Abstract
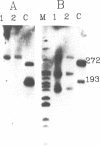
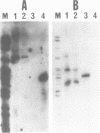
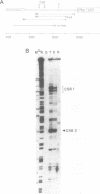
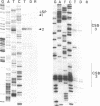
Transcription of the light strand of Xenopus laevis mitochondrial DNA initiates at two promoters located approximately 350 to 450 nucleotides upstream from the 5' ends of major D-loop DNA strands. Small RNAs within this region have been mapped by blot hybridization, primer extension and S1 nuclease protection methods. The results reveal that the large majority of RNAs within this region have 3' termini located at a sequence element, designated CSB 2, that is conserved in sequence and position in Xenopus, mouse, rat and human mtDNA. However, the X. laevis CSB 2 appears to be a site of RNA processing only, since RNA-to-DNA transitions are not detectable at this site. RNAs containing sequences downstream of CSB 2 are extremely rare. A significant fraction of these RNAs are processed by cleavage at a site just upstream of the most predominant 5' ends of D-loop DNAs. We suggest that RNA processing at this site may play a role in priming mtDNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barat M., Dufresne C., Pinon H., Tourte M., Mounolou J. C. Mitochondrial DNA synthesis in large and mature oocytes of Xenopus laevis. Dev Biol. 1977 Jan;55(1):59–68. doi: 10.1016/0012-1606(77)90319-0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Romanelli M. F. Template sequences required for transcription of Xenopus laevis mitochondrial DNA from two bidirectional promoters. Mol Cell Biol. 1988 Jul;8(7):2917–2924. doi: 10.1128/mcb.8.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Yoza B. K. Accurate in vitro transcription of Xenopus laevis mitochondrial DNA from two bidirectional promoters. Mol Cell Biol. 1986 Jul;6(7):2543–2550. doi: 10.1128/mcb.6.7.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Yoza B. K., Cairns S. S. Identification of initiation sites for transcription of Xenopus laevis mitochondrial DNA. J Biol Chem. 1986 Jun 25;261(18):8488–8494. [PubMed] [Google Scholar]
- Brown G. G., Gadaleta G., Pepe G., Saccone C., Sbisà E. Structural conservation and variation in the D-loop-containing region of vertebrate mitochondrial DNA. J Mol Biol. 1986 Dec 5;192(3):503–511. doi: 10.1016/0022-2836(86)90272-x. [DOI] [PubMed] [Google Scholar]
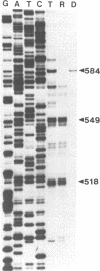
- Cairns S. S., Bogenhagen D. F. Mapping of the displacement loop within the nucleotide sequence of Xenopus laevis mitochondrial DNA. J Biol Chem. 1986 Jun 25;261(18):8481–8487. [PubMed] [Google Scholar]
- Callen J. C., Dennebouy N., Mounolou J. C. Development of the mitochondrial mass and accumulation of mtDNA in previtellogenic stages of Xenopus laevis oocytes. J Cell Sci. 1980 Feb;41:307–320. doi: 10.1242/jcs.41.1.307. [DOI] [PubMed] [Google Scholar]
- Callen J. C., Tourte M., Dennebouy N., Mounolou J. C. Changes in D-loop frequency and superhelicity among the mitochondrial DNA molecules in relation to organelle biogenesis in oocytes of Xenopus laevis. Exp Cell Res. 1983 Jan;143(1):115–125. doi: 10.1016/0014-4827(83)90114-3. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987 Mar 6;235(4793):1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Fisher R. P., Clayton D. A. Roles for a promoter and RNA processing in the synthesis of mitochondrial displacement-loop strands. Biochim Biophys Acta. 1987 Jul 14;909(2):85–91. doi: 10.1016/0167-4781(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Hauswirth W. W., Clayton D. A. Replication priming and transcription initiate from precisely the same site in mouse mitochondrial DNA. EMBO J. 1985 Jun;4(6):1559–1567. doi: 10.1002/j.1460-2075.1985.tb03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Dawid I. B. Biogenesis of mitochondria during Xenopus laevis development. Dev Biol. 1972 Apr;27(4):504–518. doi: 10.1016/0012-1606(72)90189-3. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: RNA priming during the initiation of heavy-strand synthesis. J Mol Biol. 1979 Dec 5;135(2):353–368. doi: 10.1016/0022-2836(79)90441-8. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L. Mitochondrial DNA in Xenopus laevis oocytes. I. Displacement loop occurrence. Dev Biol. 1974 Jun;38(2):346–355. doi: 10.1016/0012-1606(74)90012-8. [DOI] [PubMed] [Google Scholar]
- King T. C., Low R. L. Mapping of control elements in the displacement loop region of bovine mitochondrial DNA. J Biol Chem. 1987 May 5;262(13):6204–6213. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., Clayton D. A. Mechanism of replication of human mitochondrial DNA. Localization of the 5' ends of nascent daughter strands. J Biol Chem. 1981 May 25;256(10):5109–5115. [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Characterization of human MRP/Th RNA and its nuclear gene: full length MRP/Th RNA is an active endoribonuclease when assembled as an RNP. Nucleic Acids Res. 1990 Feb 25;18(4):793–799. doi: 10.1093/nar/18.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A. C., Smith L. D. Accumulation of mitochondrial DNA during oogenesis in Xenopus laevis. Dev Biol. 1977 Mar;56(1):219–225. doi: 10.1016/0012-1606(77)90166-x. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoza B. K., Bogenhagen D. F. Identification and in vitro capping of a primary transcript of human mitochondrial DNA. J Biol Chem. 1984 Mar 25;259(6):3909–3915. [PubMed] [Google Scholar]