Abstract
Fifty years ago, increased whole-blood serotonin levels, or hyperserotonemia, first linked disrupted 5-HT homeostasis to Autism Spectrum Disorders (ASDs). The 5-HT transporter (SERT) gene (SLC6A4) has been associated with whole blood 5-HT levels and ASD susceptibility. Previously, we identified multiple gain-of-function SERT coding variants in children with ASD. Here we establish that transgenic mice expressing the most common of these variants, SERT Ala56, exhibit elevated, p38 MAPK-dependent transporter phosphorylation, enhanced 5-HT clearance rates and hyperserotonemia. These effects are accompanied by altered basal firing of raphe 5-HT neurons, as well as 5HT1A and 5HT2A receptor hypersensitivity. Strikingly, SERT Ala56 mice display alterations in social function, communication, and repetitive behavior. Our efforts provide strong support for the hypothesis that altered 5-HT homeostasis can impact risk for ASD traits and provide a model with construct and face validity that can support further analysis of ASD mechanisms and potentially novel treatments.
Keywords: development, monoamine, neurotransmitter
Autism spectrum disorder (ASD) is a male-predominant disorder that is characterized by deficits in social interactions and communication, as well as repetitive behavior (1). Hyperserotonemia, or increased whole-blood serotonin [i.e., 5-hydroxytryptamine (5-HT)], is a well replicated biomarker that is present in approximately 30% of subjects with ASD (2, 3). Some data suggest an association of hyperserotonemia with stereotyped or self-injurious behavior, but results have been inconsistent (4, 5). Despite the high heritability of whole-blood 5-HT levels (6), a mechanistic connection between hyperserotonemia and specific components of the pathophysiology of ASD remains enigmatic. In blood, 5-HT is contained almost exclusively in platelets (7) that lack 5-HT biosynthetic capacity but accumulate the monoamine via the antidepressant-sensitive serotonin transporter (SERT; 5-HTT). A genome-wide study of whole-blood 5-HT as a quantitative trait found association with the SERT-encoding gene SLC6A4, as well as with ITGB3, which encodes the SERT-interacting protein integrin β3. In both cases, the strongest evidence for association was found in males (8–10). Linkage studies in ASD also implicate the 17q11.2 region containing SLC6A4, again with stronger evidence in males (11, 12).
As common SLC6A4 variants are only modestly associated with ASD (13), we and our colleagues previously screened SLC6A4 for rare variants in multiplex families that demonstrate strong linkage to 17q11.2. In this effort, we identified five rare SERT coding variants, each of which confers increased 5-HT transport in transfected cells as well as in lymphoblasts derived from SERT variant-expressing probands (11, 14, 15). We found the most common of these variants, Ala56 (allele frequency in subjects of European ancestry of 0.5–1%), to be overtransmitted to autism probands, and to be associated with both rigid-compulsive behavior and sensory aversion (11, 16). No such trait association is seen in families without linkage to this region (17). In transfected cells, SERT Ala56 also demonstrates increased basal phosphorylation and insensitivity to PKG- and p38 MAPK-linked signaling that normally produce increased transporter trafficking and catalytic activation, respectively (15). These findings suggest that homeostatic control of 5-HT may be impaired in some children with ASD. Importantly, model system studies indicate that 5-HT and SERT are important determinants of normal brain development and that early-life perturbations in 5-HT signaling can have enduring effects on behavior (18–21).
To explore the dependence of juvenile and adult behavior on early-life 5-HT manipulation and further understand the impact of the SERT Ala56 variant in vivo, we generated mice expressing SERT Ala56 from the native mouse Slc6a4 locus (22). Although SERT Ala56 mice exhibit normal growth and fertility (22), they display significantly increased CNS 5-HT clearance, enhanced 5-HT receptor sensitivity, and hyperserotonemia. Even more striking, SERT Ala56 animals display alterations in a number of ASD-relevant behaviors.
Results
SERT Function and Synaptic Homeostasis Is Altered in SERT Ala56 Mice.
As predicted from studies of SERT Ala56 transfected cells (11, 14, 15), midbrain SERT protein levels in Ala56 mice were found to be identical to those of WT, Gly56 littermate controls (Fig. S1A), results that are paralleled by the results of antagonist binding (Fig. S1B) and immunohistochemical studies (Fig. S2). SERT proteins exhibit significant posttranslational regulation (23), including Ser/Thr phosphorylation that involves PKG and p38 MAPK-linked pathways (24, 25). Consistent with our findings in transfected cells (14, 15), we found phosphorylation of SERT Ala56 to be significantly elevated in midbrain synaptosomes under basal conditions (P = 0.0002; Fig. 1A). Moreover, we found that activation of PKG with 8-Bromo-cGMP (8-Br-cGMP) fails to increase phosphorylation of SERT Ala56, whereas a robust increase in phosphorylation is observed for WT SERT (P = 0.0013; Fig. 1B). Basal SERT phosphorylation is dependent on p38 MAPK activity (24), and PKG activation leads to a p38 MAPK-dependent increase in SERT activity (26). The gain of SERT activity following activation of p38 MAPK is paralleled by an increased affinity for 5-HT that can support an enhanced rate of transport at low 5-HT concentrations (14, 15). When we incubated synaptosomes with the p38-MAPK inhibitor PD169316, we found significant reductions in basal phosphorylation of SERT Gly56 mice (P = 0.018; Fig. 1C). Importantly, the inhibitor also normalized the difference in phosphorylation between the WT and mutant transporters. These findings suggest that constitutive phosphorylation of SERT Ala56 precludes the flexibility exhibited by WT SERT to move between low and high activity states in parallel with changes in 5-HT release.
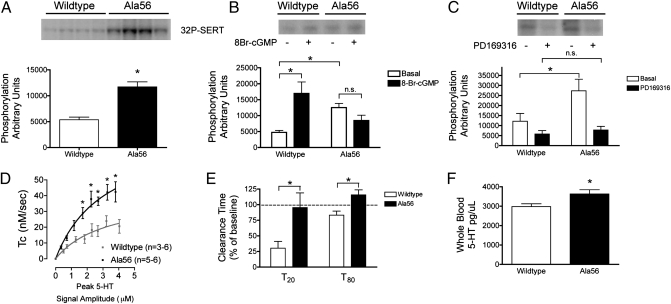
Fig. 1.
Dysregulated SERT phosphorylation, increased 5-HT uptake, and hyperserotonemia in the SERT Ala56 knock-in mice. (A) Representative gel and cumulative graph of basal phosphorylation of SERT [Bartlett test statistic, 26.49, showing unequal variances (P < 0.0001); therefore, nonparametric Mann–Whitney test was used, U = 6.00, P = 0.0002, n = 12 per genotype]. (B) Representative gel and cumulative graph of 8-Br-cGMP–induced phosphorylation (two-way repeated-measures ANOVA interaction of cGMP treatment by genotype, F = 17.51, P = 0.0013; Bonferroni post-test in WT for cGMP treatment, t = 3.66, P < 0.01; Bonferroni post-test in Ala56 for cGMP, t = 2.26, P > 0.05; WT, n = 7; Ala56, n = 7). (C) Representative gel and cumulative graph of PD169316 inhibition of phosphorylation (two-way repeated-measures ANOVA interaction of PD169316 treatment by genotype, F = 8.815, P = 0.018; Bonferroni post-test of genotype difference in basal condition, t = 4.83, P < 0.01; Bonferroni post-test of genotype difference after PD169316 treatment, t = 0.63, P > 0.05; WT, n = 5; Ala56, n = 5). (D) 5-HT clearance rates in the CA3 region of the hippocampus as a function of increasing extracellular 5-HT concentrations. Mean clearance values from multiple 5-HT pulses ± SEM with three to six mice per point. For purposes of clarity, the SEMs for signal amplitudes are not shown, but they were always within 10% of the mean. Clearance of 5-HT was significantly faster in Ala56 mice than that in WT controls (main effect genotype, F1,73 = 64.69, P < 0.0001; main effect 5-HT concentration, F7,73 = 15.86, P < 0.0001, two-way ANOVA with Bonferroni post hoc comparisons). Kinetic analysis reveals an approximate twofold increase in the apparent Vmax for 5-HT clearance (t10 = 7.248, P < 0.0001; Ala56, 82 ± 20 nM/s vs. Gly56, 41 ± 15 nM/s) with no change in apparent transporter affinity (KT) (corrected for volume fraction, α = 0.02; Ala56, 64 ± 42 nM vs. Gly56, 64 ± 28 nM). (E) Time to clear 20% (T20) and 80% (T80) of the peak 5-HT signal amplitude 10 min after application of 0.5 pmol of 8-Br-cGMP, normalized to baseline 5-HT clearance. T20 reflects 5-HT at a concentration at which SERT-mediated 5-HT clearance is near Vmax, whereas T80 provides an index of 5-HT clearance at a concentration approximating the Km for SERT-mediated 5-HT uptake. 8-Br-cGMP significantly shortened both T20 and T80 for 5-HT clearance in WT mice but was without effect in Ala56 mice (T20, t = 2.65, P = 0.022; T80: t = 3.195, P = 0.009; WT, n = 7; Ala56 n = 6). (F) HPLC measurement of 5-HT in whole blood. Unpaired t test revealed a significant increase in whole-blood 5-HT in the Ala56 animals compared with WT controls (t = 2.55, P = 0.02; WT, n = 11; Ala56, n = 9).
To complement our ex vivo phosphorylation studies and determine whether SERT Ala56 mice exhibit constitutively enhanced SERT activity in vivo, we monitored hippocampal 5-HT clearance by in vivo chronoamperometry (27). In these studies, we observed a significant increase in the rate of 5-HT clearance for Ala56 animals vs. WT littermates (P < 0.0001; Fig. 1D and Fig. S3). Paralleling our findings in synaptosomes, we observed a significant increase in 5-HT clearance following infusion of 8-Br-cGMP in the WT animals but no significant response in SERT Ala56 animals (P = 0.022 and P = 0.009 for time to clear 20% and 80% of maximum 5-HT signal, respectively; Fig. 1E). Despite the significant increase in 5-HT clearance, no change was observed in midbrain or forebrain tissue 5-HT levels (Fig. S4 A and B). In contrast, in whole blood, in which 5-HT is sequestered by platelets that lack the capability to offset 5-HT accumulation with decreased 5-HT synthesis, SERT Ala56 mice exhibited significantly increased 5-HT levels relative to WT littermates (P = 0.02; Fig. 1F).
Genetic or pharmacological reductions in SERT activity produce diminished sensitivity of multiple 5-HT receptors (28). Therefore, we hypothesized that increased CNS 5-HT clearance could lead to decreased synaptic 5-HT availability and a compensatory increase in 5-HT receptor sensitivity. Consistent with this idea, enhanced 5-HT receptor sensitivity occurs in mice overexpressing SERT (29). To explore this hypothesis in our mice, we first examined the sensitivity of animals to the 5-HT2A/2C receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), which produces a stereotyped head twitch response mediated by postsynaptic, cortical 5-HT2A receptors (30). We observed a significantly elevated head twitch response in SERT Ala56 mice compared with WT littermate controls (P < 0.01; Fig. 2A). Next, we treated animals with the 5-HT1A/7 agonist 8-hydroxy-2-(di-n-propylamino)-tetraline (8-OH-DPAT), which leads to hypothermia in mice, mediated by 5-HT1A autoreceptors located on raphe 5-HT neurons (31). As with DOI studies, SERT Ala56 mice displayed a significantly increased sensitivity to 8-OH-DPAT–induced hypothermia compared with WT controls (P < 0.0001; Fig. 2B; baseline temperature shown in Fig. S5A).
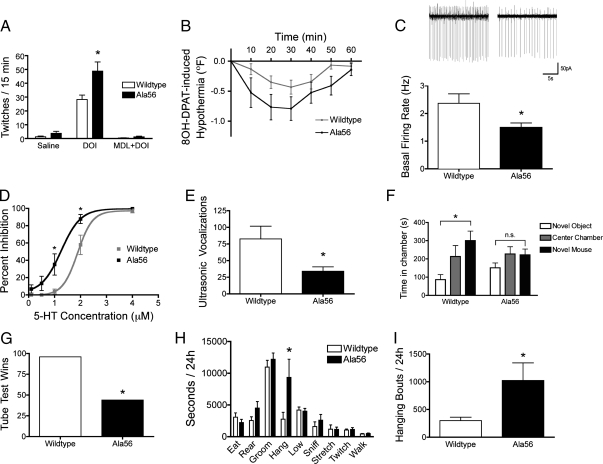
Fig. 2.
Increased receptor sensitivity and altered social, communication, and repetitive behavior in SERT Ala56 knock-in mice. (A) Head twitches recorded by two observers blind to genotype over 15 min following injection of saline solution, the 5-HT2 agonist DOI, or the specific 5-HT2A antagonist M-100907 followed by DOI. Two-way repeated-measures ANOVA revealed a significant genotype–drug interaction (F = 6.88, P = 0.0029, n = 10 per genotype), with a significant Bonferroni posttest result only for the difference between WT and SERT Ala56 animals in the DOI condition (P < 0.01). (B) Change in rectal temperature from baseline after administration of the 5-HT1A/5-HT7 receptor agonist 8-OH-DPAT. Piecewise mixed linear model analysis revealed a significant genotype–drug–time interaction over the 30 min from baseline to maximal hypothermia response (F1,177, P < 0.0001, n = 12 per genotype), reflecting a steeper slope in the SERT Ala56 animals compared with the WT controls. (C) Example traces with basal firing rates are shown for cell-attached extracellular recordings of dorsal raphe neurons in midbrain slices (n = 16 per genotype). Unpaired t test with Welch correction for unequal variances (F test to compare variances, F15,15 = 4.345, P = 0.0036) revealed a significant decrease in firing rate in the Ala56 animals compared with the WT controls (t = 2.92, P = 0.032). (D) Percent inhibition of firing of dorsal raphe neurons as a function of varying, bath-applied 5-HT concentration. Curve fit analysis against log(5-HT concentration) with variable slope reveals a significant increase in sensitivity to inhibition of firing by 5-HT in the Ala56 animals compared with the WT controls (F2,6 = 292.3, P < 0.0001). (E) Pup vocalizations upon separation from the dam for 5 min at postnatal day 7. Mann–Whitney test revealed a significant decrease in ultrasonic vocalizations in the SERT Ala56 animals in contrast with WT littermate controls (U = 85.5, P = 0.015; WT, n = 15; Ala56, n = 22). (F) Time in each chamber of the three-chamber Crawley sociability test is shown. Animals with four or fewer total entries were excluded from the analysis as a result of inactivity (Fig. S5C). Two-way repeated measures ANOVA revealed a main effect for chamber (F = 23.25, P = 0.0006) and a trend for an interaction between genotype and stimulus (F = 3.92, P = 0.058; WT, n = 11; Ala56, n = 17). Bonferroni posttest revealed a significant preference for the social chamber in the WT (P < 0.01) but not the SERT Ala56 animals (P > 0.05). (G) Wins (frontward exit) for male animals on the tube test. McNemar exact test revealed a significant decrease in wins in the SERT Ala56 animals in contrast with WT littermate controls (P < 0.0001, n = 140 pairings). (H) Time spent performing individual behaviors over 24 h in the home cage. To allow better visualization, time spent sleeping is not shown, but did not differ by genotype. Two-way repeated measures ANOVA of log10(time) revealed a significant genotype effect (F = 5.84, P = 0.027, n = 10 per genotype), with Bonferroni posttest showing a significant genotype difference only for time spent hanging (P < 0.05). (I) Number of bouts of hanging behavior in 24 h in the home cage. t test of log10(bouts) revealed a significant increase in bouts of hanging in Ala56 SERT animals in comparison with WT littermate controls (t = 2.567, P = 0.019), with a significant correlation between log10(time) and log10(bouts) (Pearson R = 0.749, P < 0.001).
To establish a physiological consequence of altered 5-HT receptor signaling in SERT Ala56 mice, we used loose-patch recordings of dorsal raphe 5-HT neurons in midbrain slices to examine basal firing rates and 5HT1A-mediated suppression of raphe neuron excitability. Location of recordings was first established by using ePET-1:EYFP transgenic mice (32), centering on neurons of the medial division of the dorsal raphe. Under basal conditions, we observed a decrease in the firing rate of these neurons in SERT Ala56 brain slices (P = 0.0036; Fig. 2C), potentially arising from increased firing suppression by inhibitory 5-HT1A autoreceptors (33). Consistent with this hypothesis, dose–response studies of raphe neuron inhibition produced by bath-applied 5-HT revealed an enhanced inhibitory potency of bath-applied 5-HT (P < 0.0001; Fig. 2D).
SERT Ala56 Mice Show Abnormal Social, Communication, and Repetitive Behavior.
Impaired social communication is often the first sign of ASD (34). To obtain a measure of early social communication, we measured ultrasonic vocalizations in pups that were separated from their dam at postnatal day 7. We observed a twofold decrease in vocalizations in SERT Ala56 pups in contrast to Gly56 littermate controls (P = 0.015; Fig. 2G). As body temperature could affect vocalization, we measured body temperatures in 7-day-old pups and found no differences between SERT Ala56 pups and Gly56 littermate controls (Fig. S5B). As adults, SERT Ala56 and WT littermates exhibit a low baseline level of ambulatory activity in novel environments (Fig. S6 A–C), typical of 129S substrains (35, 36). Thus, in analyses of adult animals that are dependent upon exploratory behavior, we included only data from mice with sufficient activity levels to allow a valid comparison between time spent in different arms or chambers (Fig. S6 B and C). In these studies, we observed no differences in anxiety-like behavior on the elevated plus-maze among mice with more than four arm entries (Fig. S6 D–F; pooled results from active and inactive animals are shown in Fig. S6G). In cognitive or behavioral assays dependent on forced movement, including the Morris water maze test of spatial learning (Fig. S7 A–C), the rotarod test (Fig. S7D), and the forced swim test (Fig. S7E), no significant differences were observed. However, when we tested SERT Ala56 mice for potential social interaction deficits in the three-chamber test of sociability (36, 37), these animals, unlike their WT littermates, failed to demonstrate preference for another mouse vs. an inanimate object (Fig. 2E; pooled results from active and inactive animals are shown in Fig. S6H).
To evaluate adult social interaction in a task that does not require high levels of ambulatory activity, we implemented the tube test of social dominance (38). Mouse models of other disorders that display ASD traits, including Rett and Fragile X syndromes (38, 39), show altered behavior on this task. After being trained to progress forward through an empty tube to be returned to their home cage, mice encounter an unfamiliar mouse that has entered from the opposite end of the tube. In these experiments, we found that SERT Ala56 animals more often withdrew from the tube upon encountering an age- and sex-matched WT littermate control (P < 0.0001; Fig. 2F).
In our studies that identified multiple, gain-of-function SERT variants in ASD subjects, we found the SERT Ala56 variant to be associated with rigid-compulsive behavior and sensory aversion in ASD (11). Several tests of sensorimotor function display deficits in subjects with ASD, including prepulse inhibition (40), a sensorimotor gating test that can be applied in mice. In a comparison with WT littermate controls, SERT Ala56 mice displayed a genotype by prepulse amplitude interaction effect on acoustic startle amplitude and prepulse inhibition of startle, reflecting an increased startle response at baseline that attenuated with increasing prepulse amplitudes (Fig. S8 A and B). To assess spontaneous repetitive behavior, we performed noninvasive, automated monitoring of animals in the home cage. Although many behaviors were found to be normal in these studies, we observed that SERT Ala56 mice spent a significantly greater time hanging from the wire cage lid relative to WT littermates (P < 0.05; Fig. 2H). Time hanging was significantly correlated with the number of bouts of hanging (Pearson R = 0.749, P < 0.001). Indeed, examination of recordings revealed that SERT Ala56 animals climbed up to hang briefly on the wire lid and then returned back to the floor of the cage, repeating this behavior an average of approximately 1,000 times over a 24-h recording period (P = 0.019; Fig. 2I). Other potential repetitive behaviors, including grooming, were not found in the home cage (Fig. 2H). We also did not see abnormalities in marble burying (Fig. S9), a test of repetitive behavior proposed to be relevant to ASD (41).
Discussion
These studies describe biochemical, physiological and behavioral traits that derive from the conversion of a single amino acid, Gly56, in SERT. Although conversion of Gly to Ala is a relatively minor change of structure, the SERT N terminus supports multiple SERT protein associations that may be impacted (23, 42–44). In this regard, SERT associates with proteins that influence the transporter's phosphorylation state, including the catalytic subunit of the Ser/Thr phosphatase PP2A (45) and PKG1 (46); although sites supporting these associations have not been defined. As the Ala56 variant does not create or alter a canonical phosphorylation site, we suspect that the alteration modifies the secondary structure of the N terminus to permit enhanced access of one or more kinases (or restricted access of a protein phosphatase) to either the N terminus itself or a nearby cytoplasmic phosphorylation site. The SERT N terminus is directly connected to transmembrane domain 1, a domain that participates in 5-HT binding during the translocation process (47, 48). We hypothesize therefore that SERT Ala56-induced changes in N-terminal structure, protein associations, or phosphorylation, lock the transporter in a high-affinity conformation for 5-HT. Such an effect could lead to diminished availability of 5-HT for signaling and effectively eliminate the flexibility needed for SERT activity to track changes in 5-HT release. As proper control of 5-HT availability is vital to normal brain development (18–21), constitutively diminished 5-HT availability could lead to alterations in brain wiring and enduring changes in behavior.
The pattern of alterations in whole blood 5-HT levels, midbrain 5-HT neuron firing, and receptor sensitivities in the SERT Ala56 mouse reflects homeostatic changes in response to the primary change in SERT. Hyperserotonemia in the Ala56 mouse is consistent with the role of SERT in platelet 5-HT uptake and with prior studies showing that mice lacking SERT show essentially no whole-blood 5-HT (9, 49). Although no changes were found in brain tissue 5-HT levels in the SERT Ala56 mice, we suspect that tryptophan hydroxylase activity can be readily modified to reduce 5-HT biosynthetic capacity. Platelets lack this mechanism of homeostatic control, as they do not synthesize 5-HT but rather accumulate 5-HT released by duodenal enterochromaffin cells as they circulate through the gut (49). Moreover, tissue levels are a poor correlate of the synaptic availability of 5-HT, which likely depends more on smaller, readily releasable pools of neurotransmitter and the inherent excitability of 5-HT neurons. Our findings of altered basal firing of raphe neurons in vitro and increased sensitivity of SERT Ala56 animals to challenge with 5HT1A and 5HT2A receptor agonists provides critical evidence that the changes seen in 5-HT clearance translate into behaviorally relevant changes in 5-HT signaling.
Substantial ethological differences exist between mice and humans, and scientists have, to date, generated only a few mouse models derived from gene variants identified in ASD (50–54). It is thus not possible to assert that a particular set of behavioral abnormalities directly models ASD in a mouse. We believe that, at this time, it is more reasonable to identify how genetic variation impacts mouse behavior, with the goal of identifying underlying changes in brain function that may be conserved in man and which can promote an understanding of the deficits arising in ASD. Given that SERT Ala56 represents a susceptibility variant, rather than a highly penetrant, monogenic cause of ASD (11, 17), we do not expect animals expressing the variant to model all aspects of ASD. The impact of susceptibility variants is expected to vary depending on the presence of other genetic or environmental factors. Thus, the biochemical, physiological, and behavioral changes seen in an animal model of a susceptibility variant could offer many, or few, parallels to the human disorder. Further, individuals with ASD show considerable heterogeneity in clinical symptoms and genetic susceptibility, and an animal model of a susceptibility variant could therefore show some features that arise in only a subset of individuals (55). Further research is needed to understand how other genetic or environmental factors modulate the phenotypes that we observed in the SERT Ala56 mice, as well as the interaction between this variant and other risk factors in individuals with ASD (11).
We find potential parallels of ASD-associated deficits in the SERT Ala56 mice. ASD is a disorder of pediatric onset. The decrease in ultrasonic vocalizations we observe in SERT Ala56 pups suggests an early emergence of the impact of 5-HT on the capacity or drive for communication. Social interaction deficits in ASD persist into adulthood. The tube test represents an ethologically valid mouse social interaction with a binary outcome that may be particularly sensitive to changes in social proficiency (39). Interestingly, Duvall and colleagues (56) identified a male-predominant, quantitative trait locus for social responsiveness in multiplex ASD families on chromosome 17q, including the SLC6A4 gene region. Consistent with this, we observed a lack of preference by adult SERT Ala56 mice for a social stimulus in the three-chamber test. Finally, SERT Ala56 has been associated with sensory aversion in ASD subjects (11). Although we observed only a modest enhancement in the startle response during prepulse inhibition tests, more sensitive studies are needed that examine the physiological properties of the sensory fields of mice and the ability of animals to integrate sensory information as these functions are known to be under the influence of 5-HT during development and in the adult (19–21).
Rigid-compulsive behavior (57) is significantly elevated in SERT Ala56 carriers and in a combined group comprising SERT Ala56 carriers and other carriers of rare, hyperfunctional SERT coding variants (11). It is difficult to predict how a genetic determinant of rigid-compulsive behavior in humans will manifest in an animal model. The repetitive bouts of hanging from the wire cage lid we observe in these mice may represent a novel parallel of the repetitive, nonfunctional routines that are common in ASD (1), although other interpretations are possible. Importantly, the repetitive hanging phenotype was identified in the context of many normal behaviors and in the animals’ home cage, suggesting that we detected a spontaneous, rather than experimentally induced, repetitive behavior.
The biochemical, physiological, and behavioral results in the SERT Ala56 animals also have some important limitations. First, it is not clear how the specific change from Gly56 to Ala56 leads to increased p38-MAPK–sensitive basal phosphorylation, whether by way of altered SERT tertiary structure or disrupted protein–protein interactions in the N-terminal domain where Gly56 is expressed. Further work is needed to understand these mechanisms, including identifying the residues at which phosphorylation occurs and the kinases or phosphatases that act at these residues. Second, we studied only homozygous animals to maximize our ability to detect phenotypes. Future studies will be needed to understand whether similar biochemical, physiological, and behavioral phenotypes are found in animals with only one copy of the SERT Ala56 variant. Third, the low activity seen in some animals in the elevated plus-maze and three-chamber sociability test limit the interpretation of these data. This low activity level appears to be a result of the inbred strain background (35, 36) and does not differ by genotype. When coupled with the ultrasonic vocalization and tube test results, however, the overall data are consistent with a change in social function. Further experiments conducted on other inbred strain backgrounds may clarify the lack of preference for social stimuli in the SERT Ala56 mice. Finally, in contrast with increased grooming behavior observed in some other mouse models of ASD-associated genetic variation (52, 54, 58), the increased climbing/hanging behavior we observed in the SERT Ala56 mice is a repetitive behavior that has not previously been described to our knowledge. The difference in the pattern of observed repetitive behavior in the SERT Ala56 mice, in contrast to other models of ASD susceptibility, could reflect the fact that expression of this susceptibility variant is limited to a single, neurochemically defined pathway. Further experiments will be necessary to connect this behavior to changes in underlying circuits.
As autism is a neurodevelopmental disorder, it will be important to now investigate the temporal profile and developmental requirements for constitutively elevated 5-HT transport in the changes we observe in the SERT Ala56 mice. Excess 5-HT clearance during early stages of development could influence neuronal migration, axonal projections, and synapse development in these mice, as indicated by other developmental manipulations that target 5-HT signaling (19, 20, 59). Our constitutively expressed variant also does not speak to the important sites of expression of SERT Ala56 in dictating phenotypes. SERT is not only expressed in the developing and mature brain but also in gut, platelet, adrenal gland, immune cells, pancreas, and lung. Moreover, Bonnin and coworkers have shown that the placenta, which expresses high levels of SERT (60, 61), is a source of forebrain 5-HT during gestation and is important for normal axonal trajectories (62). Modulating 5-HT levels or transporter function to assess the reversibility of these phenotypes could yield insight into the developmental impact of increased and dysregulated SERT function. Ultimately, studies that allow for temporal and spatial control of the SERT Ala56 variant are needed to answer these questions. Finally, ASD is a disorder with few therapies and none that consistently reverse major deficits. We believe that the SERT Ala56 model offers an opportunity to pursue genetic and pharmacological studies that can both probe ASD mechanisms and possibly identify novel therapeutic targets.
Materials and Methods
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Vanderbilt University, Medical University of South Carolina, or University of Texas Health Science Center Institutional Animal Care and Use Committee. SERT Ala56 knock-in mice were constructed as previously described (22). Details of methods related to synaptosome preparation, Western blotting, citalopram binding, SERT phosphorylation, HPLC, immunohistochemistry, in vivo electrochemical recordings, slice preparation, electrophysiological recordings, and behavioral experiments are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
Michelle Carter, Lauren Huntress, and Clinton Canal provided technical assistance; Mu Yang and Jill Silverman provided advice on behavioral data analysis; and Warren Lambert provided statistical assistance. The authors thank the families who participated in the original genetic study. This work was supported by National Institutes of Health (NIH) Grants MH081066 (to J.V.), MH094604 (to J.V.), DA07390 (to R.D.B.), MH078098 (to R.D.B.), HD065278 (to R.D.B.), and MH62612 (to S.R.); an Autism Speaks Pilot Award (to J.V.), an American Academy of Child and Adolescent Psychiatry Pilot Research Award (to J.V.), and NIH Grants HD15052 (to the Vanderbilt Kennedy Center) and RR024975 (to the Vanderbilt Institute for Clinical and Translational Research).
Footnotes
Conflict of interest statement: The authors have no direct competing financial interests. J.V. receives research funding for nonoverlapping work from Seaside Therapeutics, Roche Pharmaceuticals, and Novartis, and has served as a consultant to Novartis. R.D.B. receives research funding for nonoverlapping work from Forest Pharmaceuticals and serves on the Lundbeck Pharmaceuticals Advisory Board and as a consultant to JubilantInnovation.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112345109/-/DCSupplemental.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) 4th Ed. Washington, DC: American Psychiatric Association Press; 2000. [Google Scholar]
- 2.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 3.Mulder EJ, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kolevzon A, et al. Relationship between whole blood serotonin and repetitive behaviors in autism. Psychiatry Res. 2010;175:274–276. doi: 10.1016/j.psychres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacco R, et al. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 2010;3:237–252. doi: 10.1002/aur.151. [DOI] [PubMed] [Google Scholar]
- 6.Abney M, McPeek MS, Ober C. Broad and narrow heritabilities of quantitative traits in a founder population. Am J Hum Genet. 2001;68:1302–1307. doi: 10.1086/320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987;40:1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet. 2005;76:33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss LA, et al. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet. 2004;12:949–954. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- 11.Sutcliffe JS, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Molecular Genetic Study of Autism Consortium (IMGSAC) A genomewide screen for autism: Strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet. 2001;69:570–581. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin B, et al. CPEA Genetics Network Autism and the serotonin transporter: The long and short of it. Mol Psychiatry. 2005;10:1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- 14.Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad HC, et al. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glatt CE, et al. Screening a large reference sample to identify very low frequency sequence variants: Comparisons between two genes. Nat Genet. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai T, et al. A large-scale screen for coding variants in SERT/SLC6A4 in autism spectrum disorders. Autism Res. 2008;1:251–257. doi: 10.1002/aur.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 19.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 21.Jitsuki S, et al. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veenstra-Vanderweele J, et al. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1:158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner JA, Carneiro AM, Blakely RD. Going with the flow: Trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: Evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- 26.Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- 27.Daws LC, Toney GM, Davis DJ, Gerhardt GA, Frazer A. In vivo chronoamperometric measurements of the clearance of exogenously applied serotonin in the rat dentate gyrus. J Neurosci Methods. 1997;78:139–150. doi: 10.1016/s0165-0270(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 28.Fox MA, et al. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- 29.Jennings KA, Sheward WJ, Harmar AJ, Sharp T. Evidence that genetic variation in 5-HT transporter expression is linked to changes in 5-HT2A receptor function. Neuropharmacology. 2008;54:776–783. doi: 10.1016/j.neuropharm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- 31.Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: Role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323:477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- 32.Scott MM, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wischmeyer E, Karschin A. Receptor stimulation causes slow inhibition of IRK1 inwardly rectifying K+ channels by direct protein kinase A-mediated phosphorylation. Proc Natl Acad Sci USA. 1996;93:5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 35.Moy SS, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moy SS, et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 39.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 40.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Thomas A, et al. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller HK, Wiborg O, Haase J. Subcellular redistribution of the serotonin transporter by secretory carrier membrane protein 2. J Biol Chem. 2006;281:28901–28909. doi: 10.1074/jbc.M602848200. [DOI] [PubMed] [Google Scholar]
- 43.Binda F, Lute BJ, Dipace C, Blakely RD, Galli A. The N-terminus of the norepinephrine transporter regulates the magnitude and selectivity of the transporter-associated leak current. Neuropharmacology. 2006;50:354–361. doi: 10.1016/j.neuropharm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Ciccone MA, Timmons M, Phillips A, Quick MW. Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A. Neuropharmacology. 2008;55:763–770. doi: 10.1016/j.neuropharm.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauman AL, et al. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steiner JA, et al. cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain. 2009;2:26. doi: 10.1186/1756-6606-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl—dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 48.Adkins EM, Barker EL, Blakely RD. Interactions of tryptamine derivatives with serotonin transporter species variants implicate transmembrane domain I in substrate recognition. Mol Pharmacol. 2001;59:514–523. doi: 10.1124/mol.59.3.514. [DOI] [PubMed] [Google Scholar]
- 49.Chen JJ, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duvall JA, et al. A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry. 2007;164:656–662. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- 57.Tadevosyan-Leyfer O, et al. A principal components analysis of the Autism Diagnostic Interview-Revised. J Am Acad Child Adolesc Psychiatry. 2003;42:864–872. doi: 10.1097/01.CHI.0000046870.56865.90. [DOI] [PubMed] [Google Scholar]
- 58.Peñagarikano O, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riccio O, et al. Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry. 2009;14:280–290. doi: 10.1038/mp.2008.89. [DOI] [PubMed] [Google Scholar]
- 60.Ramamoorthy S, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad PD, et al. Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells. Placenta. 1996;17:201–207. doi: 10.1016/s0143-4004(96)90039-9. [DOI] [PubMed] [Google Scholar]
- 62.Bonnin A, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.