 ± 0.8 |
| Kidney | 14.2 ± 1.3 | 54 ± 6 | 9.2 ± 1.5 | 10 ± 2 |
| Brain | 3.1 ± 0.2 | 53 ± 13 | N.D. | 0.6 ± 0.1 |
| Testis | 5.2 ± 0.1 | 9 ± 3 | N.D. | 37 ± 7 |
n = 3. Recoveries for MT and T added to tissue homogenates are greater than 90%. The recovery of added T, however, is only greater than 90% in the presence of EDTA. T added to a liver sample binds zinc and is converted to MT that does not react with ABD-F in the absence of EDTA. Chemical equilibria are the source of zinc. In particular, we have demonstrated that in vitro T removes zinc from enzymes that zinc inhibits significantly (37). The filtrate from the ultrafiltration step shows a small fluorescent peak at the position of ABD-T in the chromatogram, suggesting that some low molecular weight species generated by the modification reagent interfere with the analysis of ABD-T and escape detection by SDS/PAGE. A correction for this interference, which can contribute up to 10% of the total fluorescence, is made by subtracting the fluorescence of the YM-3 filtrate from that of the sample. N.D., not determined.
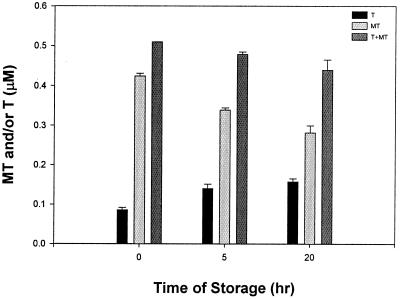
The total amount of MT in rat liver extracts decreases during storage at 4°C, so that after 20 h, only 80% of the total remains (Fig. 5). However, the amount of T increases concomitantly, and therefore the loss of MT is substantial. Some zinc must be released from MT to form T, but there likely is some degradation because the total amount decreases. These experiments emphasize the need to measure fresh samples. It was conjectured that T is not stable in vivo based on its rapid degradation by proteases in vitro (20). Our results suggest that MT is less stable than T.
Figure 5.
Stability of MT and T in rat liver extract. Rat liver extract was stored on ice for different lengths of time. The amounts of total MT (MT + T), MT, and T in the liver extract were measured as described in Materials and Methods. Analyses were performed in duplicate.
Discussion
Ever since its discovery (21), the detection, purification, and isolation of MT have presented challenges that attend the recognition and characterization of biologically essential moieties. The assays used most commonly, i.e., metal saturation† and immunological assays do not distinguish between MT and T. Apparently, such measurements were interpreted with the assumptions that the metal-loaded form, i.e., MT, is the only functionally significant constituent and that the rapid proteolytic digestion of T observed in vitro also occurs in vivo (20). Thus, it was inferred that in most cells the amount of T would be orders of magnitude lower than that of MT (23). This inference could never be tested rigorously, because there was no means to measure T directly, nor has this species ever been isolated from tissues. However, a comparison between the amounts of native Zn-MT and Cd-MT as a result of addition of cadmium suggested to some that T exists in various neoplastic tissues (24). It also was inferred that T exists in states of zinc deficiency (25) or in neoplastic tissue (26), where zinc might be limiting. The underlying assumption here was that zinc would be freely available such that T would readily form MT. Apparently, this is not the case: we found relatively large amounts of T under normal physiological conditions, thus opening additional perspectives on the functions of both T and MT. In particular, the finding suggests functions of MT that should be attributed to T instead.
The presence of T in normal tissue also suggests how this system should be dealt with, given that there are two components, not one. The MT/T system is highly dynamic and therefore several factors influence both the total amount and the ratio between the two species. First, analyses must be made on fresh tissues. In this regard, it is not so much the lability of T but of MT that biases the analysis. MT loses zinc during prolonged storage, and, hence, more T is found. Thus, for example, when an aliquot amount of rat liver was obtained from a sample stored at −80°C for 2 weeks, the T/MT + T ratio had increased from 27% to over 70%. This finding is in agreement with the observations that MT releases metal during storage of liver biopsies or homogenates (27, 28). The second factor is the cellular distribution of MT. MT is not an exclusively cytosolic protein. It is present in mitochondria (29), the nucleus (30–32), and probably other fractions as well; it could well be that different cellular fractions contain different amounts of the two species characterized by different MT/T ratios. Therefore, definition of the fraction prepared for analysis is critical. Third, the outcome of the analysis also depends on the control of the plethora of factors that induce the synthesis of T. Thus, even relatively mild stress induces T. Last, but not least, links of the MT/T system with both the redox and zinc states (33, 34) require that the redox state and metal ions in the system must be controlled. The findings also provide an additional definition of what is being purified in any attempt to isolate the protein. Isolation is commonly referred to as isolation of MT, because its metal content has proved to be one of the few characteristics that can be followed reliably during purification. Depending on the tissue, up to 90% of the protein will be discarded if the isolation is monitored solely by metal analyses. Much as the term MT has been an operational definition, the same applies to T. T does not necessarily represent only the apoform that is completely devoid of zinc with 20 reduced sulfhydryl groups. The procedure of covalently labeling T does not have the resolution at the molecular level to make this distinction. The high cooperativity of cadmium (and zinc) binding to T generally is taken as evidence for the existence of either fully zinc-loaded MT or completely metal-free T (23). If other forms exist, measurements are performed on an ensemble of molecules that possess variable numbers of free thiols and zinc and are capable of binding additional zinc.
The most important implications of the findings, however, concern our thinking about the functions of MT. The possible functional significance of T has been discussed repeatedly (35, 36). T is both a reducing and a chelating agent and is present in cells at micromolar concentrations. Thus, considering the high cysteine content of the protein (20/mol), T makes a significant contribution to the total cellular thiol redox buffering capacity. Furthermore, the high reactivity of cysteine thiols endows the protein with many possible functions, including a role as a free radical scavenger. Therefore, one may ask to what extent the antioxidant functions of MT found by in vivo studies, actually are functions of T.
Zinc is not freely available and in the presence of a strong chelating agent such as T, the concentration of “free” zinc must be very low. The presence of T in cells defines which zinc-protein interactions are likely to be significant for a regulatory role of cellular zinc. It appears highly unlikely that sites that bind zinc with micromolar binding constants would ever be occupied in the presence of T. On the other hand, low nanomolar concentrations of zinc inhibit a variety of enzymes. T activates these enzymes by removing zinc (37). It also has been shown that T inhibits the binding of zinc-containing transcription factors to DNA, presumably by removing zinc from the latter (38). Our results now suggest that a change in T/MT ratios could readily modulate such interactions.
Acknowledgments
This work was supported by the Endowment for Research in Human Biology, Inc.
Abbreviations
- ABD-F
7-fluorobenz-2-oxa-1,3-diazole-4-sulfonamide
- T
thionein
- MT
metallothionein
- ABD-T
ABD-F derivative of T
- TCEP
Tris-(2-carboxyethyl)phosphine
Footnotes
Metal saturation assays for MT are based on the strength of metal binding to MT, which is in the order Bi(III), Hg(II), Ag(I), Cu(I), Cd(II), Pb(II), Zn(II) (22). Thus, strongly binding metals that do not occur in vivo can be used to displace the native metals, the newly introduced metals are then measured either radiometrically or by atomic absorption spectroscopy, and the amount of MT is determined based on its known metal/protein stoichiometry.
References
- 1.Vallee B L, Riordan J F. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]
- 2.Fischer E H, Kent A B, Snyder E R, Krebs E G. J Am Chem Soc. 1958;80:2906–2907. [Google Scholar]
- 3.Balls A K, Jansen E F. Adv Enzymol. 1952;13:321–343. doi: 10.1002/9780470122587.ch8. [DOI] [PubMed] [Google Scholar]
- 4.Singer S J. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- 5.Toyo'oka T, Imai K. Anal Chem. 1984;56:2461–2464. [Google Scholar]
- 6.Miyairi S, Shibata S, Naganuma A. Anal Biochem. 1998;258:168–175. doi: 10.1006/abio.1998.2578. [DOI] [PubMed] [Google Scholar]
- 7.Vašák M. Methods Enzymol. 1991;205:41–44. doi: 10.1016/0076-6879(91)05082-7. [DOI] [PubMed] [Google Scholar]
- 8.Jacob C, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beattie J H, Richards M P, Self R. J Chromatogr. 1993;632:127–135. doi: 10.1016/0021-9673(93)80035-7. [DOI] [PubMed] [Google Scholar]
- 10.Otvos J D, Petering D H, Shaw C F., III Comments Inorg Chem. 1989;9:1–35. [Google Scholar]
- 11.Rüegg U T, Rudinger J. Methods Enzymol. 1977;47:111–126. doi: 10.1016/0076-6879(77)47012-5. [DOI] [PubMed] [Google Scholar]
- 12.Chin C C Q, Wold F. Anal Biochem. 1993;214:128–134. doi: 10.1006/abio.1993.1466. [DOI] [PubMed] [Google Scholar]
- 13.Han J C, Han G Y. Anal Biochem. 1994;220:5–10. doi: 10.1006/abio.1994.1290. [DOI] [PubMed] [Google Scholar]
- 14.Getz E, Xiao M, Chakrabarty T, Cooke R, Selvin P R. Anal Biochem. 1999;273:73–80. doi: 10.1006/abio.1999.4203. [DOI] [PubMed] [Google Scholar]
- 15.Barton D H R, Taran F. Tetrahedron Let. 1998;39:4777–4780. [Google Scholar]
- 16.Shaikh Z A. Methods Enzymol. 1991;205:120–130. doi: 10.1016/0076-6879(91)05094-c. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K, Suzuki K, Otaki N, Kimura M. Methods Enzymol. 1991;205:387–395. doi: 10.1016/0076-6879(91)05120-k. [DOI] [PubMed] [Google Scholar]
- 18.Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. Neuron. 1991;7:337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- 19.Hoey J G, Garrett S H, Sens M A, Todd J H, Sens D A. Toxicol Let. 1997;92:149–160. doi: 10.1016/s0378-4274(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 20.Feldman S L, Failla M L, Cousins R J. Biochim Biophys Acta. 1978;544:638–646. doi: 10.1016/0304-4165(78)90338-0. [DOI] [PubMed] [Google Scholar]
- 21.Margoshes M, Vallee B L. J Am Chem Soc. 1957;79:4813. [Google Scholar]
- 22.Kägi J H R, Kojima Y. In: Metallothionein II. Kägi J H R, Kojima Y, editors. Basel: Birkhäuser Verlag; 1987. pp. 25–61. [Google Scholar]
- 23.Kägi J H R. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser Verlag; 1993. pp. 29–55. [Google Scholar]
- 24.Pattanaik A, Shaw C F, III, Petering D H, Garvey J, Kraker A J. J Inorg Biochem. 1994;54:91–105. doi: 10.1016/0162-0134(94)80023-5. [DOI] [PubMed] [Google Scholar]
- 25.Krezoski S K, Villalobos J, Shaw C F, III, Petering D H. Biochem J. 1988;255:483–491. [PMC free article] [PubMed] [Google Scholar]
- 26.Kraker A J, Krakower G, Shaw C F, III, Petering D H, Garvey J S. Cancer Res. 1988;48:3381–3388. [PubMed] [Google Scholar]
- 27.Minkel D T, Poulsen K, Wielgus S, Shaw C F, III, Petering D H. Biochem J. 1980;191:475–485. doi: 10.1042/bj1910475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nostelbacher K, Kirchgessner M, Stangl G I. J Chromatogr B. 2000;744:273–282. doi: 10.1016/s0378-4347(00)00258-9. [DOI] [PubMed] [Google Scholar]
- 29.Ye B, Maret W, Vallee B L. Proc Natl Acad Sci USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. . (First Published February 13, 2001, 10.1073/pnas.041619198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panemangalore M, Banerjee D, Onosaka S, Cherian M G. Dev Biol. 1993;97:95–102. doi: 10.1016/0012-1606(83)90067-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsujikawa K, Imai T, Kakutani M, Kayamori Y, Mimura T, Otaki N, Kimura M, Fukuyama R, Shimizu N. FEBS Lett. 1991;283:239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- 32.Nagano T, Itoh N, Ebisutani C, Takatani T, Mioyishi T, Nakanishi T, Tanaka K. J Cell Physiol. 2000;185:440–446. doi: 10.1002/1097-4652(200012)185:3<440::AID-JCP15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maret W. J Nutr. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 35.Vallee B L. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser Verlag; 1987. pp. 5–16. [Google Scholar]
- 36.Vallee B L. Methods Enzymol. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- 37.Maret W, Jacob C, Vallee B L, Fischer E H. Proc Natl Acad Sci USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng J, Vallee B L, Kägi J H R. Proc Natl Acad Sci USA. 1991;88:9984–9988. doi: 10.1073/pnas.88.22.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]