Abstract
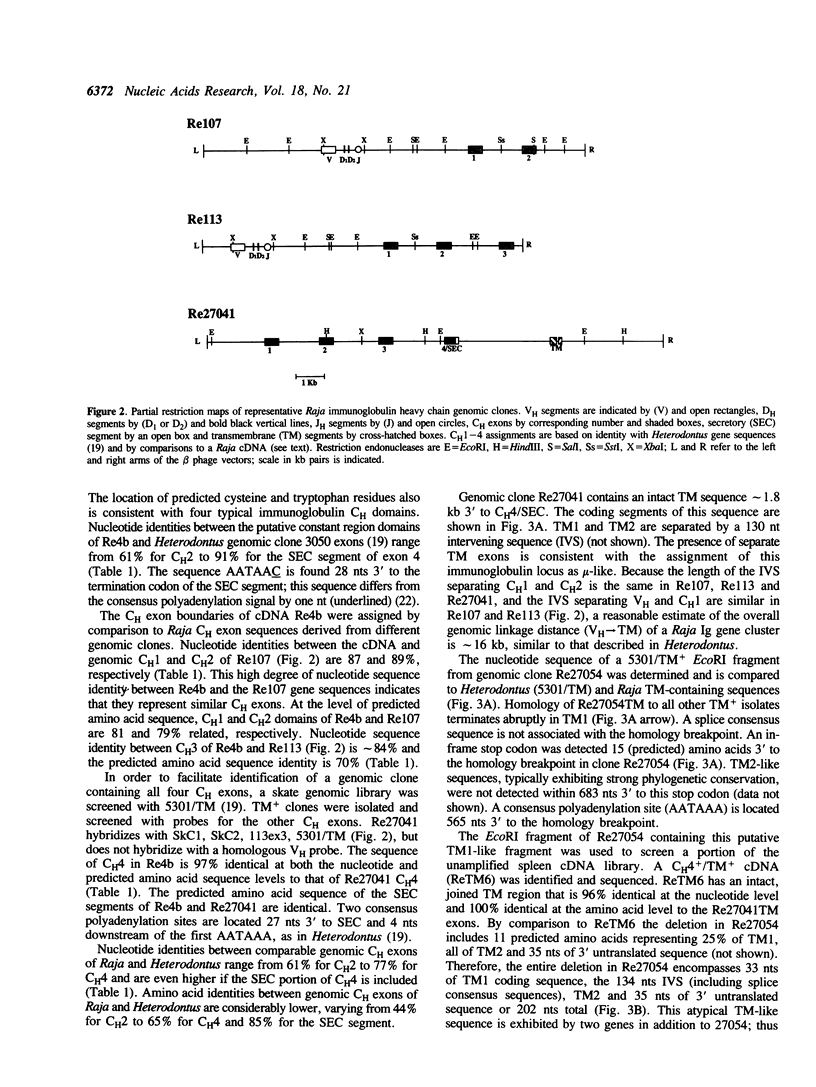
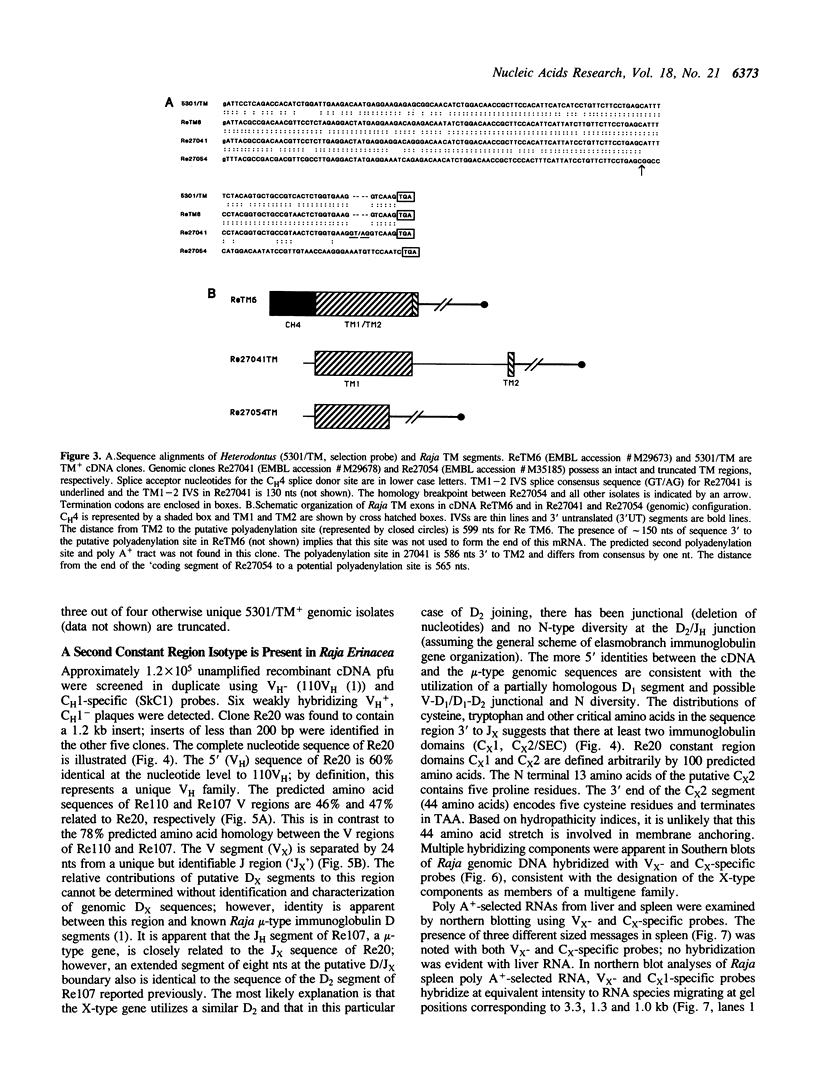
Immunoglobulin heavy chain genes in Raja erinacea (little skate) are organized in clusters consisting of VH, DH, JH segments and CH exons (1). An immunoglobulin heavy chain mu-like isotype that exhibits 61-91% nucleotide sequence identity in coding segments to the Heterodontus francisci (horned shark) mu-type immunoglobulin is described. The overall length of the mu-type clusters is approximately 16 kb; transmembrane exons (TM1 and TM2) are located 3 to CH exon 4 (CH4). In three of four TM-containing genomic clones, a significant deletion is present in TM1. A second isotype of Raja immunoglobulin heavy chain genes has been detected by screening a spleen cDNA library with homologous Raja VH- and CH1-specific probes complementing the respective regions of the mu-like isotype. Weak hybridization with VH-specific probes and no discernable hybridization with C mu-specific probes were considered presumptive evidence for a second immunoglobulin isotype that nominally is designated as X-type. The Vx region of the X-type cDNA is approximately 60% identical at the nucleotide (nt) level to other Raja VH segments and thus represents a second VH family. Putative Dx and Jx sequences also have been identified. The constant region of the X-type immunoglobulin heavy chain gene consists of two characteristic immunoglobulin domains and a cysteine-rich carboxy terminal segment that are only partially homologous with the mu-like isotype. Genomic Southern blotting indicates that the V and C segments of both immunoglobulin heavy chain isotypes are encoded by complex multigene families. Vx- and different Cx-specific probes hybridize to different length transcripts in northern blot analyses of Raja spleen RNA suggesting that the regulation of expression of the X-type genes may involve differential RNA processing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Cooper M. D., Kearney J. F., Lawton A. R., Parkhouse R. M. Sequential expression of immunoglobulin on developing mouse B lymphocytes: a systematic survey that suggests a model for the generation of immunoglobulin isotype diversity. J Immunol. 1978 Jun;120(6):2041–2049. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C., Radbruch A. Rapid induction of transcription of unrearranged S gamma 1 switch regions in activated murine B cells by interleukin 4. EMBO J. 1989 Feb;8(2):483–488. doi: 10.1002/j.1460-2075.1989.tb03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Harding F. A., Cohen N., Litman G. W. Immunoglobulin heavy chain gene organization and complexity in the skate, Raja erinacea. Nucleic Acids Res. 1990 Feb 25;18(4):1015–1020. doi: 10.1093/nar/18.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Miyata T., Honjo T. Repetitive sequences in class-switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981 Feb;23(2):357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tomonaga S., Kajii T. A second class of immunoglobulin other than IgM present in the serum of a cartilaginous fish, the skate, Raja kenojei: isolation and characterization. Mol Immunol. 1984 May;21(5):397–404. doi: 10.1016/0161-5890(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tomonaga S., Teshima K., Kajii T. Ontogenic studies on the appearance of two classes of immunoglobulin-forming cells in the spleen of the Aleutian skate, Bathyraja aleutica, a cartilaginous fish. Eur J Immunol. 1985 Sep;15(9):952–956. doi: 10.1002/eji.1830150916. [DOI] [PubMed] [Google Scholar]
- Kokubu F., Hinds K., Litman R., Shamblott M. J., Litman G. W. Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. EMBO J. 1988 Jul;7(7):1979–1988. doi: 10.1002/j.1460-2075.1988.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu F., Hinds K., Litman R., Shamblott M. J., Litman G. W. Extensive families of constant region genes in a phylogenetically primitive vertebrate indicate an additional level of immunoglobulin complexity. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5868–5872. doi: 10.1073/pnas.84.16.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu F., Litman R., Shamblott M. J., Hinds K., Litman G. W. Diverse organization of immunoglobulin VH gene loci in a primitive vertebrate. EMBO J. 1988 Nov;7(11):3413–3422. doi: 10.1002/j.1460-2075.1988.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt N., Briggs D., Gil A., Proudfoot N. J. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989 Jul;3(7):1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- Litman G. W., Berger L., Murphy K., Litman R., Hinds K., Erickson B. W. Immunoglobulin VH gene structure and diversity in Heterodontus, a phylogenetically primitive shark. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2082–2086. doi: 10.1073/pnas.82.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W., Fromme D., Chartrand S. L., Finstad J., Good R. A. Significance of heavy chain mass and antigenic relationship in immunoglobulin evolution. Immunochemistry. 1971 Apr;8(4):345–349. doi: 10.1016/0019-2791(71)90156-x. [DOI] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- Marcu K. B. Immunoglobulin heavy-chain constant-region genes. Cell. 1982 Jul;29(3):719–721. doi: 10.1016/0092-8674(82)90431-7. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Luzzati A. L. Synthesis of multiple immunoglobulin classes by single lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):175–183. doi: 10.1101/sqb.1977.041.01.023. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankey T. V., Clem L. W. Phylogeny of immunoglobulin structure and function. IX. Intramolecular heterogeneity of shark 19S IgM antibodies to the dinitrophenyl hapten. J Immunol. 1980 Dec;125(6):2690–2698. [PubMed] [Google Scholar]
- Wabl M., Meyer J., Beck-Engeser G., Tenkhoff M., Burrows P. D. Critical test of a sister chromatid exchange model for the immunoglobulin heavy-chain class switch. Nature. 1985 Feb 21;313(6004):687–689. doi: 10.1038/313687a0. [DOI] [PubMed] [Google Scholar]