Abstract
Background
Routine assessments of pain using an intensity numeric rating scale (NRS) have improved documentation, but have not improved clinical outcomes. This may be, in part, due to the failure of the NRS to adequately predict patients’ preferences for additional treatment.
Objective
To examine whether patients’ illness perceptions have a stronger association with patient treatment preferences than the pain intensity NRS.
Design
Single face-to-face interview.
Participants
Outpatients with chronic, noncancer, musculoskeletal pain.
Main Measures
Experience of pain was measured using 18 illness perception items. Factor analysis of these items found that five factors accounted for 67.1% of the variance; 38% of the variance was accounted for by a single factor labeled “pain impact.” Generalized linear models were used to examine how NRS scores and physical function compare with pain impact in predicting preferences for highly effective/high-risk treatment.
Key Results
Two hundred forty-nine subjects agreed to participate. Neither NRS nor functioning predicted patient preference (NRS: χ2 = 1.92, df = 1, p = 0.16, physical functioning: χ2 = 2.48, df = 1, p = 0.11). In contrast, pain impact was significantly associated with the preference for a riskier/more effective treatment after adjusting for age, comorbidity, efficacy of current medications and numeracy (χ2 = 4.40, df = 1, p = 0.04).
Conclusions
Tools that measure the impact of pain may be a more valuable screening instrument than the NRS. Further research is now needed to determine if measuring the impact of pain in clinical practice is more effective at triggering appropriate management than more restricted measures of pain such as the NRS.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1926-z) contains supplementary material, which is available to authorized users.
KEY WORDS: chronic pain, screening, numeric rating scale, illness perceptions, patient preferences
Chronic pain is a prevalent, disabling, and poorly managed condition. In order to improve the quality of care for patients with pain, national organizations, including the Joint Commission on Accreditation of Healthcare Organizations and the Department of Veterans Affairs1, have mandated that pain be routinely assessed for all patients. Implementation of this directive has improved the frequency of pain assessment, and pain scores are now documented for a majority of patients2,3. Improved measurement of pain intensity has not, however, translated into improved processes of care or clinical outcomes3–5.
While the Joint Commission does not specify how pain should be assessed, it is commonly measured using a 0 to 10 numeric rating scale (NRS). The pain intensity NRS has been validated as an outcome measure6–9; however, it has not been extensively tested as a screening tool, and some studies have questioned its value in triggering further management. Narasimhaswamy et al.4 found that implementation of standards to improve pain screening increased the rate of assessments, but did not affect treatment prescriptions or levels of pain. Similarly, Mularski et al.3 found that treatment was not escalated for 52% of patient reporting NRS scores of 4 or more, which is the threshold identifying patients with moderate to severe pain10.
A recent study found that the most common reason for not modifying treatment plans in response to high NRS pain scores was patient refusal to escalate care5. While the failure of the pain intensity NRS to affect outcomes is undoubtedly related to numerous factors, including limitations in physician training, patient-physician communication, and lack of effective therapies, this study suggests that NRS scores may not reliably lead to changes in management because they do not adequately reflect the patients’ experience of pain.
For physicians, there is a direct association between greater pain intensity and/or functional status and preference for escalating treatment. In contrast, studies focusing on the patient perspective have demonstrated that the relationship between pain and/or functioning and treatment preference is highly variable. O’Brien et al.11 found that willingness of rheumatoid arthritis patients to accept a risky treatment was associated with poor self-rated health status. However, other studies have failed to find a relationship between pain intensity and willingness to accept potentially risky treatment12.
While pain intensity may indeed contribute to patients’ treatment decisions, it represents only one aspect of how patients experience their illness and evaluate their treatment options. Illness perceptions refer to the organized cognitive representations and related beliefs that patients have about their illness. Studies have found that these beliefs comprise specific factors, including cause, timeline, role of treatment, personal control, and consequences13. The latter factor is directly related to how patients appraise the severity of their illness and its influence on the quality of their lives. Extant research has demonstrated that illness perceptions affect important outcomes including adherence14, coping15, self-management and regulation16,17, and treatment response13,17. Because these factors directly measure patients’ experience of pain, we hypothesized that they would exhibit a more significant association with treatment preferences than either pain intensity or physical function.
METHODS
Subjects
We recruited patients enrolled in a VA Medical Center. We generated a list of patients having had a visit with a primary care provider within the past 12 months. We subsequently performed a limited chart review of all charts (in batches of 50) to identify patients with non-cancer, musculoskeletal pain in the same location on most days of the month over the past 3 or more months and to exclude those with a diagnosis of cancer (other than basal cell carcinoma), active substance-use disorder, mental illness with psychotic features, or dementia. Letters were sent to those meeting these initial eligibility criteria. The letter notified the potential participants that they would be telephoned by a research assistant and offered them the opportunity to refuse this contact by calling an answering machine and leaving a message. The research assistant telephoned all patients who did not “opt out” in order to describe the study, confirm additional eligibility criteria (living independently or in assisted living facilities and speaking English as a primary language) and schedule interviews. Full written consent was obtained at the beginning of the in-person study interview. Patients were given $25.00 for participating in the study. The study protocol was approved by the Human Subjects Committee at our institution.
Measures
Each subject participated in a single face-to-face interview administered by a research assistant. Illness perceptions were measured using 18 items, coded on 4- or 5-point scales, drawn from three sources: the Revised Illness Perception Questionnaire18, a questionnaire developed for a concurrent longitudinal study being conducted by the principal developer of illness perception theory (HL), and two additional items to assess patients’ outlook towards the future: How satisfied are you with where your life is heading? How hopeful are you that you will be able to live a good life? Items (listed in Appendix A available online) were selected based on their relevance to chronic pain patients and their potential relationship to patient decision making.
Because of the item selection procedure, we followed Turk and Salovey’s recommendation to factor analyze the illness perception questionnaire and use factor loadings to guide the interpretation19. Principal axis factoring with an orthogonal (Varimax) rotation was used because it analyzes only shared variance, whereas principal components factoring assumes that all of the variance is common20. Five factors were extracted that had eigenvalues greater than 1. The questionnaire items, median scores and ranges, rotated factor loadings, and factor scores are contained in Appendix A (available online).
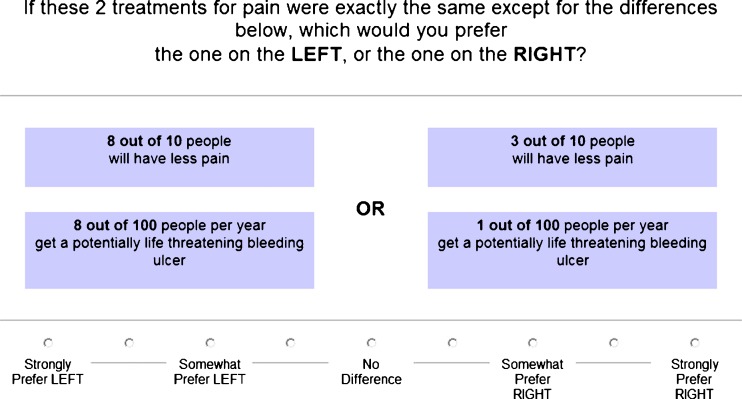
Treatment preference, the dependent variable in this study, was measured using Adaptive Conjoint Analysis (ACA) (Sawtooth Software Inc. ®, SSI Web V 6.0). Conjoint analysis is a well-established method of quantifying preferences for competing options21–23. ACA derives preferences by examining trade-offs between specific medication characteristics through a series of rating exercises21,24. It assumes that each treatment option can be broken down into specific characteristics and that each characteristic is defined by a number of levels. Levels refer to the range of plausible estimates for each characteristic. For example, the levels for the characteristic “risk of stomach upset” might be 0%, 10%, and 30%, depending on the specific medications being compared. ACA also assumes that respondents have unique values or utilities for each attribute level. In this context “utility” is a number that represents the value a respondent associates with a particular characteristic, with higher utilities indicating greater value.
The specific risks and benefits included in the ACA survey are described in Appendix B (available online). The characteristics were chosen based on outcomes commonly reported with analgesics (e.g., anti-inflammatory drugs and level III narcotics). The probabilities reflect the range of possible estimates for each characteristic. Subjects first rated the relative importance of each characteristic and subsequently rated ten paired comparisons (see, e.g., in Fig. 1). The software program applies constraints to ensure that the overall design of the questionnaire is nearly orthogonal. ACA uses the information obtained from each paired comparison to update the utility estimates and to select the next pair of options. Additional details regarding this methodology have been previously published21,24,25.
Figure 1.
Example of ACA paired-comparison task.
We measured pain intensity over the previous week using an 11-point NRS. Physical function was assessed using the physical function score of the SF-12 (a well-validated generic health-related quality of life survey)26. We also measured co-morbidities using the Charlson Co-morbidity Index, a widely used scoring system used to predict 10-year mortality based on a prespecified list of comorbid conditions27. Perceived efficacy of current pain medications was measured by asking subjects to rate how well each of their medications was working on a 3-point scale (very well, somewhat well, and not well at all). Numeracy was assessed using the Subjective Numeracy Scale, an 8-item survey of perceived ability to perform mathematical tasks and preference for the use of numerical versus narrative information28,29. Except for the Charlson Co-morbidity Index, which was obtained by chart review, all data were collected during the study interview.
Analyses
We used Sawtooth’s Software (Sawtooth Software Inc ®) Randomized First Choice simulation model to calculate participants' strength of preference for a highly effective/high-risk treatment for pain versus a mildly effective/no risk treatment for pain30. The Randomized First Choice model calculates shares of preferences where the scores of all options sum to 100. Options are defined by assigning one level per attribute for each option. In this study, preferences were estimated for a high-risk/highly effective treatment (having the maximum possible benefit and risk) versus a mildly effective/no risk treatment (smallest possible improvement with no added risk). Subjects with scores of 50 or greater for the highly effective/high-risk treatment were classified as preferring that treatment.
The factor analysis algorithm produced factor scores (one score per factor for each participant). Factor scores were examined against the dependent variable (preference for a highly effective/high-risk treatment). Given the high expected correlations between pain intensity, function and pain impact, separate tests were conducted to examine their association with the dependent variable. These factors were examined further by an adjusted model that included relevant covariates: age, co-morbidities, perceived efficacy of pain medications (summed across current pain treatments), and numeracy. Testing used the generalized linear model (GLZ) algorithm from SPSS version 1831,32. With a binomial dependent variable and a logit link function, GLZ is equivalent to binomial logistic regression analysis, with Wald chi square as the summary statistic31,32.
RESULTS
Two hundred forty-nine (67%) of invited subjects agreed to participate. Seventy-five percent of participants were male and 71% were Caucasian. Mean age (SD) was 53.5 (±19.5) and ranged from 22 to 90. Twenty-eight percent were under age 35, and 32% were age 70 and older. One hundred eighty-three (73.5%) subjects preferred a highly effective/high-risk treatment for pain versus a mildly effective/no risk treatment for pain. Participant characteristics are further described in Table 1. Two participants were excluded from the analysis because of incomplete data.
Table 1.
Participant characteristics
| Characteristic | Number (%) |
|---|---|
| Total = 249 | |
| Age [mean (SD)] | 53.5 (19.5) |
| Men | 187 (75.1) |
| Caucasian | 177 (71.1) |
| African American | 29 (11.6) |
| Latino | 29 (11.6) |
| Married (living with spouse or partner) | 122 (49) |
| Employed part or full time | 87 (34.9) |
| Current acetaminophen use | 48 (22) |
| Current nonsteroidal-anti-inflammatory use | 92 (42) |
| Current narcotic use | 110 (50) |
| Current use of exercise/physical therapy | 78 (36) |
| Charlson Comorbidity Index [mean (SD)] | 1.6 (1.5) |
| Pain intensity [mean (SD)] | 6.5 (2.1) |
| Duration of pain, years [mean (SD)] | 14.8 (14.4) |
| Physical function [mean (SD)] | 31.8 (9.3) |
| Numeracy [mean (SD)] | 4.2 (1.2) |
Factor analysis generated five factors with eigenvalues greater than 1 that accounted for 67.1% of the variance; 37.9% of the variance was accounted for by a single factor that we labeled “pain impact.” The other four factors were labeled personal control and emotion, treatment control, timeline, and vigilance (Table 2).
Table 2.
Percent variance and eigenvalue associated with each factor
| Factor label | Variance (%) | Eigenvalue |
|---|---|---|
| Impact | 37.95 | 6.83 |
| Personal control and emotion | 10.17 | 1.83 |
| Treatment control | 6.92 | 1.25 |
| Personal timeline | 6.33 | 1.14 |
| Vigilance | 5.76 | 1.04 |
| Total variance | 67.13 |
Pain impact was the only factor that was significantly associated with the preference for a riskier/more effective treatment: χ2 = 5.42, df = 1, p = 0.02, odds ratio (95% CI) = 1.43 (1.06–1.92) (Table 3). Neither NRS nor functioning predicted patient preference: NRS: χ2 = 2.31, df = 1, p = 0.13, odds ratio (95% CI) = 1.11 (0.97 = 1.27), and physical functioning, χ2 = 2.81, df = 1, p = 0.09, odds ratio (95% CI) = 0.98 (0.95–1.0). For comparison purposes, Table 4 contains adjusted GLZ models for the NRS and physical function variables. As the table shows, pain impact is significantly associated with preference for a riskier/more effective treatment after adjusting for covariates.
Table 3.
Associations between illness perception factors and treatment preference
| Factor | Wald chi square | df | P value | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Impact | Intercept | 49.49 | 1 | <0.001 | 2.81 | 2.10–3.74 |
| Factor | 5.42 | 1 | 0.02 | 1.43 | 1.06–1.92 | |
| Personal control and emotion | Intercept | 49.22 | 1 | <0.001 | 2.74 | 2.07–3.64 |
| Factor | 0.03 | 1 | 0.87 | 0.97 | 0.71–1.33 | |
| Treatment control | Intercept | 49.23 | 1 | <0.001 | 2.74 | 2.07–3.64 |
| Factor | 0.06 | 1 | 0.81 | 0.10 | 0.69–1.33 | |
| Personal timeline | Intercept | 49.39 | 1 | <0.001 | 2.79 | 2.10–3.72 |
| Factor | 3.39 | 1 | 0.07 | 0.72 | 0.51–1.02 | |
| Vigilance | Intercept | 49.23 | 1 | <0.001 | 2.74 | 2.07–3.64 |
| Factor | 0.14 | 1 | 0.70 | 1.07 | 0.73–1.57 |
Table 4.
Associations among pain impact, function, and pain intensity and treatment preference (adjusted analyses)
| Covariate | Independent variable* | Wald chi square | df | P value | Odds ratio | 95% CI |
|---|---|---|---|---|---|---|
| Intercept | 1.73 | 1 | 0.19 | 3.38 | 0.55–20.71 | |
| Age | 1.91 | 1 | 0.17 | 0.99 | 0.97–1.00 | |
| Comorbidity | 1.05 | 1 | 0.31 | 1.14 | 0.89–1.47 | |
| Perceived treatment efficacy | 0.01 | 1 | 0.94 | 1.02 | 0.57–1.83 | |
| Numeracy | 0.26 | 1 | 0.61 | 1.06 | 0.84–1.345 | |
| Pain impact | 4.4 | 1 | 0.04 | 1.39 | 1.02–1.90 | |
| Function | 2.48 | 1 | 0.11 | 0.97 | 0.94–1.01 | |
| Pain intensity | 1.92 | 1 | 0.16 | 1.11 | 0.96–1.28 |
*Each independent variable is tested in a separate generalized linear model adjusting for the covariates listed
DISCUSSION
In this study we found that impact of pain, as measured by a set of items reflecting patients’ illness perceptions, is significantly associated with patients’ treatment preference, whereas pain intensity NRS and physical function are not. These results are in keeping with a recent study by Krebs et al.33 that found that pain NRS scores were only “modestly” associated with clinically significant pain, that is pain that interferes with functioning or that motivates a physician visit. Taken together, these studies may help explain why the NRS has not affected delivery of care or outcomes.
While one might expect that patients reporting more severe pain would have stronger preferences for therapy, studies examining the relationship between pain intensity (as well as other disease activity measures such as disability) and treatment preference have found conflicting results11,12,34–36. One plausible explanation for these inconsistent findings is that the relationship among treatment preferences, pain severity, and functioning are influenced by adaptation. In this context, adaptation refers to the gradually diminished impact of a disorder or discrete event on a patient’s quality of life over time. When a painful condition initially develops, it is experienced as a loss from a previous health state. Under these circumstances, one would expect intensity of pain to be positively correlated with preference for a higher risk and more effective therapy. However, over time people adapt to their symptoms and/or functional limitations and establish a new reference point. For example, a patient who has adapted to their health state might rate their pain intensity as “5,” but not perceive a need for additional treatment, whereas a patient with a new diagnosis and the same pain rating may be more likely to prefer a high risk-high gain option.
In this study, we sought to measure patient’s experience of pain using items developed based on illness perception theory under the assumption that these items would better reflect the current impact of pain on patients’ quality of life and therefore better predict preferences for treatment. Of the five illness perception factors identified, only impact of pain was related to treatment preference. These results support the need for further research to determine whether currently available instruments used to measure similar constructs (e.g., Brief Pain Inventory-Short Form) are more effective than the NRS in improving both processes of care and outcomes in patients with chronic pain.
There are several important limitations of this study. We used ACA, a robust preference measurement tool, to quantify preferences. An advantage of using ACA in this setting is that preferences are quantified based on trade-offs between specific risks and benefits, and therefore are not biased by physicians’ preferences, subjects’ recognition of specific drug names, or personal experience with a specific product. However, we cannot conclude that the preferences measured in this survey accurately predict patients’ behavior in clinical practice. Illness perception items were drawn from the Illness Perception Questionnaire, based on their potential association with patient decision making. It is possible that additional items may also have a role in predicting treatment preferences. In addition, participants may not be representative of other patient populations as participants were from a single VA medical center. Specifically, a large number of participants were unemployed, baseline pain levels were high, and the majority preferred the highly effective/high-risk treatment option. Further studies are needed to examine the relationship between the impact of pain and treatment preferences in other patient populations. Lastly, though all patients met the criteria for chronic pain, we do not have details on their specific causes of pain or related diagnoses.
Despite the limitations of this study, our findings add to existing research questioning the value of the NRS as a screening tool and suggest that measures capturing the impact of pain may be more informative than the NRS. Further research is now needed to determine whether routinely measuring impact of pain in clinical practice is effective at triggering appropriate management. Implementation of valid measures is critical if quality of care continues to be judged against the results of screening assessments.
Electronic Supplementary Material
(PDF 31 kb)
Acknowledgement
We would like to thank Marion Michalski for her dedication to the subjects of this study. To the best of our knowledge, no conflict of interest, financial or other, exists. This work was supported by VA Health Services and Research Department grant no. IIR 07-090-3.
Conflicts of Interests
None disclosed.
Footnotes
This grant was supported by VA Health Services and Research Department grant no. IIR07-090-3.
REFERENCES
- 1.Pain assessment: The fifth vital sign. In: http://www.rn.ca.gov/pdfs/regulations/npr-b-27.pdf (Accessed October 17, 2011).
- 2.Krebs E, Bair M, Carey T, Weinberger M. Documentation of pain care processes does not accurately reflect pain management delivered in primary care. J Gen Intern Med. 2010;25:194–9. doi: 10.1007/s11606-009-1194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006;21:607–12. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narasimhaswamy S, Vedi C, Xavier Y, C-h T, Shine D. Effect of implementing pain management standards. J Gen Intern Med. 2006;21:689–93. doi: 10.1111/j.1525-1497.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubkoff L, Lorenz K, Lanto A, et al. Does Screening for pain correspond to high quality care for veterans? J Gen Intern Med. 2010;25:900–5. doi: 10.1007/s11606-010-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conno F, Caraceni A, Gamba A, et al. Pain measurement in cancer patients: A comparison of six methods. Pain. 1994;57:161–6. doi: 10.1016/0304-3959(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 7.Jensen M, Chen C, Brugger A. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–14. doi: 10.1016/S1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 9.Lund I, Lundeberg T, Sandberg L, Budh C, Kowalski J, Svensson E. Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Med Res Methodol. 2005;5:31. doi: 10.1186/1471-2288-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. Br J Anaesth. 2011;107:619–26. doi: 10.1093/bja/aer195. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien BJ, Elswood J, Calin A. Willingness to accept risk in the treatment of rheumatic disease. J Epidemiol Comm Health. 1990;44:249–52. doi: 10.1136/jech.44.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraenkel L, Bogardus ST, Jr, Concato J, Wittink DR. Treatment options in knee osteoarthritis: The patient's perspective. Arch Intern Med. 2004;164:1299–304. doi: 10.1001/archinte.164.12.1299. [DOI] [PubMed] [Google Scholar]
- 13.Petrie KJ, Weinamn JA. Perceptions of health and illness: current research and applications. Amsterdam: Harwood Academic Publishers; 1997. [Google Scholar]
- 14.Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Educ Couns. 1987;10:117–38. doi: 10.1016/0738-3991(87)90093-0. [DOI] [Google Scholar]
- 15.Scharloo M, Kaptein AA, Weinman J, et al. Illness perceptions, coping and functioning in patients with rheumatoid arthritis, chronic obstructive pulmonary disease and psoriasis. J Psychosom Res. 1998;44:573–85. doi: 10.1016/S0022-3999(97)00254-7. [DOI] [PubMed] [Google Scholar]
- 16.Halm EA, Mora P, Leventhal H. No symptoms, no asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129:573–80. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- 17.Rabin C, Leventhal H, Goodin S. Conceptualization of disease timeline predicts posttreatment distress in breast cancer patients. Health Psychol. 2004;23:407–12. doi: 10.1037/0278-6133.23.4.407. [DOI] [PubMed] [Google Scholar]
- 18.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. doi: 10.1080/08870440290001494. [DOI] [Google Scholar]
- 19.Turk DC, Rudy TE, Salovey P. Implicit models of illness. J Behav Med. 1986;9:453–74. doi: 10.1007/BF00845133. [DOI] [PubMed] [Google Scholar]
- 20.Fabrigar L, Wegener D, MacCallum R, Strahan E. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4:272–99. doi: 10.1037/1082-989X.4.3.272. [DOI] [Google Scholar]
- 21.Fraenkel L, Bogardus ST, Wittink DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care. 2001;39:1203–16. doi: 10.1097/00005650-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bridges JFP, Onukwugha E, Johnson FR, Hauber AB. Patient preference methods - A patient-centered evaluation paradigm. ISPOR Connections. 2007;13:4–7. [Google Scholar]
- 23.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green PE, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Marketing. 1990;54:3–17. doi: 10.2307/1251756. [DOI] [Google Scholar]
- 25.http://www.sawtoothsoftware.com/download/techpap/acatech.pdf. (Accessed October 17, 2011).
- 26.Stewart AL, Sherbourne C, Hays RD, et al. Summary and discussion of MOS measures. In: Stewart AL, E. Ware JE, et al., editors. Measuring functioning and well-being: The Medical Outcomes Study approach. 1992. Durham, NC: Duke University Press; 1992. pp. 345–71. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: Development of the Subjective Numeracy Scale (SNS) Med Decis Making. 2007;27:672–80. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 29.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the subjective numeracy scale (SNS): Effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27:663–71. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 30.Orme B. Getting started with conjoint analysis: strategies for product design and pricing research. Second. Madison: Research Publishers LLC; 2010. Market simulators for conjoint analysis. [Google Scholar]
- 31.SPSS advanced 18 for Windows. Chicago: SPSS Corporation; 2010.
- 32.Lindsey JK, Jones B. Choosing among generalized liner models applied to medical data. Stat Med. 1998;17:59–68. doi: 10.1002/(SICI)1097-0258(19980115)17:1<59::AID-SIM733>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Krebs E, Carey T, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22:1453–8. doi: 10.1007/s11606-007-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraenkel L, Bogardus S, Concato J, Felson D. Unwillingness of rheumatoid arthritis patients to risk adverse effects. Rheumatology. 2002;41:253–61. doi: 10.1093/rheumatology/41.3.253. [DOI] [PubMed] [Google Scholar]
- 35.Ho M, Lavery B, Pullar T. The risk of treatment: a study of rheumatoid arthritis patients' attitudes. Rheumatology. 1998;37:459–60. doi: 10.1093/rheumatology/37.4.459. [DOI] [PubMed] [Google Scholar]
- 36.Suarez-Almazor ME, Conner-Spady B, Kendall CJ, Russell AS, Skeith K. Lack of congruence in the ratings of patients' health status by patients and their physicians. Med Decis Making. 2001;21:113–21. doi: 10.1177/0272989X0102100204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 31 kb)