Abstract
The CyclinB1-Cdk1 kinase is the catalytic activity at the heart of Mitosis Promoting Factor (MPF), yet fundamental questions concerning its role in mitosis remained unresolved. It is not known when and how rapidly CyclinB1-Cdk1 is activated in mammalian cells, nor how its activation coordinates the substantial changes in the cell at mitosis. Here, we have developed a FRET biosensor specific for CyclinB1-Cdk1 that enables us to assay its activity with very high temporal precision in living human cells. We show that CyclinB1-Cdk1 is inactive in G2 phase and activated at a set time before nuclear envelope breakdown, thereby initiating the events of prophase. CyclinB1-Cdk1 levels rise to their maximum extent over the course of approximately 30 min and we demonstrate that different levels of CyclinB1-Cdk1 kinase activity trigger different mitotic events, thus revealing how the remarkable reorganisation of the cell is coordinated at mitotic entry.
Introduction
Progress through the cell cycle is driven by members of the Cyclin-dependent kinase (Cdk) family when bound to their Cyclin partner. There are two types of Cyclin that are important for mitosis in animal cells: Cyclin A, which also has an important role in DNA replication, and CyclinB. CyclinB in partnership with Cdk1 was purified as the catalytic activity in Mitosis Promoting Factor (MPF) from Xenopus and starfish eggs that upon injection drives interphase cells into mitosis (Gautier et al., 1990; Labbé et al., 1989; Lohka et al., 1988). Mammalian cells have two B-type Cyclins, Cyclins B1 and B2, but only CyclinB1 is essential in mice (Brandeis et al., 1998): CyclinB2 has a more restricted role in reorganising the Golgi apparatus at mitosis (Draviam et al., 2001). As the crucial mitotic inducer, the activity of CyclinB-Cdk complexes is carefully regulated: once a B-type Cyclin binds to Cdk1, the complex is inactivated by the Wee1 kinase family that phosphorylates Cdk1 in the ATP binding site on a tyrosine residue and, in animal cells, an adjacent threonine residue (T14 and Y15 in human Cdk1) (Morgan, 2007). Subsequently, the pool of inactive CyclinB-Cdk1 is activated by the Cdc25 phosphatase family (Cdc25A, B and C in mammals) and the active kinase phosphorylates a large number of substrates to reorganise the architecture of the cell for mitosis (reviewed in (Nigg, 1993). Thus, the activation of CyclinB-Cdk1 is generally considered to trigger entry to mitosis. This role has been challenged, however, in Drosophila, where Cyclin A rather than CyclinB is essential (Knoblich and Lehner, 1993), and by recent siRNA studies in human cells, which reported that CyclinB1 is not essential for mitotic entry and that CyclinA2 is required for nuclear envelope breakdown (NEBD) (Gong et al., 2007; Soni et al., 2008).
Both the Wee1 and Cdc25 families are targets of cell cycle checkpoints that prevent premature mitosis in the presence of unreplicated or damaged DNA (Morgan, 2007). Experiments using Xenopus eggs and mammalian cells indicate that there are feedback loops between CyclinB-Cdk1 and some of the Cdc25 isoforms (Izumi and Maller 1993, Hoffmann 1993). More recently, elegant experiments using cell-free extracts from Xenopus eggs showed that Wee1 exhibits ultrasensitivity to inactivation by CyclinB-Cdk1 (Kim and Ferrell, 2007), thereby generating bi-stable states of CyclinB-Cdk1 activity – i.e.: either very low or fully active (Pomerening et al. 2003, Sha et al. 2003, reviewed in Lindqvist 2009). The temporal resolution of experiments measuring CyclinB1-Cdk1 kinase activity until now, however, has been inherently limited because in vitro kinase assays require populations of cells that are never perfectly synchronised, and immunofluorescence cannot reveal the kinetics of activation through time. Mammalian CyclinB1-Cdk1, therefore, has been reported to be activated at various times from G2 phase through to prometaphase (Gong et al., 2007; Jackman et al., 2003; Lindqvist et al., 2007)(reviewed in (Lindqvist et al., 2009)). Thus, despite its importance to cell division, exactly when CyclinB1-Cdk1 is first activated in somatic cells and the kinetics of its activation are still unknown. To overcome this limitation, we have developed a FRET-based biosensor to measure CyclinB1-Cdk1 activity – or rather the balance between CyclinB1-Cdk1 and an antagonistic phosphatase(s) - in individual living mammalian cells as they enter mitosis. With this probe we reveal that CyclinB1-Cdk1 activation initiates prophase, and that increasing levels of CyclinB1-Cdk1 activity trigger different mitotic events. Thus, we have begun to reveal how the remarkable changes in cell architecture at mitotic entry are coordinated.
Results
We designed a biosensor to detect CyclinB1-Cdk1 kinase activity in living cells, taking as a template the Förster Resonance Energy Transfer (FRET) sensor for protein kinase A (PKA) (Zhang et al., 2001), where the donor and acceptor fluorescent proteins are linked by a phosphorylation site specific for the kinase and a phosphobinding domain (Fig.S1A). When this linker site is phosphorylated it is bound by the phosphobinding domain, thereby inducing a conformational change that alters the FRET efficiency between the donor and acceptor proteins that can be quantified by calculating the ratio of donor to acceptor emission fluorescence intensities (see Experimental Procedures). As the FRET pair we used mCerulean (Rizzo et al., 2004) and YPet (Nguyen and Daugherty, 2005) fluorescent proteins, and as the phosphobinding domain we used the Polo Box Domain (PBD) of Plk1 that binds to CDK phosphorylation sites in vivo (Elia et al., 2003). The key to any protein kinase biosensor is the specificity of the phosphorylation site and we required a site that would be phosphorylated by CyclinB1-Cdk1 but not by any other Cyclin-Cdk or related kinase. Therefore, we used 16 amino acids encompassing the autophosphorylation site from human CyclinB1 (Hagting et al., 1999). This site is only phosphorylated when CyclinB1-Cdk1 is activated (Borgne et al., 1999; Jackman et al., 2003), indicating that it is not recognised by other Cyclin-Cdks in the cell. We changed the residue at position −1 to a serine to optimise binding between the autophosphorylation site and the PBD, a position that has minimal effects on recognition by Cyclin-Cdks (Holmes and Solomon, 1996).
The biosensor specifically measures CyclinB1-Cdk1 activity
We expressed our biosensor in HeLa cells and observed that the emission ratio changed only as cells performed mitosis (Fig.1A, video 1). This change in the emission ratio was caused by an increase in the FRET efficiency because photobleaching the acceptor caused an almost two-fold higher increase in the fluorescence emission from the donor in mitosis compared with G1 or G2 phase (Fig.S1C).
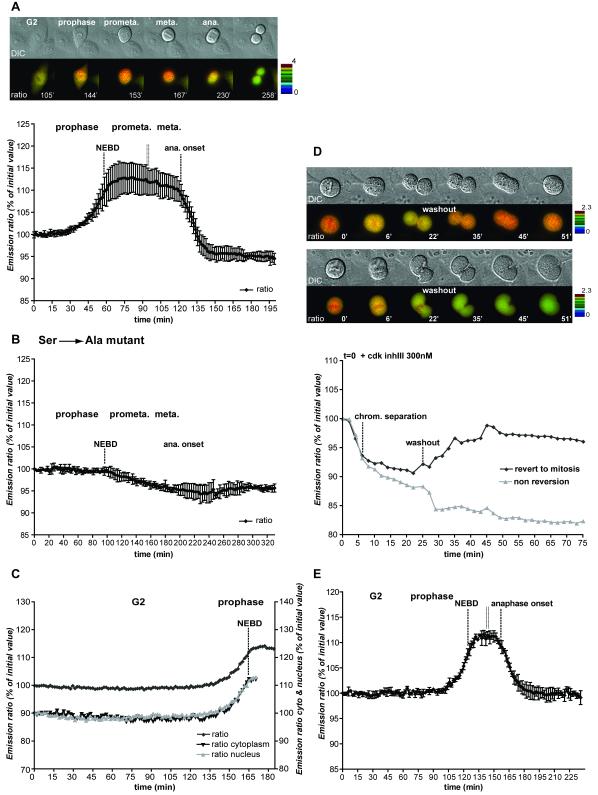
Figure 1. FRET dynamics during mitotic progression.
A. HeLa cells expressing the CyclinB1-Cdk1 activity sensor were recorded at 1 image/1’40 ”. Top: IMD representation of the FRET dynamics during mitotic progression. Bottom: Average curve of the quantification of the emission ratio over time in different cells (n=6). Quantification curves were aligned on NEBD and were eventually interrupted in prometaphase (double vertical line) to be aligned at the onset of anaphase. Note that the emission ratio is slightly lower in G1 than in G2. This seems to be due to a differential bleaching of YFP versus CFP during recording and cell rounding in some HeLa cells. Note that this decline is not seen in RPE cells (see Fig.1E). B. Average quantification curve of HeLa cells expressing the inactive CyclinB1-Cdk1 activity sensor containing the Ser126 to Ala mutation (n=5). The slight decrease in the emission ratio during mitosis is due to cell rounding up and differential bleaching of YFP versus CFP. C. Quantification of the emission ratio for the whole cell, the nucleus or the cytoplasm only in a HeLa cell progressing from G2 to mitosis. D. Dynamics of FRET signal during re-entry to mitosis. HeLa cells co-expressing the CyclinB1-Cdk1 activity sensor and a non-degradable CyclinB1L42A/R45A-mCherry were arrested in metaphase and forced to exit mitosis by adding 300 nM Cdk inhibitor III (t=0). After ~27 min the drug was extensively washed out. The top cell re-enters mitosis whereas the bottom cell does not. The quantification curves of the 2 cells are displayed. Note that in this figure the FRET level in prometaphase was set at 100%. E. Average curve of the quantification of the emission ratio during mitosis in RPE cells (n=5) expressing the CyclinB1-Cdk1 activity sensor. Curves were aligned on NEBD and the onset of anaphase as in A.
Extensive experiments demonstrated that the change in FRET efficiency of the sensor specifically responded to CyclinB1-Cdk1 kinase activity. First of all the change in FRET during mitosis required phosphorylation of the linker site because there was no change in FRET efficiency when alanine was substituted at this position (Fig.1B, video 2, n=25, 3 independent experiments), and we confirmed that the phosphorylation was mitosis-specific using an antibody that specifically recognised phosphorylation on the autophosphorylation site of CyclinB1 (Fig.S1B). Crucially, the sensor was not phosphorylated by other Cyclin-Cdk complexes in vivo, which we demonstrated in several ways. We co-expressed a DsRed1-DNA ligase I fusion protein to identify cells in S phase by their replication foci (Easwaran et al., 2004) and found that there was no change in the FRET efficiency in G1 phase (when CyclinB1 is absent) or early S phase (when CyclinE-Cdk2 is most active) (Fig.S2A, video 3 for HeLa cells; Fig.S2B for RPE cells). FRET efficiency was also constant during G2 phase where CyclinA2-Cdk2 and CyclinA2-Cdk1 activities progressively increase (Fig.1C) (Koop, 2007; Pines and Hunter, 1990). Furthermore, there was no difference in the FRET signal between the nucleus and the cytoplasm in G2 phase, whereas CyclinA2 is predominantly nuclear (Fig.1C). Moreover, adding either of two Cdk inhibitors (Cdk1/2 inhibitor III or RO3306) did not affect the FRET signal in G1, S or G2 phases, but immediately reversed it in prometaphase or metaphase cells (Fig.S3A&B). Inhibitors of other kinases (Aurora B, Plk1 and MAP kinases) had no direct effect on the FRET signal (Fig.S3B and data not shown).
Further proof that CyclinA2-Cdk activity did not contribute to the FRET signal was that the signal continued to increase in prometaphase after NEBD when CyclinA2, but not CyclinB1, is degraded (den Elzen and Pines, 2001; Geley et al., 2001) (Figs 1A, C, E). To confirm this we blocked cells in prometaphase with nocodazole and monitored CyclinA2 degradation using a CyclinA2-mCherry fusion protein. This showed that the FRET signal remained unchanged while CyclinA2 was degraded (Fig.S4A, n=5/5). Moreover, the FRET signal immediately decreased upon the addition of a Cdk inhibitor (Cdk1/2 inhibitor III, Fig.S4A) indicating that the biosensor was phosphorylated by another mitotic Cyclin-Cdk, almost certainly CyclinB1-Cdk1. Our biosensor appeared to be primarily responsive to CyclinB1-Cdk1 because the FRET profile was identical in wild-type mouse embryo fibroblasts (MEFs) and in MEFs lacking CyclinB2 (kind gift of Dr Tim Hunt) (Fig.S4B). Finally, the sensor faithfully responded to changes in CyclinB1-Cdk1 activity level. Expressing a non-degradable version of CyclinB1 (R42A/L45A) arrested cells in mitosis and the FRET signal remained high, but it dropped as soon as we added a Cdk inhibitor (Fig.1D). These cells then exited mitosis and degraded the endogenous B-type Cyclins ((Potapova et al., 2006) and data not shown). Washing out the inhibitor caused a proportion of the cells to re-enter mitosis driven specifically by the reactivation of the non-degradable CyclinB1-Cdk1, now the only mitotic Cyclin-Cdk in the cell (Potapova et al., 2006), and in these cells the FRET signal increased to its original level before addition of the inhibitor (Fig.1D, video 4, n=5/5).
Thus, we conclude that our biosensor specifically assays CyclinB1-Cdk1 activity during the cell cycle, although the final readout is clearly the balance between CyclinB1-Cdk1 activity and that of the antagonistic phosphatase(s) that recognises the biosensor (see Discussion), which in effect is the biological activity of Cyclin B1-Cdk1.
CyclinB1-Cdk1 is initially activated in prophase
With a CyclinB1-Cdk1 specific sensor we were in a position to measure exactly when, and with what kinetics, CyclinB1-Cdk1 was activated during the cell cycle. We found that CyclinB1-Cdk1 was inactive during G2 phase (Figs S2&S3), and only began to be activated at the start of prophase (Fig.1A, n=50, 6 independent experiments and video 1). CyclinB1-Cdk1 was initially activated in HeLa cells 27 +/−7 min before NEBD (Fig 1A). After its initial activation, CyclinB1-Cdk1 activity increased progressively in prophase and continued to increase for ~10 min after NEBD. We confirmed these results using hTert-RPE cells where CyclinB1-Cdk1 activity first increased 18 +/−6 min before NEBD and continued to increase for ~6.5 min in prometaphase (Fig.1E, n=18, 2 independent experiments). The maximum extent of the FRET change was independent of the expression level of the biosensor, indicating that the biosensor did not become a limiting substrate during mitosis (Fig.S5). Equally, the biosensor had no effect at any expression level on the timing of events at mitotic entry such as centrosome maturation or the accumulation of CyclinB1 in the nucleus that we have found is an excellent marker for the activation of CyclinB1-Cdk1 (Fig.S6A and Gavet and Pines, in press). At high expression levels the biosensor delayed cells in prometaphase, probably because its PBD competed with endogenous Plk1 and activated the SAC (Fig.S6B) (Seong et al., 2002).
Plk1 is not required for the amplification of human CyclinB1-Cdk1 activity
Our ability to measure the kinetics with which CyclinB1-Cdk1 was activated in living cells allowed us to begin to determine the roles of the protein kinases and phosphatases that have been implicated in the control of its activity.
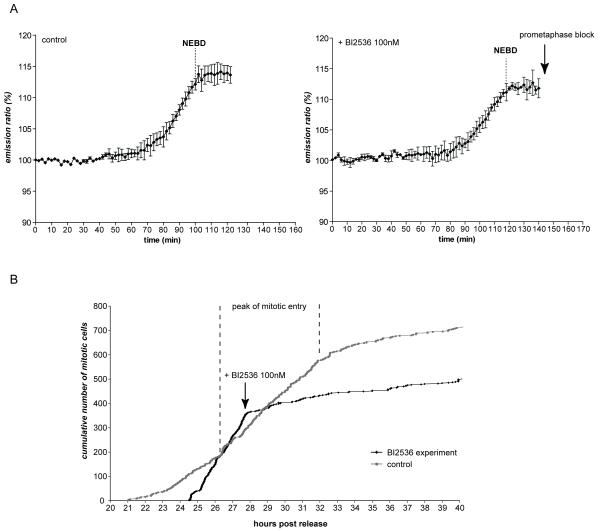
We first investigated the role of Polo-like kinase 1 (Plk1) because Plx1 (its Xenopus homologue) had been reported to form an amplification loop with Cdc25 essential for CyclinB1-Cdk1 activation (Abrieu et al., 1998). We compared precisely the kinetics of CyclinB1-Cdk1 activation in the presence and absence of Plk1 activity by recording the FRET signal before and after adding a potent Plk1 inhibitor (BI 2536) (Lenart et al., 2007) (Fig.2A). As expected, all cells entering mitosis in the presence of the Plk1 inhibitor blocked in prometaphase due to activation of the SAC (Lenart et al., 2007), demonstrating that the inhibitor was effective. In both cell types, inhibiting Plk1 induced an immediate and strong delay in mitotic entry in most cells that lasted for a few hours (Fig.2B, video 5 and data not shown), but once the cells initiated the activation of CyclinB1-Cdk1 they entered mitosis with normal kinetics (Fig.2A): there was no difference in either the kinetics or the extent of change in FRET efficiency in the presence or absence of the inhibitor (Fig.2A). Thus, in human cells, Plk1 does not appear to act in an amplification loop with human CyclinB1-Cdk1 and we conclude that Plk1 acts upstream of CyclinB1-Cdk1 activation.
Figure 2. Plk1 activity is required for mitotic entry but not for CyclinB1-Cdk1 activation per se.
A. The FRET signal was recorded (1 image/2min) during mitotic entry in HeLa cells expressing the CyclinB1-Cdk1 activity sensor before (left panel) and after (right panel) addition of Plk1 inhibitor BI2536 (100 nM) to the same dish. Average curve of 3 cells is displayed for each condition. B. RPE cells were synchronized in G2 phase by release from serum starvation and recorded at 1 image/2 min by DIC microscopy. The peak of mitotic entry is indicated by vertical dashed lines (~27-32h after serum re-addition). The addition of the Plk1 inhibitor BI2536 100 nM rapidly induces a strong delay in mitotic entry.
CyclinB1-Cdk1-dependent phosphorylation begins to be reversed at anaphase
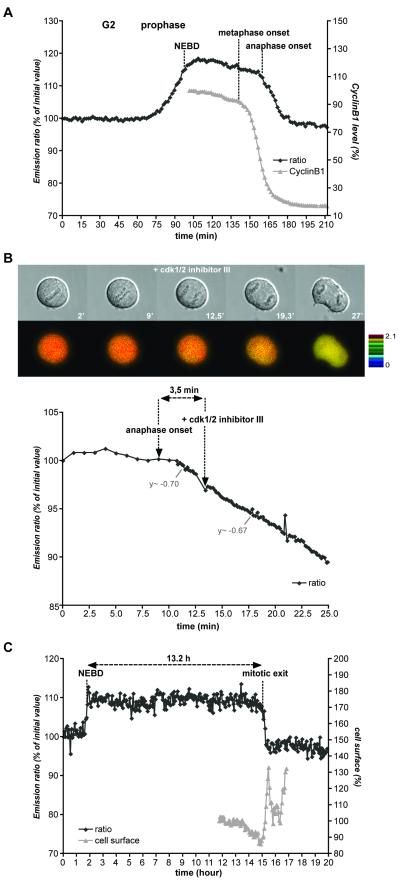
Once the FRET signal reached a maximum a few minutes after NEBD it remained almost constant throughout prometaphase and metaphase before dropping sharply at the beginning of anaphase (average 3.5 min or 1 min before visible sister chromatid separation in HeLa, n=12, and RPE, n=11, cells, respectively, Figs 1E&3A). The same result was observed in MEFs with or without CyclinB2 (Fig.S4B). Although the FRET signal remained constant throughout metaphase, the amount of kinase activity should have decreased because CyclinB1 started to be degraded from the beginning of metaphase (Clute and Pines, 1999) (Fig.3A). We reasoned that because the FRET signal represented the balance between CyclinB1-Cdk1 activity and an opposing phosphatase(s), the unchanging FRET signal in metaphase indicated that the phosphatase(s) was rate limiting in metaphase but not anaphase. In support of this, whereas adding a Cdk inhibitor in prophase, prometaphase or metaphase (Figs S3A,B) caused an immediate decrease in the FRET efficiency, adding the inhibitor in early anaphase had no effect on the rate of the FRET decrease, even in cells with measurable residual CyclinB1 (n=6/6; Fig.3B, and data not shown). This indicated that in anaphase the biosensor was primarily responsive to the antagonistic phosphatase(s). Unfortunately, we were unable to identify this phosphatase(s) despite using several different chemical inhibitors because most proved highly toxic. High concentrations (1 or 10 μM) of okadaic acid and most concentrations of calyculin A, a potent PP2A and PP1 inhibitor, almost immediately killed the cells. Nevertheless, adding 100 nM okadaic acid to inhibit primarily PP2A before or during anaphase had no effect on the FRET behaviour, allowing us to conclude that PP2A is unlikely to dephosphorylate the biosensor. The sudden decrease in FRET efficiency at anaphase did not depend on the activation of separase because the FRET signal still dropped in cells expressing a non-degradable securin mutant (n=5/5) (Hagting et al., 2002) (Fig.S7).
Figure 3. FRET dynamics during normal mitotic exit and mitotic slippage.
A. HeLa cells co-expressing the CyclinB1-Cdk1 biosensor and CyclinB1-mCherry were recorded during mitotic progression (1 image/1’40”). The FRET signal and the decrease of CyclinB1-mCherry fluorescence intensity (a measure of its proteolysis) during mitotic exit are displayed.
B. HeLa cells expressing the CyclinB1-Cdk1 activity sensor were recorded at 1 image/min until the beginning of anaphase at which point Cdk 1/2 inhibitor III (300 nM) was added and images taken at 1 image/10 sec. Note that there is no change in the rate of FRET decrease after adding the Cdk inhibitor in anaphase (n=6/6). C. RPE cells expressing the CyclinB1-Cdk1 biosensor were synchronized in G0 by serum starvation and 24h after release, 200 nM nocodazole was added. The FRET signal was recorded 4 hr after addition of nocodazole at 1 image/4 min. Quantification of the emission ratio and the cell surface area at mitotic exit in one typical cell is displayed. Note that the emission ratio is stable during ~13.2h and rapidly decreases during mitotic exit (n=5/5).
The persistent high FRET ratio in the presence of declining levels of CyclinB1-Cdk1 followed by a steep decline at mitotic exit was also observed in cells undergoing ‘mitotic slippage’ in the presence of nocodazole (Fig.3C). Previous studies showed that CyclinB1 levels gradually declined in cells arrested in mitosis with a microtubule poison until the cells eventually exited mitosis (Brito and Rieder, 2006). Our biosensor revealed that in RPE cells blocked in nocodazole, the FRET efficiency remained unchanged for up to 13 hours (n=5/5; Fig.3C, video 6) while CyclinB1 was progressively degraded (Brito and Rieder, 2006). Eventually, the FRET signal precipitously decreased over ~ 20 min (as at a normal anaphase) and this correlated with visible signs of mitotic exit (Fig.3C, video 6). We conclude that CyclinB1-Cdk1 activity must drop below a threshold before the cells exit mitosis, but once below this threshold dephosphorylation of CyclinB1-Cdk1 substrates is rapid (see Discussion).
Initial events regulated by CyclinB1-Cdk1 activation
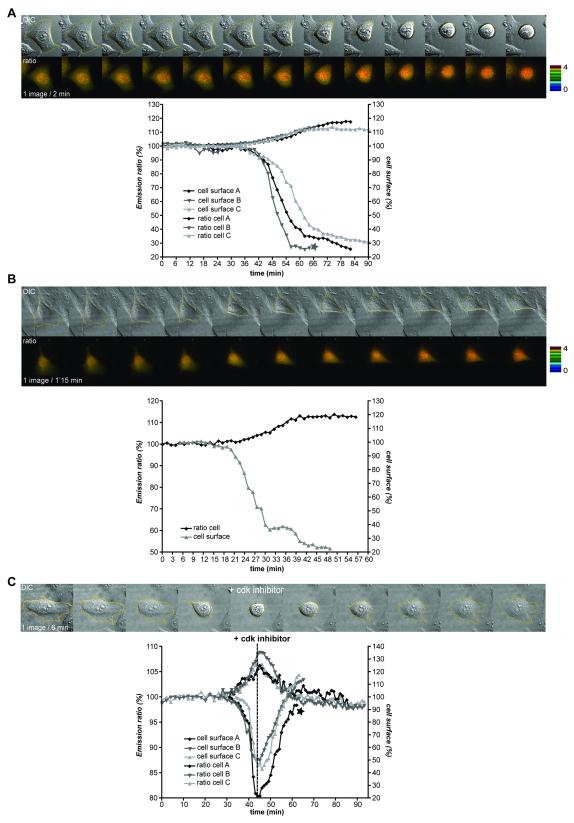
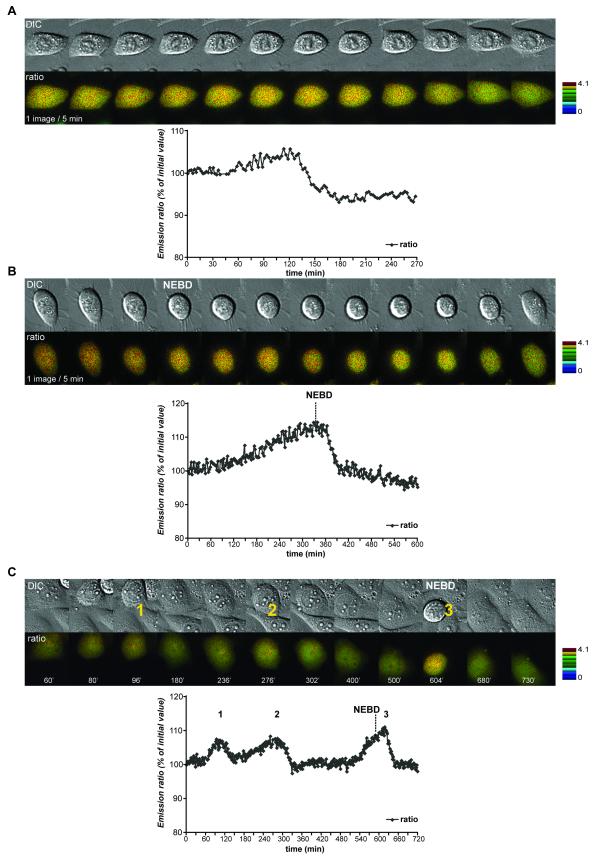
The biosensor enabled us to determine which cellular events are initiated when CyclinB1-Cdk1 is first activated. The event most closely correlated with the initial activation of CyclinB1-Cdk1 was that cells began to round up. We measured the cell surface in contact with the dish using DIC or fluorescence images, and found an excellent correlation between when the FRET signal began to increase and the cell surface began to decrease in both HeLa (n=10) and RPE cells (n=5) (Fig.4A&B, video 7). Cell rounding in prophase depended on CyclinB1-Cdk1 activity because adding a Cdk inhibitor during prophase immediately and completely reversed both the FRET signal increase and cell rounding (Fig.4C, n=5/5). Moreover, it was possible to reverse cell rounding until very late in prophase when the cell surface had decreased to its maximum extent (~80%) (Fig.4C cell A, video 8). Neither a Plk1 inhibitor (BI2536) nor an Aurora B inhibitor, ZM447439 (Ditchfield et al., 2003) reversed cell rounding (Gavet and Pines, in press). Thus, some of the earliest substrates of CyclinB1-Cdk1 are those required to reorganise the cytoskeleton to enable the cell to round up.
Figure 4. Cell rounding in prophase is concurrent with initial activation of CyclinB1-Cdk1.
A. Asynchronous HeLa cells expressing the CyclinB1-Cdk1 biosensor were recorded (1 image/2 min) and the FRET signal and surface area measured. Quantifications of the emission ratio and cell surface of 3 cells entering mitosis are displayed. B. RPE cell expressing the CyclinB1-Cdk1 sensor was followed by time-lapse fluorescence microscopy at 1 image/1’15 min. The quantification curves of the cell are displayed. C. Cell rounding up in prophase is reversed by a Cdk inhibitor. The changes in cell surface and FRET efficiency were measured on synchronized HeLa cells recorded at 1 image/2 min as they entered prophase, then every minute after addition of Cdk1/2 inhibitor III (300 nM). The star indicates the cell displayed.
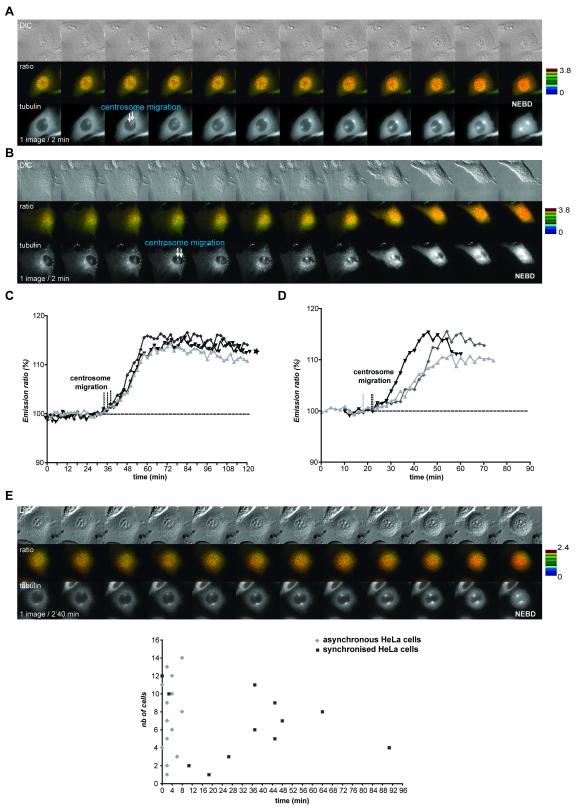
Centrosome separation is often taken as a surrogate marker for mitotic entry and for the activation of CyclinB1-Cdk1, because the Eg5 kinesin that drives centrosomes apart is phosphorylated in vitro by CyclinB1-Cdk1 to increase binding to microtubules (Blangy et al., 1995; Sawin and Mitchison, 1995). To assay this correlation in vivo we co-expressed the biosensor with mCherry-tagged α-tubulin. In asynchronous HeLa cells, centrosomes began to migrate away from each other on average 2.5 min after the FRET signal began to increase (n=14) (Fig.5A, video 9), and a similar correlation was seen in RPE cells synchronized by release from serum starvation (on average t=0, n=7) (Fig.5B). Surprisingly, in HeLa cells synchronized by a thymidine block and release regime, centrosomes often prematurely separated, with a broad variability in timing ranging from 0 to at least 90 minutes (average 35 min) before the FRET signal began to increase (Fig.5E, n>36)). Thus, centrosome separation only correlated with CyclinB1-Cdk1 activation in an unperturbed cell cycle.
Figure 5. Centrosome separation is not a consistent marker for CyclinB1 activation.
A&C. Asynchronous HeLa cells co-expressing the CyclinB1-Cdk1 biosensor and mCherry-α–tubulin were followed by time-lapse fluorescence microscopy at 1 image/2 min. The FRET quantification curves of 3 different cells are displayed and the beginning of centrosome migration for each cell is indicated by a vertical dashed line (C). B&D. Synchronized RPE cells co-expressing the CyclinB1-Cdk1 sensor and mCherry-α-tubulin were followed by time-lapse fluorescence microscopy at 1 image/2 min. The FRET quantification curves of 3 different cells are displayed. The beginning of centrosome migration for each cell is indicated by a vertical dashed line (D). E. HeLa cells co-expressing the CyclinB1-Cdk1 biosensor and mCherry- α-tubulin were synchronized in S phase by a thymidine block and release regime and assayed by time-lapse fluorescence and DIC microscopy. Upper panel: In the cell displayed the centrosomes separated >50 min before NEBD. Bottom panel: time between beginning of FRET increase and centrosome separation in typical experiments using asynchronous (n=14) or synchronized (n=12) HeLa cells. Note that centrosome separation begins at random during G2 phase in synchronised HeLa cells.
Different thresholds of CyclinB1-Cdk1 activity trigger specific events in prophase
The other event that consistently followed the activation of CyclinB1-Cdk1 was that CyclinB1 began to move into the nucleus (Pines and Hunter, 1991), and further experiments revealed that the active CyclinB1-Cdk1 kinase strongly stimulated its own nuclear import (Gavet and Pines, in press). Soon after CyclinB1-Cdk1 began to move into the nucleus we noticed progressive changes in nuclear structure, including visible chromosome condensation – as assayed by DIC or Histone H2B-GFP imaging – disassembly of the nucleolus, and lastly, NEBD. This raised the possibility that different thresholds of CyclinB1-Cdk1 activity might be required to trigger different events.
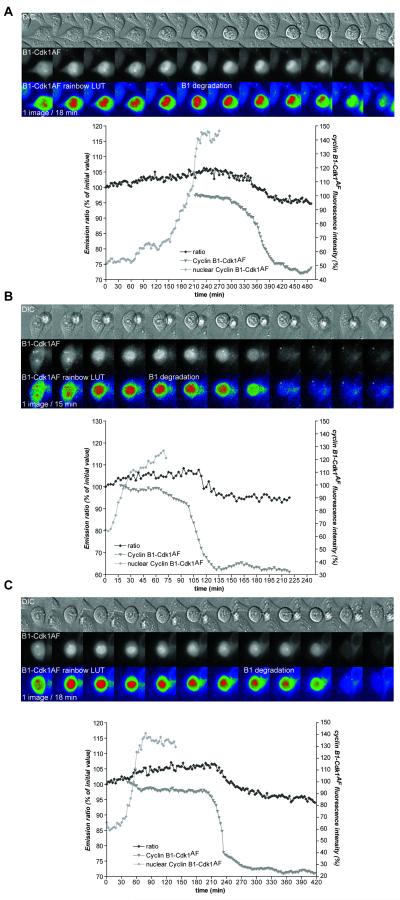
Another crucial nuclear substrate for CyclinB-Cdk1 is the Anaphase Promoting Complex/Cyclosome (APC/C), whose phosphorylation by CyclinB1-Cdk1 promotes the subsequent degradation of CyclinB1 (Kraft et al., 2003; Rudner and Murray, 2000). Pomerening and co-workers recently showed that a Cdk1 mutant that cannot be inactivated by the Wee1 kinase family (Cdk1AF) caused cells to perform several rapid cycles of CyclinB1 accumulation and destruction without chromosome segregation or cytokinesis (Pomerening et al., 2008). We hypothesised that a lower threshold of CyclinB1-Cdk1 might be required to activate the APC/C than trigger NEBD. To test this, we first co-expressed the biosensor in cells with Cdk1AF-mCherry to measure CyclinB1-Cdk1 activity and found that low levels of CyclinB1-Cdk1 activity, visualised by a weak increase in FRET activity (<10%), could trigger cell rounding but were insufficient to trigger NEBD (n=5) (Fig.6 cell A and C, two first M phase-like states; videos 10&12), whereas nucleolar disassembly and NEBD both required higher levels of kinase activity (n=6) (Fig.6 cell B & C, third M phase-like state; videos 11&12). To measure the amount of CyclinB1 protein in the nucleus and assay APC/C activity, we co-expressed the biosensor with a CyclinB1-Cdk1AF-mCherry fusion protein (Katsuno et al., 2009). In all cells analyzed (n=19), low levels of CyclinB1-Cdk1 activity, visualised by low FRET increases (<10%), could trigger CyclinB1-Cdk1 import into the nucleus and subsequently CyclinB1 degradation and mitotic exit, but not NEBD (n=10) (Fig.7, videos 13&14). NEBD and subsequently chromosome alignment and cytokinesis were only triggered in those cells with higher levels of CyclinB1-Cdk1 activity (FRET increase around 15%, Fig6 B&C and data not shown).
Figure 6. NEBD requires high levels of CyclinB1-Cdk1 activity.
HeLa cells co-expressing the CyclinB1-Cdk1 biosensor and Cdk1AF-mCherry. Three cells entering M phase-like states without cytokinesis are displayed. Low CyclinB1-Cdk1 activity, visualised by a weak increase in FRET activity, is sufficient to trigger cell rounding up (cell A and the two first M phase-like states of cell C) but higher levels are required for NEBD (cell B and third M phase-like state of cell C).
Figure 7. CyclinB1 degradation is triggered by low levels of CyclinB1-Cdk1 activity and is independent of NEBD.
HeLa cells co-expressing the CyclinB1-Cdk1 biosensor and a CyclinB1-Cdk1AF-mCherry fusion protein. Three cells entering M phase-like states without cytokinesis are displayed. Low CyclinB1-Cdk1 activity, visualised by a weak increase in FRET activity, is sufficient to trigger nuclear import of CyclinB1-Cdk1AF and its subsequent degradation in the absence of NEDB. The onset of CyclinB1-Cdk1AF degradation is indicated on the pseudo-colour (rainbow LUT) images. For each cell, the quantification of the FRET activity, the level of CyclinB1-Cdk1AF-mCherry and its nuclear accumulation are shown.
Altogether, our results show that the initial low levels of CyclinB1-cdk1 activity are sufficient to trigger cell rounding, import into the nucleus, and subsequently activate the APC/C, but higher activity is required to trigger disassembly of the nucleolus and NEBD. Thus the progressive increase in levels of CyclinB1-Cdk1 activity coordinates the reorganisation of the cell in prophase.
Discussion
Our results provide a much clearer idea for how cells coordinate cellular changes at entry to mitosis. The initial activation of CyclinB1-Cdk1 takes place about 20 to 25 min before NEBD, depending on the cell type. The time between activation of CyclinB1-Cdk1 and NEBD appears to be remarkably constant between cells of a particular cell type, and the kinetics of activation indicates that it is progressively activated over a period of approximately 30 minutes. Initially there is only a small increase in CyclinB1-Cdk1 activity that should be reversible because we and others have shown that the antephase checkpoint prevents cells completing prophase if it is activated before the nucleolus breaks down (Matsusaka and Pines, 2004; Rieder and Cole, 1998). All the events classically used to define prophase, including centrosome separation, cell rounding and chromosome condensation, are initiated only after CyclinB1-Cdk1 activity begins to rise. Thus, we propose that the rise in CyclinB1-Cdk1 activity can be used as a molecular definition of prophase (Pines and Rieder, 2001).
The time period over which human CyclinB1-Cdk1 is activated agrees well with the activation kinetics (~30 min) of CyclinB-Cdk1 measured in Xenopus egg extracts (Kim and Ferrell, 2007). The progressive activation of CyclinB1-Cdk1 might be surprising considering that the phosphorylation and inactivation of Wee1 by CyclinB1-Cdk1 exhibits ultrasensitivity, i.e.: CyclinB1-Cdk1 kinase activity has to reach a threshold before it inactivates Wee1 (Kim and Ferrell, 2007). One explanation for the extended activation period might be that during this time the feedback loops between CyclinB1-Cdk1 and its regulators could be counter-balanced by phosphatase activities. Germane to this might be the recent proposal that the Greatwall kinase participates in the feedback loops by down regulating phosphatase activities (Vigneron et al., 2009; Zhao et al., 2008), but at present the kinetics with which Greatwall is activated is unknown. In Xenopus extracts, phosphatase activities are inactivated during mitotic entry as a direct consequence of CyclinB1-Cdk1 activation (Mochida et al. 2009, Wu et al. 2009). If these phosphatases act on the CyclinB1-Cdk1 biosensor this will not affect our determination of cellular events associated with the initial activation of the kinase, but could modify the rate of the FRET increase later in mitosis.
One difference between our findings and those using Xenopus extracts (Abrieu et al., 1998) is that Plk1 does not appear to be part of the CyclinB1-Cdk1 amplification loop in mammalian cells, since inhibiting Plk1 does not influence the kinetics with which CyclinB1-Cdk1 is activated. Instead, we find that Plk1 has an important role upstream of CyclinB1-Cdk1 because in the presence of a Plk1 inhibitor, BI2536, cells delay considerably in G2 phase. A previous study using BI2536 also found a profound delay in mitotic entry but concluded that this was in prophase (Lenart et al., 2007). We, however, could not detect any sign of prophase, such as chromosome condensation, before CyclinB1-Cdk1 activation in HeLa or RPE cells in the presence of the Plk1 inhibitor. Moreover, in agreement with our results, Lenart and colleagues also found that in cells treated with BI2536, CyclinB1-Cdk1 is imported into the nucleus with normal timing before NEBD.
The role of Plk1 upstream of CyclinB1-Cdk1 is not yet clear. Plk1 regulates the activity of the FoxM1 transcription factor to transcribe genes encoding important mitotic regulators, which would, therefore, accumulate more slowly in cells treated with the BI2536 inhibitor (Fu et al., 2008). Alternatively, Plk1 might facilitate the activation of a ‘trigger’ Cdc25 phosphatase to initiate the activation of the CyclinB1-Cdk1 pool (Lobjois et al., 2009), or down regulate inhibitors of CyclinB1-Cdk1 such as Wee1 (Watanabe 2005).
The increase in CyclinB1-Cdk1 activity that we are now able to measure in mammalian cells differs from that previously suggested by a study using immunofluorescence to quantify the change in phosphorylation on tyrosine 15 of Cdk1, which concluded that CyclinB1-Cdk1 was partially activated in G2 phase. That assay, however, could not reveal the kinetics with which CyclinB1-Cdk1 is activated in an individual cell since it required fixed cells (Lindqvist et al., 2007), and our own results using an antibody recognizing the auto-phosphorylated form of Cyclin B1 as a marker for the active kinase agrees with the prophase activation we show here (Jackman et al., 2003). Nevertheless, our study does agree with Lindqvist and colleagues’ conclusion that CyclinB1-Cdk1 activity progressively increases and continues after NEBD.
We have also shown that centrosome separation should not be used as a surrogate marker for CyclinB1-Cdk1 activation. In cells synchronized by release from an S phase block centrosomes often separated more than an hour before CyclinB1 itself was activated. This could be because cells synchronized in S phase accumulate higher than normal levels of Cyclin A (Koop, 2007) and overexpression of Cyclin A-Cdk2 has previously been shown to trigger centrosome splitting (Meraldi and Nigg, 2001). In contrast we found that the import of CyclinB1 into the nucleus consistently matched the activation of CyclinB1-Cdk1 assayed by the FRET sensor. In light of these findings we would like to suggest that the observations of Gong et al. 2007 also support the role of CyclinB1 in triggering NEBD (Gong et al., 2007). Gong et al. used centrosome separation as their marker for CyclinB1-Cdk1 activity and found that NEBD was delayed in cells depleted of Cyclin A2, hence concluding that Cyclin A2 was required for NEBD. Yet in these cells CyclinB1 moved into the nucleus at the correct time before NEBD and from this we would conclude that CyclinB1-Cdk1 activation was delayed in cells depleted of Cyclin A but still eventually triggered NEBD. Indeed, this would agree with our own observations on the effects of depleting Cyclin A in somatic cells (Koop, 2007, FJ Walton & JP, unpublished results).
Using our biosensor we can for the first time correlate events triggered by the initial activation of the kinase, and thus likely to involve high affinity substrates, with those that occur only after CyclinB1-Cdk1 has reached particular levels, and are thus likely to involve lower affinity substrates (or substrates bound to antagonistic phosphatases). Since CyclinB1-Cdk1 activation immediately causes cells to begin to round up this adds weight to the evidence that CyclinB1-Cdk1 is first activated in the cytoplasm (Jackman et al., 2003; Lindqvist et al., 2007). Moreover, a proportion of active CyclinB1-Cdk1 kinase immediately begins to move into the nucleus, due to a change in the nuclear transport machinery (Gavet and Pines, in press), after which the chromosomes condense, the APC/C is activated, the nucleolus disappears, and finally the nuclear envelope breaks down. Our experiments have revealed that both nucleolar disassembly and NEBD require a higher threshold of Cdk activity than does cell rounding or the activation of the APC/C. Thus, the increasing level of CyclinB1-Cdk1 activity coordinates events during prophase. Our observations also constitute (to our knowledge) the first direct demonstration that cell cycle events can be coordinated by changing thresholds of a single Cyclin-Cdk, as first proposed by Stern and Nurse for the fission yeast cell cycle (Stern and Nurse, 1996)
CyclinB1-Cdk1 activity reaches its maximum shortly after NEBD and this maintains the cell in its mitotic state until CyclinB1-Cdk1 substrates begin to be dephosphorylated in anaphase, after CyclinB1-Cdk1 itself has begun to be inactivated by the destruction of CyclinB1. The rapid and abrupt inactivation of our biosensor may indicate that this dephosphorylation is only effective when CyclinB1 has dropped below a threshold, or that there is a positive signal that triggers anaphase. If there is a positive signal it does not require active separase, unlike the Mitotic Exit Network in budding yeast (D’Amours and Amon, 2004). Instead, we suspect that the rapid decrease in the FRET signal at anaphase is likely to reflect the activation of a phosphatase(s). Our okadaic acid results tend to exclude a role for PP2A and genetically the PP1 family of phosphatases has been most clearly shown to be required for mitotic exit (Ohkura et al., 1989). Moreover, PP1 is repressed in part by phosphorylation by CyclinB1-Cdk1 from early mitosis until anaphase (Kwon et al., 1997; Wu et al., 2009; Yamano et al., 1994), and Wu and co-workers recently showed that PP1 auto-activates by dephosphorylating its Cdk-dependent phosphorylation once Cdk1 levels have dropped (Wu et al., 2009).
Our newfound ability to measure CyclinB1-Cdk1-specific phosphorylation should help us to dissect how the remarkable reorganisation of the cell as it enters and exits mitosis is achieved.
Experimental Procedures
Cell culture and synchronisation
HeLa cells were cultured in Advanced DMEM (Invitrogen) supplemented with 2% FBS (Invitrogen), Glutamax-1 (200 μM), Penicillin (100U/ml), Streptomycin (100 μg/ml) and Fungizone (250 ng/ml) at 37°C, 10% CO2. MEF and hTert-RPE1 cells (BD Biosciences) were cultured in 50:50 DMEM Nutrient Mixture + Ham’s F-12 (Sigma-Aldrich) supplemented with 10% FBS, Glutamax-1, Penicillin, Streptomycin and Fungizone at 37°C, 5% CO2. Medium for hTert-RPE cells was supplemented with 0.25% sodium bicarbonate. For time-lapse imaging, cells were cultured on glass bottom dishes (Willco Wells) precoated with fibronectin (Sigma-Aldrich) at 1 μg/cm2 for 2h before using. HeLa cells were synchronised in S phase with 2.5 mM thymidine for 24 hours then released into fresh medium. hTert-RPE cells were synchronized by serum starvation for 24 hours (DMEM:F-12 supplemented with 0.1% serum) then released into the same medium with 10% FBS. MEFs were not synchronized.
Transfection
HeLa cells were electroporated (3× 106 cells, 10 to 20 μg of DNA) at 250V, 1500 μF in 4 mm cuvettes with an Easyject plus electroporator (Equibio). Cells were immediately plated on coated glass-bottom dishes and synchronized by a thymidine block and release regime 7 hours after transfection. Asynchronous cells were recorded 24 hours post-transfection. MEF and hTert-RPE cells were electroporated (5× 105 cells, 4 μg DNA) using a Microporator MP-100 (NanoEnTek Inc, Korea) according to manufacturer’s instructions.
Time-lapse imaging
Time-lapse imaging was performed on cells in L15 medium with 10% serum using a Deltavision microscope (Applied Precision, USA) equipped with either a CoolSNAP HQ or a Cascade II EMCCD 512K camera (Photometrics, USA) and an Olympus 40x UApo NA 1.35 objective. A GC400 filter was used to eliminate UV. FRET imaging was performed using a CFP/YFP filter set: CFPex 436/10, CFPem 470/30, YFPem 535/30, CFP/YFP dichroic C41258 or a CFP/YFP/mCherry dichroic C85363 (Chroma Technologies). The bleed-through of the CFP emission signal in the YFP emission channel was ~ 55% or ~100% using the CFP/YFP and CFP/YFP/mCherry dichroics respectively. The use of one or the other dichroic mirror did not affect the behaviour of the FRET signal during mitosis but affected the initial value of the emission ratio (~2 for CFP/YFP dichroic, ~3 for CFP/YFP/mCherry dichroic). To facilitate comparison of the different FRET curves, we expressed the emission ratio as a percentage of the initial value. The same exposure time was used for CFPex/CFPem and CFPex/YFPem (between 100 and 200 ms). For the centrosome separation experiments, 3 z-sections 2 μm apart were recorded for each cell and excitation wavelength. All quantifications were performed using ImageJ software. For emission ratio measurements, we used the following formula:
Whole cell signal = sum of the intensity of the pixels for one cell
Background signal = average signal per pixel for a region selected just beside the cell.
Whole cell signal corrected = Whole cell signal-(number of pixels for the selected cell = surface selected × Background)
Emission ratio = whole cell YFP signal corrected/ whole cell CFP signal corrected
Intensity-Modulated Display (IMD) representations were performed using Metamorph Software. Briefly, for each IMD, we used 8 colour hues with 32 intensities ranking from dark to bright. The max and min values were fixed manually and are indicated on each figure. The colour intensities displayed for each hue were determined automatically by the software using one of the CFPex/YFPem images as a reference (see Tsien and Harootunian 1990).
For photobleaching experiments, cells were illuminated under YFP excitation light (YFPex 500/20; mercury bulb 100W) without neutral density filter for 5 minutes. This resulted in a >80% decrease of the YFP emission. The increase of CFP emission under CFP excitation light was recorded and normalized for 100% bleaching of YFP emission. Using cells expressing YPet alone, we did not observe any photoconversion of YFP molecules to CFP-like species under our experimental conditions (Valentin and al, 2005; Thaler and al. 2006).
For reversion to mitosis experiments, we used a FCS2 chamber (Bioptechs, USA). The volume used was 150 μl and for washout the pump speed was calibrated in order to have 3 washes per min during 2-3 min.
Supplementary Material
Acknowledgements
We are grateful to Tim Hunt and Jane Kirk for MEF CyclinB2−/− cells, Dr L. Vassilev and Prof. A. Giannis for RO3306, Nick Keen for ZM447439, Dave Piston for the Cerulean cDNA, Patrick Daugherty for the YPet cDNA, Cristina Cardoso for pDsRed1 DNA ligase I cDNA and Rob Wolthuis for Cdk1AF-YFP. We thank all members of our laboratory, in particular Takahiro Matsusaka, for help in experiments and stimulating discussions. OG was supported by a Marie Curie fellowship and MRC project grants. Work in JP’s laboratory is funded by a programme grant from Cancer Research UK.
References
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M. The polo-like kinase plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in xenopus eggs. Journal of cell science. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Borgne A, Ostvold AC, Flament S, Meijer L. Intra-M Phase-promoting Factor Phosphorylation of CyclinB at the Prophase/Metaphase Transition. The Journal of biological chemistry. 1999;274:11977–11986. doi: 10.1074/jbc.274.17.11977. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, Gannon J, Hunt T. CyclinB2-null mice develop normally and are fertile whereas CyclinB1-null mice die in utero. Proc Natl Acad Sci USA. 1998;95:4344–4349. doi: 10.1073/pnas.95.8.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via CyclinB destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P, Pines J. Temporal and spatial control of CyclinB1 destruction in metaphase. Nature cell biology. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- D’Amours D, Amon A. At the interface between signaling and executing anaphase--Cdc14 and the FEAR network. Genes & development. 2004;18:2581–2595. doi: 10.1101/gad.1247304. [DOI] [PubMed] [Google Scholar]
- den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. The Journal of cell biology. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. The Journal of cell biology. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam VM, Orrechia S, Lowe M, Pardi R, Pines J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. The Journal of cell biology. 2001;152:945–958. doi: 10.1083/jcb.152.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran HP, Schermelleh L, Leonhardt H, Cardoso MC. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004;5:1181–1186. doi: 10.1038/sj.embor.7400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science (New York, NY. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindal DJ, Chen J. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nature cell biology. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of MPF from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Geley S, Kramer E, Gieffers C, Gannon J, Peters J-M, Hunt T. APC/C-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Pomerening JR, Myers JW, Gustavsson C, Jones JT, Hahn AT, Meyer T, Ferrell JE., Jr. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of CyclinB1. Curr Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. The Journal of cell biology. 2002;157:1125–1137. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Jackman M, Simpson K, Pines J. Translocation of CyclinB1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr Biol. 1999;9:680–689. doi: 10.1016/s0960-9822(99)80308-x. [DOI] [PubMed] [Google Scholar]
- Holmes JK, Solomon MJ. A predictive scale for evaluating cyclin-dependent kinase substrates. A comparison of p34cdc2 and p33cdk2. The Journal of biological chemistry. 1996;271:25240–25246. doi: 10.1074/jbc.271.41.25240. [DOI] [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active CyclinB1-Cdk1 first appears on centrosomes in prophase. Nature cell biology. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Kim SY, Ferrell JE., Jr. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–1145. doi: 10.1016/j.cell.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclins A and B during the G2-M transition. The EMBO journal. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop L. The role of Cyclin A in the entry to mitosis. Department of Zoology, University of Cambridge; Cambridge, UK: 2007. [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. The EMBO journal. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YG, Lee SY, Choi Y, Greengard P, Nairn AC. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci USA. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé JC, Picard A, Peaucellier G, Cavadore JC, Nurse P, Doree M. Purification of MPF from starfish: identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell. 1989;57:253–263. doi: 10.1016/0092-8674(89)90963-x. [DOI] [PubMed] [Google Scholar]
- Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009 doi: 10.1083/jcb.200812045. jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, van Zon W, Karlsson Rosenthal C, Wolthuis RM. CyclinB1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 2007;5:e123. doi: 10.1371/journal.pbio.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobjois V, Jullien D, Bouche JP, Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim Biophys Acta. 2009;1793:462–468. doi: 10.1016/j.bbamcr.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Hayes MK, Maller JL. Purification of maturation -promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci. 1988;85:3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. The Journal of cell biology. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Nigg EA. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. Journal of cell science. 2001;114:3749–3757. doi: 10.1242/jcs.114.20.3749. [DOI] [PubMed] [Google Scholar]
- Morgan DO. The Cell Cycle: Principles of Control. Oxford University Press; Oxford: 2007. [Google Scholar]
- Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60, and behaves differently from CyclinB. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. Human cyclins A and B are differentially located in the cell and undergo cell cycle dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Rieder CL. Re-staging mitosis: a contemporary view of mitotic progression. Nature cell biology. 2001;3:E3–E6. doi: 10.1038/35050676. [DOI] [PubMed] [Google Scholar]
- Pomerening JR, Ubersax JA, Ferrell JE., Jr. Rapid Cycling and Precocious Termination of G1 Phase in Cells Expressing CDK1AF. Molecular biology of the cell. 2008;19:3426–3441. doi: 10.1091/mbc.E08-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–958. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. The Journal of cell biology. 1998;142:1013–1022. doi: 10.1083/jcb.142.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- Rudner AD, Murray AW. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. The Journal of cell biology. 2000;149:1377–1390. doi: 10.1083/jcb.149.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T, Lee KS. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. The Journal of biological chemistry. 2002;277:32282–32293. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- Soni DV, Sramkoski RM, Lam M, Stefan T, Jacobberger JW. CyclinB1 is rate limiting but not essential for mitotic entry and progression in mammalian somatic cells. Cell cycle (Georgetown, Tex. 2008;7:1285–1300. doi: 10.4161/cc.7.9.5711. [DOI] [PubMed] [Google Scholar]
- Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends-Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. The EMBO journal. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Guo JY, Tang W, Yang C-S, Freel CD, Chen C, Nairn AC, Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nature cell biology. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Ishii K, Yanagida M. Phosphorylation of dis2 protein phosphatase at the C-terminal cdc2 consensus and its potential role in cell cycle regulation. The EMBO journal. 1994;13:5310–5318. doi: 10.1002/j.1460-2075.1994.tb06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, Goldberg ML. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Molecular biology of the cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.