Abstract
Human ABCG2 is a member of the ATP-binding cassette (ABC) transporter superfamily and is known to contribute to multidrug resistance (MDR) in cancer chemotherapy. Among ABC transporters that are known to cause MDR, ABCG2 is particularly interesting for its potential role in protecting cancer stem cells and its complex oligomeric structure. Recent studies have also revealed that the biogenesis of ABCG2 could be modulated by small molecule compounds. These modulators, upon binding to ABCG2, accelerate the endocytosis and trafficking to lysosome for degradation and effectively reduce the half-life of ABCG2. Hence, targeting ABCG2 stability could be a new venue for therapeutic discovery to sensitize drug resistant human cancers. In this report, we review recent progress on understanding the structure, function, biogenesis, as well as physiological and pathophysiological functions of ABCG2.
Keywords: Human ABCG2, structure, function, multidrug resistance, ATP-binding cassette, cancer, chemotherapy
Introduction
Chemotherapy has been a major form of treatment for various cancers since 1940s. However, ineffectiveness and failure of chemotherapy with single agent was soon observed. This is probably due to the ability of cancer cells to mutate spontaneously at a rate of approximately 10-7 cells per generation and acquire resistance to the single agent in response to the pressures imposed by the drug treatment via a selection process [1]. In order to resolve this issue, the break-through concept of combinational therapy was introduced in the 1960s, which was based on the premise that the emergence of resistant cancers could be prevented with an alternating combination of drugs that have different targets. Nevertheless, multidrug resistance (MDR), which refers to the ability of organisms and cells to display resistance to a wide range of drugs that are structurally and functionally unrelated, has become a pervasive clinical problem in a majority of cancers ever since the introduction of combinational therapy.
Cellular and molecular mechanisms of MDR have been extensively studied. Studies with drug-selected model cell lines have repeatedly demonstrated that over-expression of some members of the ATP-binding cassette (ABC) transporter superfamily including breast cancer resistance protein (BCRP or ABCG2), P-glycoprotein (Pgp or ABCB1), and multidrug resistance associated protein 1 (MRP1 or ABCC1) is one of the major mechanisms responsible for MDR. The increased expressions of these ABC transporters on plasma membranes cause increased efflux and decreased intracellular accumulation of many unrelated anti-cancer drugs, leading to MDR.
ABC transporters represent one of the largest families of transporter proteins. In human alone, there are 48 ABC transporters and they have been divided into seven distinct subfamilies from ABCA through ABCG, based on their gene structure similarities and sequence homology [2]. Human ABC transporters are exclusively exporters. They use energy from ATP hydrolysis and are predominantly involved in the efflux of endogenous materials such as metabolic products, vitamins, lipids and sterols, as well as exogenous drugs and toxins from cytoplasm into extracellular space or intracellular compartments such as endoplasmic reticulum and peroxisomes. Therefore, human ABC transporters play essential roles in a majority of physiological, pathological, and pharmacological processes.
ABCG2 is one of the human ABC transporters that have been implicated in MDR in cancer chemotherapy [3,4]. ABCG2 gene was cloned independently from both drug-selected model cell lines and human cDNA library in 1998. ABCG2 cloned by Ross’s group from a drug-selected human breast cancer cell line MCF-7/ AdVp3000 was termed as BCRP [5]. Simultaneously, Dean’s group cloned a nearly identical transporter as an expressed sequence tag and named it ABCP for its high expression in placenta [6]. Shortly after, the cDNA of ABCG2 was cloned independently from a mitoxantrone-selected human colon carcinoma cell line, S1- M1-80, and was designated MXR for mitoxantrone resistance [7]. Human ABCG2 is an important molecule in both innate and acquired MDR, in regulation of drug bioavailability, in prognosis prediction of both hematopoietic and solid malignancies, and in protecting cancer stem cells. In this article, we will review recent progresses on the study of human ABCG2 regarding its structure, function, role in MDR, and its substrates as well as modulators and biogenesis.
Structure of ABCG2
All human ABC transporters have a distinctive modular architecture, consisting of at least one hydrophilic nucleotide binding domain (NBD) located in cytoplasm and one hydrophobic membrane-spanning domain (MSD). Based on the structure and arrangement of NBD and MSD, they can be grouped into ‘full transporters’, ‘half transporters’ and non-transporter type ABC proteins [8]. Typically, full transporters, such as ABCB1, comprise two homologous halves and are characterized by two MSDs and two NBDs with an arrangement of MSD1-NBD1- MSD2-NBD2. Other types of full transporters, such as ABCC1, have an extra MSD (MSD0) at the amino terminus with a domain structure of MSD0-MSD1-NBD1-MSD2-NBD2. Half transporters contain only one MSD and one NBD, which are about half the size of a full transporter. These half transporters include members of the ABCD subfamily and some of the ABCB subfamily with a domain structure of MSD-NBD, and members of the ABCG subfamily with a reversed NBD-MSD configuration. The non-transporter ABC proteins include members of the ABCE and ABCF subfamilies that do not have MSDs.
Human ABCG2 is a half transporter with a domain structure of NBD-MSD and the MSD consists of 6 putative transmembrane (TM) segments (Figure 1). This topological folding of ABCG2 with 6 TM segments has been demonstrated using epitope tagging although the exact location of the TM segments is slightly different from the original prediction [9]. The previously predicted TM2 and TM5 are shifted to the extracellular and intracellular loops in the new model, respectively. Further studies are needed to verify the exact location of these two TM segments and the new sequences that now function as TM2 and TM5. Nevertheless, future studies of the MSD of ABCG2 need to take into consideration of the possible alteration in the TM segment assignments.
Figure 1.
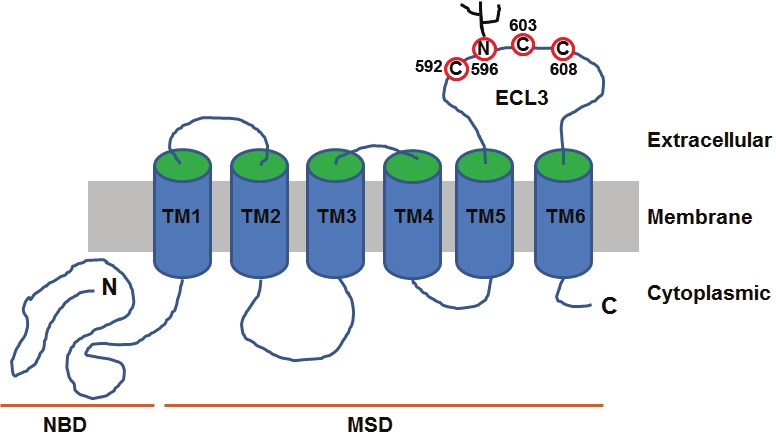
Membrane topology model of ABCG2. The schematic topological model was constructed on the basis of sequence analysis and the available experimental data. Nucleotide- binding domain (NBD), membrane-spanning domain (MSD), transmembrane (TM) segments, and extracellular loop 3 (ECL3) are indicated. The cysteine residues and N-linked glycosylation sites in ECL3 are also shown.
Because of its half size nature, ABCG2 has been thought to exist and work as a homo-dimer. This hypothesis is supported by a study showing that co-expression of an ATPase-dead ABCG2 with the wild-type ABCG2 resulted in reduction of the ABCG2 transport activity and that ABCG2 migrated as monomers on SDS-PAGE under reducing conditions but as a dimer complex in the absence of reducing agents [10]. It was also found that human ABCG2 expressed in insect or bacterial cells retains its function, which argues against the necessity of other mammalian protein partners for ABCG2 function [11].
However, emerging evidence suggest that ABCG2 may exist as a higher order of homo-oligomer on plasma membranes. Using chemical crosslinking and non-reducing SDS-PAGE, Litman et al. first detected higher forms of oligomers in addition to dimeric proteins [12]. Using various biochemical methods such as nondenaturing PAGE, perfluoro-octanoic acid-PAGE (PFO-PAGE), gel filtration chromatography, sucrose gradient sedimentation, chemical crosslinking as well as co-immunoprecipitation, we unambiguously demonstrated that the major oligomeric unit of human ABCG2 in plasma membranes is a homo-dodecamer with a minimum stable unit of homo-tetramer [13] (Figure 2). No monomeric or dimeric ABCG2 was found under non-denaturing conditions using all methods mentioned above, suggesting that the major form of ABCG2 is likely bigger than a homodimer, possibly a dodecamer. Using chemical cross-linking, Bhatia et al. also showed the existence of higher order oligomers of ABCG2 in both isolated cell membranes and whole cell preparations [14]. Furthermore, using fluorescence resonance energy transfer (FRET) analysis of CFP/YFP tagged ABCG2 in whole cells, Wang et al. also showed the existence of oligomeric ABCG2 although the exact size of the complex could not be assessed with this method [15].
Figure 2.
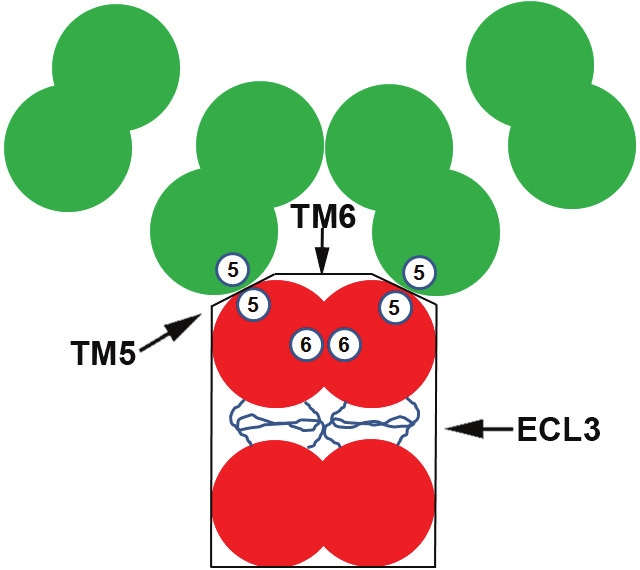
Schematic model of the dodecameric ABCG2. Three different possible interaction sites contributed by TM5 (5), TM6 (6), and ECL3 are shown.
Later, examining purified human ABCG2 using cryo-electron microscopy revealed that purified human ABCG2 may exist as a homo-octamer consisting of four homo-dimeric ABCG2 complexes [16]. Yet, in another study using improved purification method preserving lipid environment, it was found that the purified ABCG2 in the presence of all solubilized membrane components may be a tetrameric complex when expressed in Sf9 cells [17]. In a third study of purified ABCG2 using electron microscopy, it was also found that the major oligomeric complex of ABCG2 is a tetramer [18]. Although the causes for the discrepancy between the later three studies of purified ABCG2 are unknown, it is clear that all these studies have demonstrated the existence of an oligomeric ABCG2 complex bigger than a dimer. However, use of different detergents and methods of purification in these studies may affect the outcome considering that the minimal stable ABCG2 complex is a tetramer and manipulation with various chemical or physical forces will break down the higher forms of oligomers to tetramers [13]. The observation of tetramers in two of the three studies of purified ABCG2 is consistent with this conclusion.
Using delet ion mapping and coimmunoprecipitation of differentially tagged ABCG2 constructs, Xu et al. [19] mapped the oligomerization domain of human ABCG2 to its MSD consisting of extracellular loop 3 (ECL3) with its flanking TM segments (TM5 and TM6). The polypeptide consisting of TM5-ECL3-TM6 not only forms a homo-dodecameric complex by itself but also exerts a dominant negative effect on the drug transport function of wild-type ABCG2, possibly by forming hetero-complexes with the wild-type molecule. We recently found that ECL3, TM5, and TM6 all contain oligomerization activities [20], suggesting that each of these three segments may be responsible for three different inter-molecular contacts responsible for the formation of a homododecamer [13,19] (see also Figure 2). However, each segment plays a different role in ABCG2 drug transport function [20]. While TM5 is essential for ABCG2 function in drug transport, TM6 and ECL3 are replaceable.
ECL3 is an interesting loop containing three cysteine residues that may involve in formation of intra- and inter-molecular disulfide bonds. While Cys603 was identified to form the intermolecular disulfide bond [21] and it may potentially contribute to dimer formation as shown using non-reducing PAGE [10,21], formation of the oligomeric ABCG2 does not depend on the inter-molecular disulfide bonds [13]. Furthermore, it appears that Cys603 is not required for the expression or localization of ABCG2, nor is it essential for the ATPase or transport activity of ABCG2 [14,21,22]. Considering the possibility that the inter-molecular disulfide bonds may be due to oxidation during sample preparation [13], the above studies suggest that the functional ABCG2 does not necessarily need an intermolecular disulfide bridge for its function and/or oligomerization.
Regulation of ABCG2 expression
In normal human tissues, ABCG2 is prominently expressed in placental syncytiotrophoblasts, epithelium of small intestine and colon, liver canalicular membranes, and ducts and lobules of mammary tissue [23]. ABCG2 expression has also been detected in the luminal membrane of epithelial cells in normal gallbladder [24], alveolar pneumocytes, sebaceous glands, interstitial cells of testes, prostate epithelium, endocervical cells of uterus, squamous epithelium of cervix, islet and acinar cells of pancreas, zona reticularis layer of adrenal gland, kidney cortical tubules and hepatocytes [25,26]. ABCG2 is also present in veinous and capillary endothelium. Furthermore, ABCG2 is predominantly localized to the plasma membranes of cells in the above mentioned tissues [27], many of which harbor secretory or barrier function. This specific distribution profile of ABCG2 is closely related to the physiological role of human ABCG2 (see below). Increased expression of ABCG2 is frequently seen in both drug resistant cancer cell lines and clinical tumor tissues. The expression of ABCG2 in normal and cancer cells appears to be regulated at different levels including gene amplification, epigenetic modifications, transcriptional and posttranscriptional regulation.
Gene amplification
Gene amplification has long been recognized as a major contributor to the increased expression of ABCB1 [28]. It was, thus, thought that the increased expression of ABCG2 in drug resistant cancer cells might also be due to gene amplification. The first report on ABCG2 gene amplification was a study of a few mitoxantrone-selected derivative cell lines of MCF-7 using Southern blot analysis [29]. This finding was later verified using comparative genomic hybridization (CGH) and Southern blot in MCF-7/ MX cells as well as in Adriamycin-selected MCF- 7/AdVp3000 cells [30]. The mitoxantrone-sensitive parental cell line MCF-7, on the other hand, had no amplification or chromosome translocation of the ABCG2 gene. It has also been shown that the resistance level to SN-38 in colorectal cancer cells positively correlates with ABCG2 gene amplification [31], suggesting that gene amplification of ABCG2 is not restricted to MCF-7 breast cancer cells.
Using stepwise-selected glioblastoma cell line SF295 against mitoxantrone in combination with Southern blot and fluorescence in situ hybridization (FISH), Rao et al. [32] examined the mechanisms of ABCG2 gene amplification during drug selection. It was found that double minute chromosomes was responsible for ABCG2 gene amplification in the drug resistant SF395 derivative cell lines selected with low concentration of mitoxantrone (50 and 100nM). However, in the derivative cell lines selected with high concentration of mitoxantrone (250 and 500nM), ABCG2 gene amplification appears to be due to chromosomal reintegration of the amplicon at multiple chromosomes to generate a more stable genotype.
Epigenetic regulation
In addition to gene amplification, ABCG2 gene has also been found to be regulated epigenetically. Demethylation of the ABCG2 gene has been found to contribute to increased ABCG2 expression in human multiple myeloma cells [33]. Methylation of ABCG2 gene was also found in renal carcinoma cell lines and treatment with demethylating agents increases ABCG2 expression [34]. Chromatin immunoprecipitation assay showed that the methylated promoter of ABCG2 interacted with methyl CpG island-binding proteins MBD2 and MeCP2, which further recruited histone deacetylase 1 and a co-repressor, resulting in interruption of ABCG2 transcription. Moreover, inhibition of DNA methylation in PC-6 lung cancer cells greatly increased both mRNA and protein levels of ABCG2 and the promoter methylation of ABCG2 is inversely correlated with ABCG2 expression in both SCLC and NSCLC cells [35], indicating that promoter demethylation of ABCG2 could be a common regulatory mechanism for ABCG2 up-regulation in cancer cells.
Histone acetylation has also been shown to possibly regulate ABCG2 promoter activity [36]. Following drug selection in several cancer cell lines and subsequent over-expression of ABCG2, the authors observed an increase in acetylated histone H3 but a decrease in class I HDACs associated with the ABCG2 promoter. The increased ABCG2 expression requires three prerequisites, removal of the repressive histone marker (trimethylated histone H3 lysine), recruitment of RNA polymerase II and recruitment of a chromatin-remodeling factor to the ABCG2 promoter. These observations suggest that the regulation of ABCG2 expression is complex at both genetic and epigenetic levels.
Transcriptional regulation of ABCG2
In addition to the regulation at genetic and epigenetic levels, transcriptional regulation of ABCG2 expression has also been reported. The human ABCG2 gene is located on chromosome 4q22 and spans more than 66 kbp [30]. It contains 16 exons and 15 introns [37]. While the first exon contains most of the 5’-untranslated region (5’-UTR), the translation initiation site is located in exon 2. The ABCG2 gene has a TATA-less promoter with its basal promoter activity conferred by a sequence ~312 bases upstream from the transcription start site. A CCAAT box is present at about 274 bases upstream from the transcription start site and its removal reduces the transcription activity of the ABCG2 gene. There are five putative Sp1 sites downstream from a putative CpG island, a common feature of promoters lacking a TATA box. In pancreatic cancer cells, it was found that the homeobox gene MSX2 helps recruit SP1 to the Sp1 binding sites in the ABCG2 promoter and increases ABCG2 gene transcription [38].
In addition to SP1, several other transcription factors have been shown to involve in regulating ABCG2 expression. These transcription factors include but not limited to estrogen receptor alpha (ERα), hypoxia-inducible factor 1 (HIF-1), peroxisome proliferator-activated receptor gamma (PPARγ), progesterone receptor (PGR) and aryl hydrocarbon receptor (AHR).
Sequence analysis of the 5'-flanking region of the ABCG2 gene has led to the discovery of a putative estrogen response element (ERE) between positions -188 to -172 of the ABCG2 promoter [39]. Deletion and site-directed mutagenesis analysis confirmed the existence of ERE in this region. It is further demonstrated that 17β-estradiol (E2) could promote the mRNA expression of ABCG2 through activation of estrogen receptor alpha (ERα), which directly binds to the ERE located in the ABCG2 promoter.
A study by Krishnamurthy et al. [40] showed that hypoxia also increases the mRNA level of ABCG2 in three different human cell lines. Analysis of the 5’-flanking sequence of human ABCG2 gene revealed three putative hypoxia response elements (HREs), all located upstream of the transcription start site. Using site-directed mutagenesis and electrophoretic mobility shift assays (EMSA), it was found that the HIF-1 complex specifically binds to the ABCG2 promoter through the only functional HRE at -116, bases upstream of the transcription start site and promotes ABCG2 transcription. This up-regulation of ABCG2 transcription by activated HIF-1 under hypoxia condition may stand for one of the mechanisms in some tumors to facilitate drug resistance. Moreover, IL-6 or ER stress inducer could synergistically increase ABCG2 expression through the site overlapping with XBP-1 and HIF- 1 binding sites on the ABCG2 promoter, indicating that HRE might be involved in the effect of ER stress on ABCG2 expression as well [41].
Another nuclear receptor transcription factor that was thought to regulate ABCG2 expression is PPARγ. It was first observed that the mRNA level of ABCG2 was increased in human myeloid lineage monocyte-derived dendritic cells upon treatment with PPARγ agonist rosiglitazone [42]. This effect was completely abolished by PPARγ antagonist or PPARγ siRNA, indicating that PPARγ is likely involved. To elucidate the mechanisms of the above finding, the promoter sequence of the ABCG2 gene was analyzed and three potential PPAR response elements were found in a conserved region of ~150 bp in length (-3946 to -3796). EMSA analysis further demonstrated that all three putative elements were able to bind PPARγ-RXR heterodimers specifically, suggesting that this genomic region likely plays an important role in the PPARγ- dependent transcriptional regulation of ABCG2 gene.
More recently, a novel progesterone response element (PRE) has also been identified between -243 to -115 of the ABCG2 promoter region [43,44]. Progesterone significantly increases ABCG2 mRNA level in progesterone receptor B (PGR-B)- but not PGR-A-transfected cells. Although EMSA confirmed the direct binding of PRE in the ABCG2 promoter with either PGR-B or PGR-A, mutations in PRE only decreased the progesterone- response in PGR-B-transfected but not PGR-A- transfected cells. Further deletion of the PRE nearly completely abrogated the progesterone effect on ABCG2 promoter activity. Interestingly, co-expressing PGR-A and PGR-B significantly decreased the progesterone-response compared with PGR-B alone, indicating that progesterone induces ABCG2 transcription through PGR-B, while PGR-A may inhibit the effect of PGR-B via an undefined mechanism.
Two other elements have also been found in the ABCG2 promoter and they are proximal dioxin-response element (DRE) between -194 and - 190, bases upstream of the transcription start site of the human ABCG2 gene and an antioxidant response element (ARE) at -431 to -420 [45-48]. While the first one may be responsible for direct binding of AHR and the subsequent induction of ABCG2 transcription, the second one is possibly responsible for Nrf2-mediated ABCG2 expression through interaction with Nrf2. However, whether these elements work in concert or compete with each other is largely unknown and further investigation may provide valuable information for the characterization of transcriptional regulation of ABCG2.
Posttranscriptional regulation of ABCG2
ABCG2 is also under posttranscriptional regulation by microRNAs. In CD34+/CD38- hematopoietic stem cells isolated from human umbilical cord blood, it was found that hsa-miR-520h inhibits ABCG2 expression and possibly promotes the differentiation of these stem cells [49]. hsa-miR- 520h has also been shown to downregulate ABCG2 expression, resulting in inhibition of migration and invasion of pancreatic cancer cells [50]. Another miRNA, miR-328, also targets ABCG2 gene and decreases ABCG2 mRNA and protein levels through mRNA cleavage [51]. The proximal miRNA response element (MRE) of ABCG2 is located in the 3’-UTR of ABCG2 mRNA in various cancer cell lines [52]. Interestingly, it was found that this putative MRE of ABCG2 was lost in drug resistant cells and, therefore, the drug resistant cancer cells can evade ABCG2 mRNA degradation and protein synthesis repression mediated by miRNAs, leading to over-expression of ABCG2 [53].
While misfolded ABCG2 proteins, such as Cys592 and Cys608 mutants that lack the intramolecular disulfide bond and lose proper membrane trafficking and the Asn596 mutant that lose N-linked glycosylation, have been shown to be removed from ER by retrograde translocation to the cytosol compartment, ubiquitinated by ubiquitin ligase and degraded in proteasome, wild-type ABCG2 is degraded in lysosomes [54-57].
Endocytosis and degradation of wild type ABCG2 in lysosome can also be accelerated by ABCG2 inhibitors. Recently, it was found that a new ABCG2 inhibitor, PZ-39, not only inhibits ABCG2 function, but also causes conformational change and accelerates degradation of ABCG2 via endocytosis and trafficking to lysosomes [58]. Later, it was found that there are a group of dynamic ABCG2 inhibitors that could accelerate ABCG2 degradation in lysosomes [59] (also see below). These inhibitors may also hijack newly synthesized ABCG2 from ER and direct them to lysosome for degradation or cause ER-associated degradation (ERAD). Indeed, in a recent study it was found that Derlin-1, a protein component of a complex that mediates ERAD, promotes the degradation of wild-type ABCG2 through suppression of ER to Golgi transport [60].
ABCG2 in MDR
Although human ABCG2 is widely expressed in normal tissues, over-expression of ABCG2 has been frequently found in various drug-selected cancer cell lines and contributes to the clinical MDR of hematopoietic malignancies and solid tumors. Increased ABCG2 expression has also been linked to cancer stem cells.
ABCG2 in MDR of cancer cell lines
As mentioned earlier, ABCG2 was originally cloned from an Adriamycin-selected breast cancer cell line MCF-7/AdVp3000, which exhibited resistance to a range of cytotoxic agents, including mitoxantrone, doxorubicin and daunorubicin, but had no increased expression of ABCB1 or ABCC1 [5]. over-expression of ABCG2 has been found in and correlated to the MDR phenotypes of numerous drug-selected cancer cell lines derived from various tumor types, including topotecan-selected ovarian tumor cell line T8 [61], mitoxantrone-selected colon cancer cell lines S1-M1-80 [7] and HT29 [62], SN-38- selected human small cell lung cancer cells PC- 6/SN2-5 [63], mitoxantrone-selected human gastric carcinoma cell line EPG85-257RNOV [64], gefitinib-resistant non-small cell lung cancer (NSCLC) cells [65], epirubicin-resistant human hepatocyte carcinoma cells HLE-EPI [66], as well as topotecan and doxorubicin-selected human multiple myeloma cells [33]. However, the ABCG2-mediated drug resistance profiles found in these cell lines vary, which might be attributed to different cell origin or the involvement of other resistance factors selected by different drugs. Recently, several other mechanisms of drug resistance have also be identified in the Adriamycin-selected MCF7/AdVp3000 cells. These new mechanisms of resistance in MCF7/AdVp3000 include but are not limited to increased expression of other ABC transporters [67], 14-3-3σ and HSP27 [68], as well as fatty acid synthase [69]. Likely, alteration in other factors such as DNA repair proteins [70] and apoptosis [71] also contribute to the observed differences in level of resistance in different cell lines. Therefore, ABCG2 may be only one of the multiple factors responsible for MDR and a more complex model is required for better evaluation of MDR cell lines.
ABCG2 in hematopoietic malignancies
Although the cellular models are powerful tools to examine the MDR phenotype mediated by ABCG2, the clinical relevance of ABCG2 was established using clinical samples. Many studies have provided evidence demonstrating over-expression of ABCG2 in many different hematopoietic malignancies and its association with patient response. Early studies by Ross et al. have indicated relatively high levels of ABCG2 expression in 33% of acute myeloid leukemia (AML) blast cells [72]. Several follow-up studies have also demonstrated that ABCG2 expression correlates with prognosis and survival of AML patients (Table 1). However, such an association was not found in some other studies (Table 1). Currently, the reason for different findings of these studies is unknown. However, it is possible that the method used for detecting ABCG2 expression, sample size, as well as patient stratification may all play some role in different interpretation of the findings in these studies. Expression of other ABC transporters in these patients may also affect the outcome. It has been shown that AML patients expressing one or none of functional ABCB1, ABCC3 or ABCG2 have better prognosis than those patients expressing two or all of the above transporters [73]. Thus, analysis of a group of ABC transporters rather than a single one may provide a better picture regarding prediction of prognosis and chemotherapy response of AML patients. These data also suggest that modulation of all susceptible ABC transporters may be necessary for better treatment of AML patients.
Table 1.
ABCG2 expression in human hematopoietic malignancies
| Type | Correlation | Methods | N1 | Reference |
|---|---|---|---|---|
| AML | Yes | RT-PCR | 20 | [72] |
| AML | No (mitoxantrone, topotecan or doxorubicin based therapy) | ICC (BXP34) | 20 | [162] |
| AML | Yes (daunorubicin based therapy) | ICC (BXP34) | 20 | [162] |
| AML | No | FCM (BXP34 & BXP21) | 20 | [163] |
| AML | Yes (relapse/refractory) | RT-PCR | 20 | [164] |
| AML (child) | Yes (prognosis, relapse) | RT-PCR | 59 | [165] |
| AML (adult) | No | RT-PCR | 40 | [166] |
| AML | No | RT-PCR | 51 | [167] |
| AML (adult) | Yes (prognosis on daunorubicin and mitoxantrone therapy) | RT-PCR | 149 | [168] |
| AML (adult) | Yes (complete remission, DFS, overall survival) | FCM (BXP21) | 85 | [73] |
| AML | Yes (relapse and DFS) | FCM (BXP34) | 73 | [169] |
| AML | Yes (complete response rate) | RT-PCR | 154 | [170] |
| AML | Yes (DFS, relapse, overall survival to fludarabine-basd therapy) | 138 | [171] | |
| ALL (child) | No (prognosis) | RT-PCR | 67 | [76] |
| ALL | Yes (B-lineage) | FCM (BXP34) | 46 | [75] |
| ALL (adult) | Yes (DFS) | ICC (BXP21) | 30 | [74] |
Number of subjects studied
The role of ABCG2 expression in acute lymphocytic leukemia (ALL) has also been implicated but stays inconclusive (Table 1). A correlation between ABCG2 expression and prognosis in adult ALL patients was reported in two different studies [74,75]. However, this association was not found in a third study of childhood ALL [76]. Although it is not yet clear, the discordance between these studies might be due to differences in adult or childhood ALL (Table 1). Thus, more studies to compare adult and childhood ALL may help address this potential issue.
ABCG2 in solid tumors
Correlations between ABCG2 expression and prognosis of solid tumors have also been studied (Table 2). Similar to the studies of hematopoietic malignancies, some studies of solid tumors showed correlation between ABCG2 expression and prognosis while others did not. For example, in one study of breast cancer patients it was found that ABCG2 expression correlates with response to anthracycline-based chemotherapy [77]. However, in another study of breast cancers, no such correlation was identified [78]. Thus, whether ABCG2 over-expression contributes to MDR in solid tumors is currently inconclusive. More studies are clearly needed to investigate the role of ABCG2 in drug resistance and chemotherapy response of solid tumors.
Table 2.
ABCG2 expression in human solid tumors
| Type | Correlation | Methods | N1 | Reference |
|---|---|---|---|---|
| Breast carcinoma | No (doxorubicin-based treatment) | RT-PCR | 43 | [78] |
| Breast carcinoma | No (anthracycline-based therapy) | IHC (BXP21 & BXP34) | 52 | [172] |
| Breast cancer | Yes (anthracycline-based therapy) | RT-PCR | 59 | [77] |
| Digestive tract tumors | Yes | IHC (BXP21) | 32 | [173] |
| Colorectal & cervical cancer | Yes (downregulation) | IHC | 154 | [174] |
| Endometrial carcinoma | Yes | IHC (BXP21) | 5 | [173] |
| Lung tumors | Yes | IHC (BXP21) | 10 | [173] |
| NSCLC | Yes (PFS and overall survival to platinum-based therapy) | IHC (BXP21) | 72 | [175] |
| NSCLC | Yes (short survival to cisplatin-based therapy) | IHC (BXP21) | 156 | [176] |
| SCLC | Yes (response and PFS to platinum-based therapy) | IHC (BXP21) | 130 | [177] |
| Melanoma | Yes | IHC (BXP21) | 5 | [173] |
| Melanoma | No | RT-PCR | 18 | [178] |
| Retinoblastoma | Yes (invasion) | IHC (5D3) | 39 | [104] |
| Retinoblastoma | No | IHC (BXP21) | 18 | [179] |
| Esophageal carcinoma | Yes | RT-PCR | 100 | [180] |
| T/NK-cell lymphoma | Yes | IHC | 45 | [181] |
| Diffuse large B-cell lymphoma | Yes (prognosis) | IHC (BXP21) | 67 | [182] |
Number of subjects studied
Physiological functions of ABCG2
Although ABCG2 appears to play an important role in MDR of human cancer cells, its expression and distribution pattern in normal tissues (see above) implicates that it must play some important physiological functions such as protecting the organism as a first line of defense against environmental insults. Data collected from ABCG2-null mice help appreciate this first line of defense role of ABCG2 [79]. ABCG2-null mice are more susceptible to phototoxic skin lesions, which are caused by accumulation of pheophorbide a, a chlorophyll degradation product found in food and supplements [79].
GI tract
Human ABCG2 is physiologically expressed in the apical membrane of epithelial cells in the gastrointestinal (GI) tract, with maximal expression in the duodenum and a gradual decrease along the GI tract to the rectum [80]. ABCG2 is also constitutively expressed in the liver canalicular membranes, which supports a possible protective role of ABCG2 against xenobiotic absorption and towards toxic metabolites excretion. Indeed, it has been shown that administration of GF120918, a dual inhibitor of ABCB1 and ABCG2, resulted in a significant increase in the bioavailability and systemic concentration of topotecan after oral administration [81]. It was also found that GF120918 could markedly reduce the biliary and renal excretion of topotecan after intravenous administration. Considering that topotecan has higher affinity with ABCG2 than ABCB1, inhibition of ABCG2 by GF120918 may be the major mechanism responsible for the increased intestinal absorption and decreased biliary and renal excretion of topotecan. ABCG2 also appears to be responsible for the efflux of sulfate and glucuronide conjugates of xenobiotics and hormones, which are mostly products of phase II metabolism [82], suggesting that ABCG2 has a major role in extruding toxic metabolites via the biliary pathway.
Blood-brain barrier
ABCG2 is constitutively expressed at the blood-brain barrier mainly at the luminal cell surface of microvessel endothelium, serving as a crucial barrier to drug access into the brain [83-85]. It is now clear that ABCG2 works with ABCB1 in the blood-brain barrier and is responsible for restricting numerous xenobiotics into the brain. The positive impact of this function is that ABCG2 protects brain from the toxicity of xenobiotics while the negative impact is that ABCG2 impedes therapeutic agents to reach their intracerebral targets for treating brain tumors.
Placenta
ABCG2 expression is highest on the plasma membranes of the chorionic villi in placenta [12]. This cellular localization indicates that ABCG2 may play a major role in protecting fetus against toxic materials ingested by the mother. In ABCG2-null mice, the fetal exposure to topotecan and other dietary toxins increased significantly [86], further confirming the protective role of ABCG2 in the placenta.
Stem cells
A fascinating property of hematopoietic stem cells is their ability to actively extrude Hoechst 33342, a fluorescent dye. The low Hoechst 33342-staining cells isolated by subsequent fluorescence-activated cell sorting (FACS) are termed as ‘side population’ (SP) [87], which have been shown to possess stem cell-like characteristics in a variety of tissues [88-91]. ABCG2 expression is high in SP cells than non-SP cells, and has been characterized to be the Hoechst 33342 efflux pump in SP [92,93]. Moreover, ectopic over-expression of ABCG2 conferred a SP phenotype in HEK293 cells, indicating that ABCG2 may serve as an attractive candidate marker for stem cells [92]. On the other hand, ABCG2 is differentially expressed during hematopoiesis, with the highest levels in the primitive bone marrow stem cell populations, followed by a sharp reduction in response to stem cell differentiation, suggesting a possible dual role of ABCG2 in maintaining human pluripotent stem cells in an undifferentiated state and in protecting these stem cells from xenobiotics or other toxins in vivo. [88,89,92].
Studies with ABCG2-null mice further confirmed that ABCG2 is necessary for SP phenotype, since loss of ABCG2 expression resulted in a drastic decrease in SP cells in the bone marrow and skeletal muscle. Notably, it has also been shown that the hematopoietic cells of ABCG2- null mice became more sensitive to the cytotoxicity of mitoxantrone, confirming the physiological protection function of ABCG2 in hematopoietic cells [94].
Based on similar concept, ABCG2 has also been proposed to play a role in protecting putative cancer stem cells. ABCG2 has been shown to be responsible for extrusion of Hoechst dye in SP cells of breast [95-97], lung [96,98], prostate [97], GI tract [99], head and neck [100], pancreas [101], nasopharyngeal carcinoma [102] neuroblastoma and glioblastoma [96], glioma [97], leukemia [97], and retinoblastoma [103,104]. It has been shown that the cancer SP cells with higher ABCG2 expression was capable of sustained expansion ex vivo, asymmetric division, higher rate of tumorigenesis in vivo. However, in some studies it was reported that the cancer cells with or without ABCG2 expression are equally tumorigenic [97] and, thus, raising a question whether ABCG2 plays any role in the stemness of cancer cells. Further studies are clearly needed to elucidate the expression and role of ABCG2 in putative cancer stem cells.
Substrates of ABCG2
The substrates of ABCG2, identified directly by cellular or vesicular transport assays, or indirectly by substrate-stimulated ATPase activity or cytotoxicity assays, comprise a broad spectrum of anticancer drugs, sulfate and glucuronide conjugates of sterols and xenobiotics, natural compounds and toxins, fluorescent dyes, photosensitizers, and antibiotics (Table 3).
Table 3.
Summary of ABCG2 substrates
| Substrates | Reference |
|---|---|
| Topoisomerase inhibitors | |
| Mitoxantrone (topoisomerase II inhibitor) | [183] |
| Bisantrene (topoisomerase II inhibitor) | [108] |
| Etoposide (topoisomeriase II inihibitor) | [184] |
| Becatecarin (topoisomerase II inhibitor) | [185] |
| NB-506, J-107088 (topoisomerase I inhibitors) | [186] |
| Anthracyclines (Topoisomerase II inhibitors) | |
| Daunorubicin | [11] |
| Doxobucincin | [11] |
| Epirubicin | [112] |
| Pirarubicin | [184] |
| Camptothecin analogs (Topoisomerase I inhibitors) | |
| Topotecan | [61] |
| SN-38 | [61] |
| CPT-11 | [127] |
| 9-aminocamptothecin | [127] |
| NX211 | [127] |
| DX-8951f | [187] |
| Homocamptothecins | [188] |
| BN80915 (diflomotecan) | [188] |
| Gimatecan | [189] |
| Belotecan | [190] |
| Tyrosine kinase inhibitors | |
| Gefitinib | [141] |
| Dasatinib | [191] |
| Erlotinib | [192] |
| Vandetanib | [193] |
| Nilotinib | [194] |
| Sorafenib | [195] |
| Tandutinib | [196] |
| CI1033 (Pan-HER TKI) | [133] |
| CP-724,714 (HER2 TKI) | [197] |
| Symadex (fms-like tyrosine kinase 3 inhibitor) | [198] |
| Antimetabolites | |
| MTX, MTX diglutamate, MTX triglutamate (antifolate) | [115] |
| GW1843, Tomudex (antifolates) | [199] |
| Trimetrexatte, piritrexim, metoprine, pyrimethamine (lipophilic antifolates)* | [200] |
| 5-fluorouracil (pyrimidine analog) | [184] |
| CdAMP (nucleotide), cladribine (nucleoside) | [201] |
| Other anticancer drugs | |
| Flavopiridol (cyclin-dependent kinase inhibitor) | [119] |
| JNJ-7706621 (CDK and aurora kinases inhibitor) | [202] |
| Bicalutamide (non-steroidal anti-androgen) | [203] |
| NSC73306 | [204] |
| Phenethyl isothiocyanate (PEITC) | [205] |
| TH-337 (indazole-based tubulin inhibitors) | [206] |
| Sufate and glucuronide conjugates of xenobiotics | |
| Estrone 3-sulfate (E1S) | [207] |
| 17beta-estradiol sulfate | [208] |
| DHEAS | [207] |
| 4[35S]-methylumbelliferone sulfate | [207] |
| E3040 sulfate | [207] |
| Troglitazone sulfate | [209] |
| 3-O-sulfate conjugate of 17alpha-ethinylestradiol | [210] |
| SN-38-glucuronide | [211] |
| [3H]17beta-estradiol-17beta-D-glucuronide | [207] |
| [14C]4-methylumbelliferone glucuronide | [207] |
| BP-3-sulfate and BP-3-glucuronide | [45] |
| Phenolic MPA glucuronide | [212] |
| Photosensitizers | |
| Pheophorbide a | [105] |
| Pyropheophorbide a methyl ester | [106] |
| Chlorine E6 | [106] |
| 5-aminolevulinic acid | [106] |
| Phytoporphyrin | [213] |
| HPPH | [214] |
| Natural compounds and toxins | |
| Folic acid | [115] |
| Urate | [215] |
| Genistein | [145] |
| Riboflavin (vitamin B2) | [216] |
| Vitamin K3, plumbagin | [217] |
| Glutathione (GSH) | [218] |
| Sphingosine 1-phosphate | [219] |
| PhIP (carcinogen) | [220] |
| PPIX (heme precursor) | [221] |
| Fluorescent dyes | |
| Rhodamine 123 | [108] |
| Hoechst 33342 | [111] |
| Lysotracker green | [112] |
| BODIPY-prazosin | [222] |
| D-luciferin (firefly luciferase substrate) | [223] |
| Cholyl-L-lysyl-fluorescein (fluorescent bile salt derivative) | [224] |
| BODIPY-FL-dihydropyridine | [225] |
| Others | |
| [(125)I]lodoarylazidoprazosin (IAAP), [(3)H]azidopine | [225] |
| Sulfasalazine (anti-inflammatory) | [226] |
| Erythromycin (macrolide antibiotic) | [227] |
| Ciprofloxacin, ofloxacin, norfloxacin,enrofloxacin, grepafloxacin, ulifloxacin (fluoroquinolone antibiotics) | [228-230] |
| Nitrofurantoin (urinary tract antibiotic) | [231] |
| Moxidectin (parasiticide) | [232] |
| Albendazole suloxide and oxfendazole (anthelmintics) | [233] |
| Ganciclovir (antiviral drug) | [234] |
| Zidovudine (NRTI) | [235] |
| Lamivudine (NRTI) | [236] |
| Leflunomide and A771726 (antirheumatic drugs) | [237] |
| Diclofenac (analgesic and anti-inflammatory drug) | [238] |
| Cimetidine (histamine H2-receptor antagonist) | [220] |
| ME3277 (hydrophilic glycoprotein IIb/IIIa antagonist) | [239] |
| Pitavastatin (HMG-CoA reductase inhibitor) | [240] |
| Rosuvastatin (HMG-CoA reductase inhibitor) | [241] |
| Dipyridamole (thromboxane synthase inhibitor) | [242] |
| Glyburide (hypoglycemic agent) | [243] |
| Nicardipine, nifedipine, nitrendipine (Ca2+ channel blocker) | [225] |
| Olmesartan medoxomil (angiotensin II AT1-R antagonist) | [244] |
| Befloxatone (selective monoamine oxidase inhibitor) | [245] |
| Prazosin (alpha-1-adrenergic receptor antagonist) | [108] |
| Riluzole (Na+ channels blocker) | [246] |
| Amyloid-beta | [247] |
| Zoledronic acid (osteotropic compound) | [248] |
| Hesperetin conjugates (flavonoid) | [249] |
| Kaempferol (flavonoid) | [250] |
One of the major constituents of ABCG2 substrates is anticancer drugs, including topoisomerase inhibitors, anthracyclines, camptothecin (CPT) analogs, tyrosine kinase inhibitors (TKI), and antimetabolites. ABCG2 also transports many sulfate and glucuronide conjugates of steroids and xenobiotics, which are two common products of mammalian Phase II metabolism, suggesting that ABCG2 is important in drug metabolic pathways. ABCG2 also mediates the efflux of Pheophorbide a (PhA), a chlorophyll catabolite [105], and many other photosensitizers [106], implicating ABCG2 as a possible cause for photodynamic therapy resistance. Interestingly, ABCG2 may also be involved in the transport of Aβ peptides at the blood-brain barrier and up-regulation of ABCG2 correlates with Aβ deposition in cerebrovessels, leading to cerebral amyloid angiopathy in Alzheimer’s disease patients [107].
Interestingly, in early studies with MCF-7/ AdVp3000 or mitoxantrone-selected S1-M1-80 cell lines, transport of rhodamine 123 has been observed [108]. However, the transport ability of rhodamine 123 was not seen in several other ABCG2 over-expressing cell lines [109]. This inconsistency led to discovery of a gain of function ABCG2 mutant with R482G/T mutation [109,110] Both the R482G and R482T mutants and the wild-type ABCG2 are able to efflux mitoxantrone, topotecan, SN-38, Hoechst 33342 [111] and BODIPY-prazosin [112]. However, the R482G and R482T mutants have higher affinity with anthracyclines, including doxorubicin, daunorubicin, epirubicin, as well as bisantrene, fluorescence dye rhodamine 123 and lysotracker green [112]. Only the wild type ABCG2 can effectively transport methotrexate (MTX) [113,114], MTX diglutamate and triglutamate, as well as folic acid [115]. These data suggest that amino acid 482 may be a ‘hot spot’ for mutation and this mutation affects substrate specificity of ABCG2.
Nevertheless, a study using IAARh123, the photoreactive analogue of rhodamine 123, has surprisingly shown that the wild type ABCG2 along with the two R482G and R482T mutants can all bind directly to IAARh123 although the wild type ABCG2 could not transport rhodamine [116]. This observation not only shows the direct binding of substrates to ABCG2 but also suggests the inability of the wild type ABCG2 to transport rhodamine 123 may not occur at the initial binding step. In a follow-up study of nine R482 mutations, it was found that the R482 mutations induce major changes in both substrate specificity and transport activity of ABCG2 [117]. Although R482 mutations have not been identified in any clinical samples [110,118,119], R482 mutants provide a superior tool to explore ABCG2 functions and to assist the modification of ABCG2 substrates and the development of ABCG2 inhibitors.
Modulation of ABCG2 function and expression for chemosensitization
Because of the possible role of ABCG2 in causing MDR, considerable efforts have been paid to identify chemosensitizing agents targeting ABCG2 to sensitize ABCG2-mediated MDR for better treatment of human cancers. Some of these ABCG2 modulators are listed in Table 4.
Table 4.
ABCG2 Modulators
| Modulators | Dynamic/Static | Reference |
|---|---|---|
| ABCB1 inhibitors | ||
| Elacridar (GF120918) | [126,127] | |
| Cyclosporin A | [11,129] | |
| PSC0833 | [130] | |
| Pyridines derivatives | [131] | |
| Tariquidar (XR9576) | [105] | |
| Chromanone derivatives | [132] | |
| Tyrosine kinase inhibitors (TKI) | ||
| CI1033 | [133] | |
| Gefitnib (Iressa, ZD1839) | [134,141] | |
| Imatinib mesylate (Gleevec, STI571) | [135] | |
| Nilotinib | [137] | |
| Erlotinib | [138] | |
| Lapatinib | [139] | |
| Sunitinib | [140] | |
| Other inhibitors | ||
| Flavonoids | [143-146,251] | |
| Curcumin | Static | [148,149] |
| PZ compounds | Dynamic | [58,59] |
| Xanthine | Dynamic | [125] |
| FTC | Static | [120] |
| Pipecolinate derivative (VX-710) | [252] | |
| Taxane derivatives | [253,254] | |
| Tetrahydroisoquinolin-ethyl-phenylamines | [255,256] | |
| Novobiocin | [150] | |
| UCN-01 | [105] |
Modulators of ABCG2 function
The first inhibitor of ABCG2, fumitremorgin C (FTC), was reported before ABCG2 had been discovered and was shown to sensitize the resistance in the mitoxantrone-selected S1-M1- 3.2 colon cancer cell line [120]. Later, FTC was also shown to specifically inhibit ABCG2 mediated transport of chemotherapeutic agents [121]. Unfortunately, clinical development of FTC was not possible due to its neurotoxicity, which led to the development of a new tetracyclic analogue of FTC, Ko143 [122]. Ko143 appeared to be a specific and potent inhibitor of both human and murine ABCG2. Most importantly, Ko143 was nontoxic in vitro at therapeutic concentrations and in vivo in mice either through oral or intraperitoneal administration [122]. Subsequently, other FTC-type inhibitors, including the indolyl diketopiperazines [123] and tryprostatin A [124] were identified and studies on these inhibitors are ongoing.
Recently, we identified a specific ABCG2 inhibitor with dual modes of action, PZ-39, from screening of a commercial chemical compound library [58]. Unlike FTC and its derivatives, PZ- 39 not only can effectively inhibit ABCG2 function, but also can bind to ABCG2 and cause its degradation by accelerating its endocytosis and trafficking to lysosomes. PZ-39 can effectively reduce the half-life of ABCG2 from ~54 hrs to ~5 hrs. Interestingly, in a follow-up study it was found that there are probably two classes of ABCG2 inhibitors with dynamic and static properties [59]. The dynamic inhibitors including PZ- 39 can inhibit ABCG2 activity and induce ABCG2 degradation while the static inhibitors such as FTC only inhibit ABCG2 function. This conclusion was further confirmed by a later finding that xanthines can also cause ABCG2 degradation via lysosomes [125]. Thus, targeting ABCG2 degradation may provide a novel mechanism of ABCG2 inhibition that may become an effective way of reversing ABCG2-mediated MDR.
Numerous ABCB1 inhibitors were shown to also inhibit ABCG2. These inhibitors include elacridar (GF120918) [126,127], reserpine [88,128], cyclosporin A [11,129], tariquidar (XR9576) [105], PSC-833 [130], a series of newly synthesized 1,4-dihygropyridines and pyridines, such as dihydropyridines, niguldipine, nicardipine and nitrendipine [131], and chromanone derivatives, such as piperazinobenzopyranones and phenalkylaminobenzopyranones [132].
Similarly, several tyrosine kinase inhibitors (TKIs) were shown to also inhibit ABCG2. For example, CI1033 was found to reverse ABCG2- mediated resistance to SN-38 and topotecan [133]. Gefitinib (Iressa; ZD1839) has also been shown to inhibit ABCG2-mediated drug resistance, similar as imatinib mesylate (Gleevec, STI571), EKI-785, nilotinib, erlotinib, lapatinib and sunitinib [134-140]. Since ABCG2 can directly transport or confer resistance to CI1033, gefitinib, and imatinib [141,142], it is possible that these TKIs may act as competitive inhibitors of ABCG2.
Flavonoids, a class of polyphenolic compounds widely present in foods and herbal products, are another class of ABCG2 inhibitors. Silymarin, hesperetin, quercetin, and daidzein, as well as the stilbene resveratrol, were shown to increase intracellular accumulation of mitoxantrone and BODIPY-prazosin in ABCG2 over-expressing cells [143]. Similarly, chrysin and biochanin A have also been shown to potently inhibit ABCG2 [144]. In addition, genestein, naringenin, acacetin, kaempferol and glycosylated flavonoids reversed resistance to SN-38 and mitoxantrone in ABCG2-overexpressing K562 cells [145]. It is thought that flavonoid inhibition of ABCG2 may be via interaction with its NBD [146]. Treatment with multiple flavonoids has revealed an additive effect in ABCG2 inhibition, implying that the approach of ‘flavonoid cocktails’ might achieve ideal effects on reversing ABCG2-mediated MDR [147].
Curcumin represents another group of compounds that have ABCG2 inhibitory activities. Curcumin could inhibit the transport and resistance of mitoxantrone or PhA in ABCG2 over-expressing cells, without affecting ATP binding activity or expression of ABCG2 [148]. Tetrahydrocurcumin (THC), a major metabolite of curcumin, exhibited potent inhibition of ABCG2, ABCB1 and ABCC1 [149]. More importantly, THC inhibited the binding of IAAP with ABCG2, inferring that THC may inhibit ABCG2 function through direct interaction with drug binding site of ABCG2.
Several other potent ABCG2 inhibitors including novobiocin, a coumermycin antibiotic, could decrease resistance to topotecan, SN-38 and mitoxantrone at low concentrations through competitive inhibition [150]. UCN-01, a cyclin-dependent kinase inhibitor, inhibited ABCG2- mediated transport of xenobiotics [105]. Some HIV protease inhibitors including ritonavir, saquinavir, nelfinavir, and iopinavir could effectively inhibit the transport activity of wild type ABCG2 but with less effect on R482T/G mutants [151,152]. However, none of the HIV inhibitors tested were ABCG2 substrates.
Modulators of ABCG2 expression
In addition to modulating ABCG2 function, directly inhibiting the expression of ABCG2 gene has been considered. For example, hammerhead ribozyme or antisense oligonucleotide-based treatment has been demonstrated to be a powerful therapeutic strategy to overcome drug resistance mediated by ABC transporters [153,154]. Six hammerhead ribozymes directed against ABCG2 gene have been designed and one of them, RzB1, showed high endoribonucleolytic cleavage activity at physiological pH and temperature in a cell free system [155]. Upon introduction into cultured cells, RzB1 successfully decreased both mRNA and protein levels of ABCG2 as well as reversed the drug-resistant phenotype of a human gastric carcinoma cell line with moderate ABCG2 expression [156] and MCF-7/MX cells with high level of ABCG2 [157]. An antisense oligonucleotide targeting ABCG2 was also able to reduce ABCG2 expression and cellular resistance to topotecan [158]. Recently, RNA interference has been considered to specifically knockdown ABCG2 expression to reverse drug resistance [159-161].Thus, clearly inhibiting ABCG2 expression may serve as an alternative route to sensitize ABCG2-mediate drug resistance in cancer chemotherapy.
Acknowledgement
This work was supported in part by an NIH grant R01 CA120221. WM was a recipient of predcotoral fellowship (W81XWH-08-1-0228) from Department of Defense Breast Cancer Research Program.
References
- 1.Boesen JJ, Niericker MJ, Dieteren N, Simons JW. How variable is a spontaneous mutation rate in cultured mammalian cells? Mutat Res. 1994;307:121–129. doi: 10.1016/0027-5107(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang JT. Use of arrays to investigate the contribution of ATP-binding cassette transporters to drug resistance in cancer chemotherapy and prediction of chemosensitivity. Cell Res. 2007;17:311–323. doi: 10.1038/cr.2007.15. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Peng H, Zhang JT. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JT. Biochemistry and pharmacology of the human multidrug resistance gene product, ABCG2. J. Cent. South Univ. (Med Sci) 2007;32:531–541. [PubMed] [Google Scholar]
- 5.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta- specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 7.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 8.Mo W, Liu JY, Zhang JT. Biochemistry and pharmacology of human ABCC1/MRP1 and its role in detoxification and in multidrug resistance of cancer chemotherapy. In: Pestka S, Shi Y, Liu XY, editors. Recent Advances on Cancer Research and Therapy. Elsvier; in press. [Google Scholar]
- 9.Wang H, Lee EW, Cai X, Ni Z, Zhou L, Mao Q. Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry. 2008;47:13778–13787. doi: 10.1021/bi801644v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. Dominant- negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 11.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 12.Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, Abati A, Hansen PR, Horn T, Skovsgaard T, Bates SE. Use of peptide antibodies to probe for the mitoxantrone resistance- associated protein MXR/BCRP/ABCP/ ABCG2. Biochim Biophys Acta. 2002;1565:6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia A, Schafer HJ, Hrycyna CA. Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry. 2005;44:10893–10904. doi: 10.1021/bi0503807. [DOI] [PubMed] [Google Scholar]
- 15.Ni Z, Mark ME, Cai X, Mao Q. Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int J Biochem Mol Biol. 2010;1:1–11. [PMC free article] [PubMed] [Google Scholar]
- 16.McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D Structural Analysis of Oligomeric Human Multidrug Transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Dezi M, Fribourg PF, Di Cicco A, Arnaud O, Marco S, Falson P, Di Pietro A, Levy D. The multidrug resistance half-transporter ABCG2 is purified as a tetramer upon selective extraction from membranes. Biochim Biophys Acta. 2010;1798:2094–2101. doi: 10.1016/j.bbamem.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg MF, Bikadi Z, Chan J, Liu X, Ni Z, Cai X, Ford RC, Mao Q. The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure. 2010;18:482–493. doi: 10.1016/j.str.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res. 2007;67:4373–4381. doi: 10.1158/0008-5472.CAN-06-3169. [DOI] [PubMed] [Google Scholar]
- 20.Mo W, Qi J, Zhang JT. Different roles of TM5, TM6, and ECL3 in oligomerization and function of human ABCG2. doi: 10.1021/bi300301a. Submitted 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280:36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Yang Y, Qi J, Peng H, Zhang JT. Effect of cysteine mutagenesis on the function and disulfide bond formation of human ABCG2. J Pharmacol Exp Ther. 2008;326:33–40. doi: 10.1124/jpet.108.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 24.Aust S, Obrist P, Jaeger W, Klimpfinger M, Tucek G, Wrba F, Penner E, Thalhammer T. Subcellular localization of the ABCG2 transporter in normal and malignant human gallbladder epithelium. Lab Invest. 2004;84:1024–1036. doi: 10.1038/labinvest.3700127. [DOI] [PubMed] [Google Scholar]
- 25.Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006;235:84–92. doi: 10.1016/j.canlet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 27.Rocchi E, Khodjakov A, Volk EL, Yang CH, Litman T, Bates SE, Schneider E. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun. 2000;271:42–46. doi: 10.1006/bbrc.2000.2590. [DOI] [PubMed] [Google Scholar]
- 28.Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817–823. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- 29.Ross DD, Yang W, Abruzzo LV, Dalton WS, Schneider E, Lage H, Dietel M, Greenberger L, Cole SP, Doyle LA. Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J Natl Cancer Inst. 1999;91:429–433. doi: 10.1093/jnci/91.5.429. [DOI] [PubMed] [Google Scholar]
- 30.Knutsen T, Rao VK, Ried T, Mickley L, Schneider E, Miyake K, Ghadimi BM, Padilla-Nash H, Pack S, Greenberger L, Cowan K, Dean M, Fojo T, Bates S. Amplification of 4q21-q22 and the MXR gene in independently derived mitoxantrone-resistant cell lines. Genes, chromosomes & cancer. 2000;27:110–116. [PubMed] [Google Scholar]
- 31.Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, Orsetti B, Scheffer GL, Ychou M, Khan QA, Pommier Y, Pau B, Martineau P, Del Rio M. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–854. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 32.Rao VK, Wangsa D, Robey RW, Huff L, Honjo Y, Hung J, Knutsen T, Ried T, Bates SE. Characterization of ABCG2 gene amplification manifesting as extrachromosomal DNA in mitoxantrone-selected SF295 human glioblastoma cells. Cancer Genet Cytogenet. 2005;160:126–133. doi: 10.1016/j.cancergencyto.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood. 2006;108:3881–3889. doi: 10.1182/blood-2005-10-009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To KK, Zhan Z, Bates SE. Aberrant promoter methylation of the ABCG2 gene in renal carcinoma. Mol Cell Biol. 2006;26:8572–8585. doi: 10.1128/MCB.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano H, Nakamura Y, Soda H, Kamikatahira M, Uchida K, Takasu M, Kitazaki T, Yamaguchi H, Nakatomi K, Yanagihara K, Kohno S, Tsukamoto K. Methylation status of breast cancer resistance protein detected by methylation- specific polymerase chain reaction analysis is correlated inversely with its expression in drug-resistant lung cancer cells. Cancer. 2008;112:1122–1130. doi: 10.1002/cncr.23285. [DOI] [PubMed] [Google Scholar]
- 36.To KK, Polgar O, Huff LM, Morisaki K, Bates SE. Histone modifications at the ABCG2 promoter following treatment with histone deacetylase inhibitor mirror those in multidrug -resistant cells. Mol Cancer Res. 2008;6:151–164. doi: 10.1158/1541-7786.MCR-07-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanzaki A, Toi M, Neamati N, Miyashita H, Oubu M, Nakayama K, Bando H, Ogawa K, Mutoh M, Mori S, Terada K, Sugiyama T, Fukumoto M, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human breast carcinoma. Jpn J Cancer Res. 2002;93:70–77. doi: 10.1111/j.1349-7006.2002.tb01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H, Masamune A, Kikuta K, Kume K, Shimosegawa T. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2011 doi: 10.1002/jcp.22781. [DOI] [PubMed] [Google Scholar]
- 39.Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64:1247–1251. doi: 10.1158/0008-5472.can-03-3583. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy P, Ross DD, Nakanishi T, Bailey -Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 41.Nakamichi N, Morii E, Ikeda J, Qiu Y, Mamato S, Tian T, Fukuhara S, Aozasa K. Synergistic effect of interleukin-6 and endoplasmic reticulum stress inducers on the high level of ABCG2 expression in plasma cells. Lab Invest. 2009;89:327–336. doi: 10.1038/labinvest.2008.157. [DOI] [PubMed] [Google Scholar]
- 42.Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B, Nagy L. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281:23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Lee EW, Zhou L, Leung PC, Ross DD, Unadkat JD, Mao Q. Progesterone receptor (PR) isoforms PRA and PRB differentially regulate expression of the breast cancer resistance protein in human placental choriocarcinoma BeWo cells. Mol Pharmacol. 2008;73:845–854. doi: 10.1124/mol.107.041087. [DOI] [PubMed] [Google Scholar]
- 44.Vore M, Leggas M. Progesterone acts via progesterone receptors A and B to regulate breast cancer resistance protein expression. Mol Pharmacol. 2008;73:613–615. doi: 10.1124/mol.107.044289. [DOI] [PubMed] [Google Scholar]
- 45.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a] pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–1763. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 46.Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a] pyrene-3-sulfate. Toxicol Sci. 2007;96:227–236. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 47.Tan KP, Wang B, Yang M, Boutros PC, Macaulay J, Xu H, Chuang AI, Kosuge K, Yamamoto M, Takahashi S, Wu AM, Ross DD, Harper PA, Ito S. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2) Mol Pharmacol. 2010;78:175–185. doi: 10.1124/mol.110.065078. [DOI] [PubMed] [Google Scholar]
- 48.Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol Cancer Ther. 2010;9:2365–2376. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao R, Sun J, Zhang L, Lou G, Chen M, Zhou D, Chen Z, Zhang S. MicroRNAs play a role in the development of human hematopoietic stem cells. J Cell Biochem. 2008;104:805–817. doi: 10.1002/jcb.21668. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z, Miao Y. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR- 328, -519c and -520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–792. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.To KK, Robey RW, Knutsen T, Zhan Z, Ried T, Bates SE. Escape from hsa-miR-519c enables drug-resistant cells to maintain high expression of ABCG2. Mol Cancer Ther. 2009;8:2959–2968. doi: 10.1158/1535-7163.MCT-09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakabayashi K, Nakagawa H, Tamura A, Koshiba S, Hoshijima K, Komada M, Ishikawa T. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J Biol Chem. 2007;282:27841–27846. doi: 10.1074/jbc.C700133200. [DOI] [PubMed] [Google Scholar]
- 55.Wakabayashi-Nakao K, Tamura A, Furukawa T, Nakagawa H, Ishikawa T. Quality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradation. Adv Drug Deliv Rev. 2009;61:66–72. doi: 10.1016/j.addr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa H, Tamura A, Wakabayashi K, Hoshijima K, Komada M, Yoshida T, Kometani S, Matsubara T, Mikuriya K, Ishikawa T. Ubiquitin- mediated proteasomal degradation of non-synonymous SNP variants of human ABC transporter ABCG2. Biochem J. 2008;411:623–631. doi: 10.1042/BJ20071229. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T. Disruption of N-linked glycosylation enhances ubiquitin- mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J. 2009;276:7237–7252. doi: 10.1111/j.1742-4658.2009.07423.x. [DOI] [PubMed] [Google Scholar]
- 58.Peng H, Dong Z, Qi J, Yang Y, Liu Y, Li Z, Xu J, Zhang JT. A Novel Two Mode-Acting Inhibitor of ABCG2-Mediated Multidrug Transport and Resistance in Cancer Chemotherapy. PLoS ONE. 2009;4:e5676. doi: 10.1371/journal.pone.0005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng H, Qi J, Dong Z, Zhang JT. Dynamic vs Static ABCG2 Inhibitors to Sensitize Drug Resistant Cancer Cells. PLoS One. 2010;5:e15276. doi: 10.1371/journal.pone.0015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiyama T, Shuto T, Suzuki S, Sato T, Koga T, Suico MA, Kusuhara H, Sugiyama Y, Cyr DM, Kai H. Posttranslational negative regulation of glycosylated and non-glycosylated BCRP expression by Derlin-1. Biochem Biophys Res Commun. 2011;404:853–858. doi: 10.1016/j.bbrc.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp- Helmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 62.Perego P, De Cesare M, De Isabella P, Carenini N, Beggiolin G, Pezzoni G, Palumbo M, Tartaglia L, Pratesi G, Pisano C, Carminati P, Scheffer GL, Zunino F. A novel 7-modified camptothecin analog overcomes breast cancer resistance protein-associated resistance in a mitoxantrone-selected colon carcinoma cell line. Cancer Res. 2001;61:6034–6037. [PubMed] [Google Scholar]
- 63.Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, Soda H, Fukuda M, Ikegami Y, Sugahara K, Yamada Y, Kamihira S, Doyle LA, Ross DD, Kohno S. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280:1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 64.Stein U, Lage H, Jordan A, Walther W, Bates SE, Litman T, Hohenberger P, Dietel M. Impact of BCRP/MXR, MRP1 and MDR1/P-Glycoprotein on thermoresistant variants of atypical and classical multidrug resistant cancer cells. Int J Cancer. 2002;97:751–760. doi: 10.1002/ijc.10131. [DOI] [PubMed] [Google Scholar]
- 65.Nagashima S, Soda H, Oka M, Kitazaki T, Shiozawa K, Nakamura Y, Takemura M, Yabuuchi H, Fukuda M, Tsukamoto K, Kohno S. BCRP/ABCG2 levels account for the resistance to topoisomerase I inhibitors and reversal effects by gefitinib in non-small cell lung cancer. Cancer Chemother Pharmacol. 2006;58:594–600. doi: 10.1007/s00280-006-0212-y. [DOI] [PubMed] [Google Scholar]
- 66.Kamiyama N, Takagi S, Yamamoto C, Kudo T, Nakagawa T, Takahashi M, Nakanishi K, Takahashi H, Todo S, Iseki K. Expression of ABC transporters in human hepatocyte carcinoma cells with cross-resistance to epirubicin and mitoxantrone. Anticancer research. 2006;26:885–888. [PubMed] [Google Scholar]
- 67.Liu Y, Peng H, Zhang JT. Expression Profiling of ABC Transporters in a Drug-Resistant Breast Cancer Cell Line Using AmpArray. Mol Pharmacol. 2005;68:430–438. doi: 10.1124/mol.105.011015. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Liu H, Han B, Zhang JT. Identification of 14-3-3sigma as a contributor to drug resistance in human breast cancer cells using functional proteomic analysis. Cancer Res. 2006;66:3248–3255. doi: 10.1158/0008-5472.CAN-05-3801. [DOI] [PubMed] [Google Scholar]
- 69.Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7:263–270. doi: 10.1158/1535-7163.MCT-07-0445. [DOI] [PubMed] [Google Scholar]
- 70.Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu X, Yang Y, Mo W, Huang W, Khoo SK, Chen J, Petillo D, Teh BT, Qian CN, Zhang JT. Role of eIF3a in regulating cisplatin sensitivity and in translational control of nucleotide excision repair of nasopharyngeal carcinoma. Oncogene. 2011;30:4814–4823. doi: 10.1038/onc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jaeger W. Classical resistance mechanisms. Int J Clin Pharmacol Ther. 2009;47:46–48. [PubMed] [Google Scholar]
- 72.Ross DD, Karp JE, Chen TT, Doyle LA. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96:365–368. [PubMed] [Google Scholar]
- 73.Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, Morjani H, Marzac C, Marie JP, Legrand O. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–7772. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 74.Suvannasankha A, Minderman H, O'Loughlin KL, Nakanishi T, Ford LA, Greco WR, Wetzler M, Ross DD, Baer MR. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br J Haematol. 2004;127:392–398. doi: 10.1111/j.1365-2141.2004.05211.x. [DOI] [PubMed] [Google Scholar]
- 75.Plasschaert SL, van der Kolk DM, de Bont ES, Kamps WA, Morisaki K, Bates SE, Scheffer GL, Scheper RJ, Vellenga E, de Vries EG. The role of breast cancer resistance protein in acute lymphoblastic leukemia. Clin Cancer Res. 2003;9:5171–5177. [PubMed] [Google Scholar]
- 76.Sauerbrey A, Sell W, Steinbach D, Voigt A, Zintl F. Expression of the BCRP gene (ABCG2/ MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br J Haematol. 2002;118:147–150. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- 77.Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, Stoter G, Nooter K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–836. [PubMed] [Google Scholar]
- 78.Kanzaki A, Toi M, Nakayama K, Bando H, Mutoh M, Uchida T, Fukumoto M, Takebayashi Y. Expression of multidrug resistance-related transporters in human breast carcinoma. Jpn J Cancer Res. 2001;92:452–458. doi: 10.1111/j.1349-7006.2001.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol. 2005;70:695–699. doi: 10.1016/j.bcp.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 81.Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 82.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52:1788–1795. doi: 10.1136/gut.52.12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 84.Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- 85.Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, Scheper RJ, van der Valk P, Leenstra S, Baayen JC, Spliet WG, Troost D. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- 86.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 87.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 89.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 90.Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293:670–674. doi: 10.1016/S0006-291X(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 91.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 92.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 93.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 94.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco MM, Dale TC, Smalley MJ. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raaijmakers MH, de Grouw EP, Heuver LH, van der Reijden BA, Jansen JH, Scheper RJ, Scheffer GL, de Witte TJ, Raymakers RA. Breast cancer resistance protein in drug resistance of primitive CD34+38- cells in acute myeloid leukemia. Clin Cancer Res. 2005;11:2436–2444. doi: 10.1158/1078-0432.CCR-04-0212. [DOI] [PubMed] [Google Scholar]
- 98.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 99.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 100.Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–406. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 101.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 102.Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 103.Seigel GM, Campbell LM, Narayan M, Gonzalez- Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- 104.Mohan A, Kandalam M, Ramkumar HL, Gopal L, Krishnakumar S. Stem cell markers: ABCG2 and MCM2 expression in retinoblastoma. Br J Ophthalmol. 2006;90:889–893. doi: 10.1136/bjo.2005.089219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–1246. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 106.Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther. 2005;4:187–194. [PubMed] [Google Scholar]
- 107.Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, Zhang W. ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1- 40) peptides. J Neurosci. 2009;29:5463–5475. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC halftransporter, MXR (ABCG2) J Cell Sci. 2000;113(Pt 11):2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 109.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61:6635–6639. [PubMed] [Google Scholar]
- 110.Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, Bates SE. Single-Nucleotide Polymorphism (SNP) Analysis in the ABC Half- Transporter ABCG2 (MXR/BCRP/ABCP1) Cancer Biol Ther. 2002;1:696–702. doi: 10.4161/cbt.322. [DOI] [PubMed] [Google Scholar]
- 111.Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277:47980–47990. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- 112.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Volk EL, Rohde K, Rhee M, McGuire JJ, Doyle LA, Ross DD, Schneider E. Methotrexate cross-resistance in a mitoxantrone-selected multidrug-resistant MCF7 breast cancer cell line is attributable to enhanced energy-dependent drug efflux. Cancer Res. 2000;60:3514–3521. [PubMed] [Google Scholar]
- 114.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–5040. [PubMed] [Google Scholar]
- 115.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–4054. [PubMed] [Google Scholar]
- 116.Alqawi O, Bates S, Georges E. Arginine482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem J. 2004;382:711–716. doi: 10.1042/BJ20040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozvegy-Laczka C, Koblos G, Sarkadi B, Varadi A. Single amino acid (482) variants of the ABCG2 multidrug transporter: major differences in transport capacity and substrate recognition. Biochim Biophys Acta. 2005;1668:53–63. doi: 10.1016/j.bbamem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, Schuetz EG. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 119.Nakanishi T, Karp JE, Tan M, Doyle LA, Peters T, Yang W, Wei D, Ross DD. Quantitative analysis of breast cancer resistance protein and cellular resistance to flavopiridol in acute leukemia patients. Clin Cancer Res. 2003;9:3320–3328. [PubMed] [Google Scholar]
- 120.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 121.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 122.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 123.van Loevezijn A, Allen JD, Schinkel AH, Koomen GJ. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg Med Chem Lett. 2001;11:29–32. doi: 10.1016/s0960-894x(00)00588-6. [DOI] [PubMed] [Google Scholar]
- 124.Woehlecke H, Osada H, Herrmann A, Lage H. Reversal of breast cancer resistance protein- mediated drug resistance by tryprostatin A. Int J Cancer. 2003;107:721–728. doi: 10.1002/ijc.11444. [DOI] [PubMed] [Google Scholar]
- 125.Ding R, Shi J, Pabon K, Scotto KW. Xanthines Down-Regulate the Drug Transporter ABCG2 and Reverse Multidrug Resistance. Mol Pharmacol. 2012;81:328–337. doi: 10.1124/mol.111.075556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 127.Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, Pluim D, Beijnen JH, Schellens JH. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7:935–941. [PubMed] [Google Scholar]
- 128.Wierdl M, Wall A, Morton CL, Sampath J, Danks MK, Schuetz JD, Potter PM. Carboxylesterase- mediated sensitization of human tumor cells to CPT-11 cannot override ABCG2-mediated drug resistance. Mol Pharmacol. 2003;64:279–288. doi: 10.1124/mol.64.2.279. [DOI] [PubMed] [Google Scholar]
- 129.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, Baer MR. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 130.Garcia-Escarp M, Martinez-Munoz V, Sales- Pardo I, Barquinero J, Domingo JC, Marin P, Petriz J. Flow cytometry-based approach to ABCG2 function suggests that the transporter differentially handles the influx and efflux of drugs. Cytometry A. 2004;62:129–138. doi: 10.1002/cyto.a.20072. [DOI] [PubMed] [Google Scholar]
- 131.Zhou XF, Yang X, Wang Q, Coburn RA, Morris ME. Effects of dihydropyridines and pyridines on multidrug resistance mediated by breast cancer resistance protein: in vitro and in vivo studies. Drug Metab Dispos. 2005;33:1220–1228. doi: 10.1124/dmd.104.003558. [DOI] [PubMed] [Google Scholar]
- 132.Boumendjel A, Nicolle E, Moraux T, Gerby B, Blanc M, Ronot X, Boutonnat J. Piperazinobenzopyranones and phenalkylaminobenzopyranones: potent inhibitors of breast cancer resistance protein (ABCG2) J Med Chem. 2005;48:7275–7281. doi: 10.1021/jm050705h. [DOI] [PubMed] [Google Scholar]
- 133.Erlichman C, Boerner SA, Hallgren CG, Spieker R, Wang XY, James CD, Scheffer GL, Maliepaard M, Ross DD, Bible KC, Kaufmann SH. The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10- hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res. 2001;61:739–748. [PubMed] [Google Scholar]
- 134.Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, Itoh A, Ikegami Y, Tsurutani J, Nakatomi K, Kitazaki T, Doi S, Yoshida H, Kohno S. Gefitinib ("Iressa", ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res. 2005;65:1541–1546. doi: 10.1158/0008-5472.CAN-03-2417. [DOI] [PubMed] [Google Scholar]
- 135.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 136.Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 137.Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267–1275. doi: 10.1038/sj.leu.2404638. [DOI] [PubMed] [Google Scholar]
- 138.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR, Jr. , Fu LW, Ambudkar SV, Chen ZS. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 139.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR, Jr. , Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, Chen ZS, Tong XZ, Fu LW. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 141.Elkind NB, Szentpetery Z, Apati A, Ozvegy- Laczka C, Varady G, Ujhelly O, Szabo K, Homolya L, Varadi A, Buday L, Keri G, Nemet K, Sarkadi B. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib) Cancer Res. 2005;65:1770–1777. doi: 10.1158/0008-5472.CAN-04-3303. [DOI] [PubMed] [Google Scholar]
- 142.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 143.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317:269–275. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 145.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 146.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 147.Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 148.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 149.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 150.Shiozawa K, Oka M, Soda H, Yoshikawa M, Ikegami Y, Tsurutani J, Nakatomi K, Nakamura Y, Doi S, Kitazaki T, Mizuta Y, Murase K, Yoshida H, Ross DD, Kohno S. Reversal of breast cancer resistance protein (BCRP/ ABCG2)-mediated drug resistance by novobiocin, a coumermycin antibiotic. Int J Cancer. 2004;108:146–151. doi: 10.1002/ijc.11528. [DOI] [PubMed] [Google Scholar]
- 151.Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- 152.Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. Modulation of human BCRP (ABCG2) activity by anti- HIV drugs. The Journal of antimicrobial chemotherapy. 2007;59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 153.Stuart DD, Kao GY, Allen TM. A novel, long-circulating, and functional liposomal formulation of antisense oligodeoxynucleotides targeted against MDR1. Cancer Gene Ther. 2000;7:466–475. doi: 10.1038/sj.cgt.7700145. [DOI] [PubMed] [Google Scholar]
- 154.Kobayashi H, Dorai T, Holland JF, Ohnuma T. Reversal of drug sensitivity in multidrug-resistant tumor cells by an MDR1 (PGY1) ribozyme. Cancer Res. 1994;54:1271–1275. [PubMed] [Google Scholar]
- 155.Kowalski P, Wichert A, Holm PS, Dietel M, Lage H. Selection and characterization of a high-activity ribozyme directed against the antineoplastic drug resistance-associated ABC transporter BCRP/MXR/ABCG2. Cancer Gene Ther. 2001;8:185–192. doi: 10.1038/sj.cgt.7700294. [DOI] [PubMed] [Google Scholar]
- 156.Kowalski P, Stein U, Scheffer GL, Lage H. Modulation of the atypical multidrug-resistant phenotype by a hammerhead ribozyme directed against the ABC transporter BCRP/ MXR/ABCG2. Cancer Gene Ther. 2002;9:579–586. doi: 10.1038/sj.cgt.7700471. [DOI] [PubMed] [Google Scholar]
- 157.Kowalski P, Farley KM, Lage H, Schneider E. Effective knock down of very high ABCG2 expression by a hammerhead ribozyme. Anti-cancer research. 2004;24:2231–2235. [PubMed] [Google Scholar]
- 158.Jia P, Wu SB, Xu Q, Wu MF, Gao QL, Liao GN, Lu YP, Ma D. [Antisense oligonucleotide reverses topotecan-resistant ovarian cancer cells] . Ai Zheng. 2003;22:1296–1300. [PubMed] [Google Scholar]
- 159.Ee PL, He X, Ross DD, Beck WT. Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther. 2004;3:1577–1583. [PubMed] [Google Scholar]
- 160.Li WT, Zhou GY, Song XR, Chi WL, Ren RM, Wang XW. Modulation of BCRP mediated atypical multidrug resistance phenotype by RNA interference. Neoplasma. 2005;52:219–224. [PubMed] [Google Scholar]
- 161.Priebsch A, Rompe F, Tonnies H, Kowalski P, Surowiak P, Stege A, Materna V, Lage H. Complete reversal of ABCG2-depending atypical multidrug resistance by RNA interference in human carcinoma cells. Oligonucleotides. 2006;16:263–274. doi: 10.1089/oli.2006.16.263. [DOI] [PubMed] [Google Scholar]
- 162.Sargent JM, Williamson CJ, Maliepaard M, Elgie AW, Scheper RJ, Taylor CG. Breast cancer resistance protein expression and resistance to daunorubicin in blast cells from patients with acute myeloid leukaemia. Br J Haematol. 2001;115:257–262. doi: 10.1046/j.1365-2141.2001.03122.x. [DOI] [PubMed] [Google Scholar]
- 163.van der Kolk DM, Vellenga E, Scheffer GL, Muller M, Bates SE, Scheper RJ, de Vries EG. Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood. 2002;99:3763–3770. doi: 10.1182/blood.v99.10.3763. [DOI] [PubMed] [Google Scholar]
- 164.van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B, Pieters R, Sonneveld P. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML) Leukemia. 2002;16:833–839. doi: 10.1038/sj.leu.2402496. [DOI] [PubMed] [Google Scholar]
- 165.Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia. 2002;16:1443–1447. doi: 10.1038/sj.leu.2402541. [DOI] [PubMed] [Google Scholar]
- 166.Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–4601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 167.Galimberti S, Guerrini F, Palumbo GA, Consoli U, Fazzi R, Morabito F, Santini V, Petrini M. Evaluation of BCRP and MDR-1 co-expression by quantitative molecular assessment in AML patients. Leuk Res. 2004;28:367–372. doi: 10.1016/j.leukres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 168.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 169.Damiani D, Tiribelli M, Calistri E, Geromin A, Chiarvesio A, Michelutti A, Cavallin M, Fanin R. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica. 2006;91:825–828. [PubMed] [Google Scholar]
- 170.van den Heuvel-Eibrink MM, van der Holt B, Burnett AK, Knauf WU, Fey MF, Verhoef GE, Vellenga E, Ossenkoppele GJ, Lowenberg B, Sonneveld P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol. 2007;86:329–337. doi: 10.1007/s00277-007-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Damiani D, Tiribelli M, Michelutti A, Geromin A, Cavallin M, Fabbro D, Pianta A, Malagola M, Damante G, Russo D, Fanin R. Fludarabine- based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk Res. 2010;34:942–945. doi: 10.1016/j.leukres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 172.Faneyte IF, Kristel PM, Maliepaard M, Scheffer GL, Scheper RJ, Schellens JH, van de Vijver MJ. Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res. 2002;8:1068–1074. [PubMed] [Google Scholar]
- 173.Diestra JE, Scheffer GL, Catala I, Maliepaard M, Schellens JH, Scheper RJ, Germa-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 174.Gupta N, Martin PM, Miyauchi S, Ananth S, Herdman AV, Martindale RG, Podolsky R, Ganapathy V. Down-regulation of BCRP/ ABCG2 in colorectal and cervical cancer. Biochem Biophys Res Commun. 2006;343:571–577. doi: 10.1016/j.bbrc.2006.02.172. [DOI] [PubMed] [Google Scholar]
- 175.Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M, Ochiai A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 176.Ota S, Ishii G, Goto K, Kubota K, Kim YH, Kojika M, Murata Y, Yamazaki M, Nishiwaki Y, Eguchi K, Ochiai A. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small- cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 177.Kim YH, Ishii G, Goto K, Ota S, Kubota K, Murata Y, Mishima M, Saijo N, Nishiwaki Y, Ochiai A. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65:105–111. doi: 10.1016/j.lungcan.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 178.Deichmann M, Thome M, Egner U, Hartschuh W, Kurzen H. The chemoresistance gene ABCG2 (MXR/BCRP1/ABCP1) is not expressed in melanomas but in single neuroendocrine carcinomas of the skin. J Cutan Pathol. 2005;32:467–473. doi: 10.1111/j.0303-6987.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 179.Wilson MW, Fraga CH, Fuller CE, Rodriguez- Galindo C, Mancini J, Hagedorn N, Leggas ML, Stewart CF. Immunohistochemical detection of multidrug-resistant protein expression in retinoblastoma treated by primary enucleation. Invest Ophthalmol Vis Sci. 2006;47:1269–1273. doi: 10.1167/iovs.05-1321. [DOI] [PubMed] [Google Scholar]
- 180.Tsunoda S, Okumura T, Ito T, Kondo K, Ortiz C, Tanaka E, Watanabe G, Itami A, Sakai Y, Shimada Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology. 2006;71:251–258. doi: 10.1159/000106787. [DOI] [PubMed] [Google Scholar]
- 181.Saglam A, Hayran M, Uner AH. Immunohistochemical expression of multidrug resistance proteins in mature T/NK-cell lymphomas. APMIS. 2008;116:791–800. doi: 10.1111/j.1600-0463.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 182.Kim JE, Singh RR, Cho-Vega JH, Drakos E, Davuluri Y, Khokhar FA, Fayad L, Medeiros LJ, Vega F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- 183.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 184.Yuan J, Lv H, Peng B, Wang C, Yu Y, He Z. Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009;63:1103–1110. doi: 10.1007/s00280-008-0838-z. [DOI] [PubMed] [Google Scholar]
- 185.Robey RW, Obrzut T, Shukla S, Polgar O, Macalou S, Bahr JC, Di Pietro A, Ambudkar SV, Bates SE. Becatecarin (rebeccamycin analog, NSC 655649) is a transport substrate and induces expression of the ATP-binding cassette transporter, ABCG2, in lung carcinoma cells. Cancer Chemother Pharmacol. 2009;64:575–583. doi: 10.1007/s00280-008-0908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Komatani H, Kotani H, Hara Y, Nakagawa R, Matsumoto M, Arakawa H, Nishimura S. Identification of breast cancer resistant protein/ mitoxantrone resistance/placenta-specific, ATP-binding cassette transporter as a transporter of NB-506 and J-107088, topoisomerase I inhibitors with an indolocarbazole structure. Cancer Res. 2001;61:2827–2832. [PubMed] [Google Scholar]
- 187.van Hattum AH, Hoogsteen IJ, Schluper HM, Maliepaard M, Scheffer GL, Scheper RJ, Kohlhagen G, Pommier Y, Pinedo HM, Boven E. Induction of breast cancer resistance protein by the camptothecin derivative DX-8951f is associated with minor reduction of antitumour activity. Br J Cancer. 2002;87:665–672. doi: 10.1038/sj.bjc.6600508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bates SE, Medina-Perez WY, Kohlhagen G, Antony S, Nadjem T, Robey RW, Pommier Y. ABCG2 mediates differential resistance to SN-38 (7-ethyl-10-hydroxycamptothecin) and homocamptothecins. J Pharmacol Exp Ther. 2004;310:836–842. doi: 10.1124/jpet.103.063149. [DOI] [PubMed] [Google Scholar]
- 189.Marchetti S, Oostendorp RL, Pluim D, van Eijndhoven M, van Tellingen O, Schinkel AH, Versace R, Beijnen JH, Mazzanti R, Schellens JH. In vitro transport of gimatecan (7-t-butoxyiminomethylcamptothecin) by breast cancer resistance protein, P-glycoprotein, and multidrug resistance protein 2. Mol Cancer Ther. 2007;6:3307–3313. doi: 10.1158/1535-7163.MCT-07-0461. [DOI] [PubMed] [Google Scholar]
- 190.Li H, Jin HE, Kim W, Han YH, Kim DD, Chung SJ, Shim CK. Involvement of P-glycoprotein, multidrug resistance protein 2 and breast cancer resistance protein in the transport of belotecan and topotecan in Caco- 2 and MDCKII cells. Pharm Res. 2008;25:2601–2612. doi: 10.1007/s11095-008-9678-0. [DOI] [PubMed] [Google Scholar]
- 191.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, Eadie L, To LB, Melo J, Kumar S, Hughes TP, White DL. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 192.Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1 -/-/Mdr1a/1b-/- (triple-knockout) and wildtype mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 193.Azzariti A, Porcelli L, Simone GM, Quatrale AE, Colabufo NA, Berardi F, Perrone R, Zucchetti M, D'Incalci M, Xu JM, Paradiso A. Tyrosine kinase inhibitors and multidrug resistance proteins: interactions and biological consequences. Cancer Chemother Pharmacol. 2010;65:335–346. doi: 10.1007/s00280-009-1039-0. [DOI] [PubMed] [Google Scholar]
- 194.Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L, Keri G, Katona M, Takats Z, Varadi A, Szakacs G, Sarkadi B. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336:223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang JJ, Milton MN, Yu S, Liao M, Liu N, Wu JT, Gan L, Balani SK, Lee FW, Prakash S, Xia CQ. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab Lett. 2010;4:201–212. doi: 10.2174/187231210792928279. [DOI] [PubMed] [Google Scholar]
- 197.Feng B, Xu JJ, Bi YA, Mireles R, Davidson R, Duignan DB, Campbell S, Kostrubsky VE, Dunn MC, Smith AR, Wang HF. Role of hepatic transporters in the disposition and hepatotoxicity of a HER2 tyrosine kinase inhibitor CP- 724,714. Toxicol Sci. 2009;108:492–500. doi: 10.1093/toxsci/kfp033. [DOI] [PubMed] [Google Scholar]
- 198.Bram EE, Adar Y, Mesika N, Sabisz M, Skladanowski A, Assaraf YG. Structural determinants of imidazoacridinones facilitating antitumor activity are crucial for substrate recognition by ABCG2. Mol Pharmacol. 2009;75:1149–1159. doi: 10.1124/mol.109.054791. [DOI] [PubMed] [Google Scholar]
- 199.Shafran A, Ifergan I, Bram E, Jansen G, Kathmann I, Peters GJ, Robey RW, Bates SE, Assaraf YG. ABCG2 harboring the Gly482 mutation confers high-level resistance to various hydrophilic antifolates. Cancer Res. 2005;65:8414–8422. doi: 10.1158/0008-5472.CAN-04-4547. [DOI] [PubMed] [Google Scholar]
- 200.Bram E, Ifergan I, Shafran A, Berman B, Jansen G, Assaraf YG. Mutant Gly482 and Thr482 ABCG2 mediate high-level resistance to lipophilic antifolates. Cancer Chemother Pharmacol. 2006;58:826–834. doi: 10.1007/s00280-006-0230-9. [DOI] [PubMed] [Google Scholar]
- 201.de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, Beijnen J, Borst P. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7:3092–3102. doi: 10.1158/1535-7163.MCT-08-0427. [DOI] [PubMed] [Google Scholar]
- 202.Seamon JA, Rugg CA, Emanuel S, Calcagno AM, Ambudkar SV, Middleton SA, Butler J, Borowski V, Greenberger LM. Role of the ABCG2 drug transporter in the resistance and oral bioavailability of a potent cyclin-dependent kinase/Aurora kinase inhibitor. Mol Cancer Ther. 2006;5:2459–2467. doi: 10.1158/1535-7163.MCT-06-0339. [DOI] [PubMed] [Google Scholar]
- 203.Colabufo NA, Pagliarulo V, Berardi F, Contino M, Inglese C, Niso M, Ancona P, Albo G, Pagliarulo A, Perrone R. Bicalutamide failure in prostate cancer treatment: involvement of Multi Drug Resistance proteins. Eur J Pharmacol. 2008;601:38–42. doi: 10.1016/j.ejphar.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 204.Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ji Y, Morris ME. Effect of organic isothiocyanates on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:2261–2269. doi: 10.1007/s11095-004-7679-1. [DOI] [PubMed] [Google Scholar]
- 206.Meng F, Cai X, Duan J, Matteucci MG, Hart CP. A novel class of tubulin inhibitors that exhibit potent antiproliferation and in vitro vessel-disrupting activity. Cancer Chemother Pharmacol. 2008;61:953–963. doi: 10.1007/s00280-007-0549-x. [DOI] [PubMed] [Google Scholar]
- 207.Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278:22644–22649. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]
- 208.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64:610–618. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 209.Enokizono J, Kusuhara H, Sugiyama Y. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the biliary excretion and intestinal efflux of troglitazone sulfate, the major metabolite of troglitazone with a cholestatic effect. Drug Metab Dispos. 2007;35:209–214. doi: 10.1124/dmd.106.012567. [DOI] [PubMed] [Google Scholar]
- 210.Han YH, Busler D, Hong Y, Tian Y, Chen C, Rodrigues AD. Transporter studies with the 3- O-sul fate conjugate of 17alpha-ethinylestradiol: assessment of human liver drug transporters. Drug Metab Dispos. 2010;38:1072–1082. doi: 10.1124/dmd.109.031518. [DOI] [PubMed] [Google Scholar]
- 211.Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, Shiozawa K, Kawabata S, Soda H, Ishikawa T, Tanabe S, Kohno S. Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem Biophys Res Commun. 2001;288:827–832. doi: 10.1006/bbrc.2001.5850. [DOI] [PubMed] [Google Scholar]
- 212.Miura M, Kagaya H, Satoh S, Inoue K, Saito M, Habuchi T, Suzuki T. Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit. 2008;30:559–564. doi: 10.1097/FTD.0b013e3181838063. [DOI] [PubMed] [Google Scholar]
- 213.Robey RW, Fetsch PA, Polgar O, Dean M, Bates SE. The livestock photosensitizer, phytoporphyrin (phylloerythrin), is a substrate of the ATP-binding cassette transporter ABCG2. Res Vet Sci. 2006;81:345–349. doi: 10.1016/j.rvsc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 214.Zheng X, Morgan J, Pandey SK, Chen Y, Tracy E, Baumann H, Missert JR, Batt C, Jackson J, Bellnier DA, Henderson BW, Pandey RK. Conjugation of 2-(1'-hexyloxyethyl)-2- devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo. J Med Chem. 2009;52:4306–4318. doi: 10.1021/jm9001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol. 2007;27:1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shukla S, Wu CP, Nandigama K, Ambudkar SV. The naphthoquinones, vitamin K3 and its structural analogue plumbagin, are substrates of the multidrug resistance linked ATP binding cassette drug transporter ABCG2. Mol Cancer Ther. 2007;6:3279–3286. doi: 10.1158/1535-7163.MCT-07-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brechbuhl HM, Min E, Kariya C, Frederick B, Raben D, Day BJ. Select cyclopentenone prostaglandins trigger glutathione efflux and the role of ABCG2 transport. Free Radic Biol Med. 2009;47:722–730. doi: 10.1016/j.freeradbiomed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1- phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo (4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312:144–152. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]
- 221.Zhou S, Zong Y, Ney PA, Nair G, Stewart CF, Sorrentino BP. Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood. 2005;105:2571–2576. doi: 10.1182/blood-2004-04-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cygalova LH, Hofman J, Ceckova M, Staud F. Transplacental pharmacokinetics of glyburide, rhodamine 123, and BODIPY FL prazosin: effect of drug efflux transporters and lipid solubility. J Pharmacol Exp Ther. 2009;331:1118–1125. doi: 10.1124/jpet.109.160564. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y, Byun Y, Ren YR, Liu JO, Laterra J, Pomper MG. Identification of inhibitors of ABCG2 by a bioluminescence imaging-based high-throughput assay. Cancer Res. 2009;69:5867–5875. doi: 10.1158/0008-5472.CAN-08-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Waart DR, Hausler S, Vlaming ML, Kunne C, Hanggi E, Gruss HJ, Oude Elferink RP, Stieger B. Hepatic transport mechanisms of cholyl-L-lysyl-fluorescein. J Pharmacol Exp Ther. 2010;334:78–86. doi: 10.1124/jpet.110.166991. [DOI] [PubMed] [Google Scholar]
- 225.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4- dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–8951. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 226.van der Heijden J, de Jong MC, Dijkmans BA, Lems WF, Oerlemans R, Kathmann I, Scheffer GL, Scheper RJ, Assaraf YG, Jansen G. Acquired resistance of human T cells to sulfasalazine: stability of the resistant phenotype and sensitivity to non-related DMARDs. Ann Rheum Dis. 2004;63:131–137. doi: 10.1136/ard.2003.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Janvilisri T, Shahi S, Venter H, Balakrishnan L, van Veen HW. Arginine-482 is not essential for transport of antibiotics, primary bile acids and unconjugated sterols by the human breast cancer resistance protein (ABCG2) Biochem J. 2005;385:419–426. doi: 10.1042/BJ20040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34:690–695. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- 229.Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther. 2006;29:279–287. doi: 10.1111/j.1365-2885.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 230.Ando T, Kusuhara H, Merino G, Alvarez AI, Schinkel AH, Sugiyama Y. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos. 2007;35:1873–1879. doi: 10.1124/dmd.107.014969. [DOI] [PubMed] [Google Scholar]
- 231.Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–1764. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- 232.Perez M, Blazquez AG, Real R, Mendoza G, Prieto JG, Merino G, Alvarez AI. In vitro and in vivo interaction of moxidectin with BCRP/ ABCG2. Chem Biol Interact. 2009;180:106–112. doi: 10.1016/j.cbi.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 233.Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ ABCG2) Drug Metab Dispos. 2005;33:614–618. doi: 10.1124/dmd.104.003319. [DOI] [PubMed] [Google Scholar]
- 234.Hu W, Liu W. Side populations of glioblastoma cells are less sensitive to HSV-TK/GCV suicide gene therapy system than the non-side population. In Vitro Cell Dev Biol Anim. 2010;46:497–501. doi: 10.1007/s11626-010-9274-6. [DOI] [PubMed] [Google Scholar]
- 235.Wang X, Furukawa T, Nitanda T, Okamoto M, Sugimoto Y, Akiyama S, Baba M. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003;63:65–72. doi: 10.1124/mol.63.1.65. [DOI] [PubMed] [Google Scholar]
- 236.Kim HS, Sunwoo YE, Ryu JY, Kang HJ, Jung HE, Song IS, Kim EY, Shim JC, Shon JH, Shin JG. The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007;64:645–654. doi: 10.1111/j.1365-2125.2007.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kis E, Nagy T, Jani M, Molnar E, Janossy J, Ujhellyi O, Nemet K, Heredi-Szabo K, Krajcsi P. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: implications for drug resistance. Ann Rheum Dis. 2009;68:1201–1207. doi: 10.1136/ard.2007.086264. [DOI] [PubMed] [Google Scholar]
- 238.Lagas JS, van der Kruijssen CM, van de Wetering K, Beijnen JH, Schinkel AH. Transport of diclofenac by breast cancer resistance protein (ABCG2) and stimulation of multidrug resistance protein 2 (ABCC2)-mediated drug transport by diclofenac and benzbromarone. Drug Metab Dispos. 2009;37:129–136. doi: 10.1124/dmd.108.023200. [DOI] [PubMed] [Google Scholar]
- 239.Kondo C, Onuki R, Kusuhara H, Suzuki H, Suzuki M, Okudaira N, Kojima M, Ishiwata K, Jonker JW, Sugiyama Y. Lack of improvement of oral absorption of ME3277 by prodrug formation is ascribed to the intestinal efflux mediated by breast cancer resistant protein (BCRP/ABCG2) Pharm Res. 2005;22:613–618. doi: 10.1007/s11095-005-2487-9. [DOI] [PubMed] [Google Scholar]
- 240.Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–807. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- 241.Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738–742. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- 242.Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, Mao Q. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res. 2005;22:2023–2034. doi: 10.1007/s11095-005-8384-4. [DOI] [PubMed] [Google Scholar]
- 243.Gedeon C, Anger G, Piquette-Miller M, Koren G. Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta. 2008;29:39–43. doi: 10.1016/j.placenta.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 244.Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, Nakazawa H, Okano T, Adachi M, Schuetz JD, Adachi Y, Hu Z, Kusuhara H, Sugiyama Y. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35:2166–2176. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- 245.Tournier N, Valette H, Peyronneau MA, Saba W, Goutal S, Kuhnast B, Dolle F, Scherrmann JM, Cisternino S, Bottlaender M. Transport of selected PET radiotracers by human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2): an in vitro screening. J Nucl Med. 2011;52:415–423. doi: 10.2967/jnumed.110.079608. [DOI] [PubMed] [Google Scholar]
- 246.Milane A, Vautier S, Chacun H, Meininger V, Bensimon G, Farinotti R, Fernandez C. Interactions between riluzole and ABCG2/ BCRP transporter. Neurosci Lett. 2009;452:12–16. doi: 10.1016/j.neulet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 247.Tai LM, Loughlin AJ, Male DK, Romero IA. P-glycoprotein and breast cancer resistance protein restrict apical-to-basolateral permeability of human brain endothelium to amyloid-beta. J Cereb Blood Flow Metab. 2009;29:1079–1083. doi: 10.1038/jcbfm.2009.42. [DOI] [PubMed] [Google Scholar]
- 248.Kars MD, Iseri OD, Ural AU, Gunduz U. In vitro evaluation of zoledronic acid resistance developed in MCF-7 cells. Anticancer research. 2007;27:4031–4037. [PubMed] [Google Scholar]
- 249.Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, Rietjens IM. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 250.An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos. 2011;39:426–432. doi: 10.1124/dmd.110.035212. [DOI] [PubMed] [Google Scholar]
- 251.Zhang SZ, Yang XN, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharmaceutical Research. 2004;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 252.Minderman H, O'Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–1834. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 253.Minderman H, Brooks TA, O'Loughlin KL, Ojima I, Bernacki RJ, Baer MR. Broad-spectrum modulation of ATP-binding cassette transport proteins by the taxane derivatives ortataxel (IDN-5109, BAY 59-8862) and tRA96023. Cancer Chemother Pharmacol. 2004;53:363–369. doi: 10.1007/s00280-003-0745-2. [DOI] [PubMed] [Google Scholar]
- 254.Brooks TA, Minderman H, O'Loughlin KL, Pera P, Ojima I, Baer MR, Bernacki RJ. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2:1195–1205. [PubMed] [Google Scholar]
- 255.Jekerle V, Klinkhammer W, Reilly RM, Piquette -Miller M, Wiese M. Novel tetrahydroisoquinolin- ethyl-phenylamine based multidrug resistance inhibitors with broad-spectrum modulating properties. Cancer Chemother Pharmacol. 2007;59:61–69. doi: 10.1007/s00280-006-0244-3. [DOI] [PubMed] [Google Scholar]
- 256.Jekerle V, Klinkhammer W, Scollard DA, Breitbach K, Reilly RM, Piquette-Miller M, Wiese M. In vitro and in vivo evaluation of WKX- 34, a novel inhibitor of P-glycoprotein and BCRP, using radio imaging techniques. Int J Cancer. 2006;119:414–422. doi: 10.1002/ijc.21827. [DOI] [PubMed] [Google Scholar]


