Abstract
Liposomes, phospholipid vesicles with a bilayered membrane structure, have been widely used as pharmaceutical carriers for drugs and genes, in particular for treatment of cancer. To enhance the efficacy of the liposomal drugs, drug-loaded liposomes are targeted to the tumors by means of passive (enhanced permeability and retention mediated) targeting, based on the longevity of liposomes in blood and its accumulation in pathological sites with compromised vasculature, and active targeting, based on the attachment of specific ligands to the liposomal surface to bind certain antigens on the target cells. Antibody-targeted liposomes loaded with anticancer drugs demonstrate high potential for clinical applications. This review highlights evolution of liposomes for both passive and active targeting and challenges in development of targeted liposomal therapeutics specifically antibody-targeted liposomes.
Key words: active targeting, immunoliposomes, passive targeting, stimuli sensitive, targeted liposomes
INTRODUCTION: DRUG TARGETING AND THE EVOLUTION OF LIPOSOMES
The majority of the drugs currently used for cancer treatment are broadly cytotoxic molecules. Upon administration, these drugs are generally distributed within the whole body and may result in substantial toxicity to normal tissues, thus limiting their clinical application. Drug targeting using site-specific pharmaceutical nanocarriers has been extensively studied and can provide the following advantages: altered drug distribution dynamics, increased drug concentration in the required sites without negative effects on nontarget compartments, simplification of drug administration protocols, reduction in the quantity of drug required to achieve a therapeutic effect, and reduction in the cost of therapy (1).
The most common and well-investigated nanocarriers are liposomes, which are artificial phospholipid vesicles with sizes of approximately 50–1,000 nm that can be loaded with a variety of drugs (2). For drug delivery purposes, liposomes have several advantageous properties such as biocompatibility, biodegradability, low toxicity, a capacity to modify the pharmacokinetic profile of the loaded drug, all of which can help in the delivery of a drug preferentially to a desired target tissue. Although, liposomes have attracted extensive attention during the past 30 years as pharmaceutical carriers, still, the currently available marketed liposomal formulations are not capable of selective targeting of cancer cells at a molecular level (3).
The first generation of liposomes underwent rapid clearance by the reticuloendothelial system (RES). The progressive optimization lead to more stable and longer-circulating liposomes with an increased accumulation at desired target sites via the enhanced permeability and retention (EPR) effect (4, 5). The EPR effect involves the phenomenon of enhanced extravasation of macromolecules from tumor blood vessels, and their retention in tumor tissues, infarcts, and inflamed regions compared to normal tissues. The incorporation of polyethyleneglycol–lipid conjugates (e.g., methoxy polyethylene glycol (mPEG)–distearoylphosphatidylethanolamine (DSPE)) within the bilayer membrane results in prolonged blood circulation half-life of the liposomes and thus promotes liposome accumulation at sites with a leaky vasculature by the EPR effect (6). The next generation of liposomes offered direct molecular targeting by the attachment of site-specific ligands to the liposomal surface (7). Although this strategy increases intracellular drug levels in target areas following the receptor-mediated endocytosis, the endocytosed material is subjected to the acidic lysosomal compartment and hydrolysis by various enzymes, resulting in reduced biological activity. This problem is particularly critical for drugs that are sensitive to such degradation, for example, nucleic acid and peptidic drugs (8). For such molecules, methods enabling the release of the entrapped cargo into the cytosol are advantageous. Later development of liposomal formulations involved attempts to combine long-circulation properties and targetability in single liposomal formulation (9, 10). However, sometimes inability of the polyethylene glycol (PEG)ylated liposomes to readily release the drug and kill tumor cells upon target cell accumulation may be unfavorable for drug delivery purpose. To solve this problem, the chemistry has been developed to promote the detachment of PEG from the lipid anchor under conditions characteristic of therapeutic target (11, 12).
Most recently, the concept of “stimuli-sensitive” nanocarriers has developed within the field of drug delivery. Such a stimulus-sensitive nanocarrier bearing site-specific targeting ligands are expected to release their contents in targeted tissues or cell compartments and greatly increase the drug’s efficacy when exposed to a certain internal or external stimulus such as low pH (13), elevated temperature (14, 15), a magnetic field (16, 17), or altered redox potential (18). Going one step further, we introduced the concept of “smart multifunctional” nanocarriers bearing various functionalities (such as a protective PEG coat, targeting ligands and a cell-penetrating function). These nanocarriers are designed so that particular functions can be shielded to prevent their exposure until, under particular local stimulus conditions, they are de-shielded in an orchestrated fashion.
Here, we focus on the evolution of liposomes for both passive and active targeting and the challenges in development of targeted liposomal therapeutics, specifically antibody-targeted liposomes.
PASSIVE TARGETING OF LIPOSOMES AND THE EPR EFFECT
EPR Effect
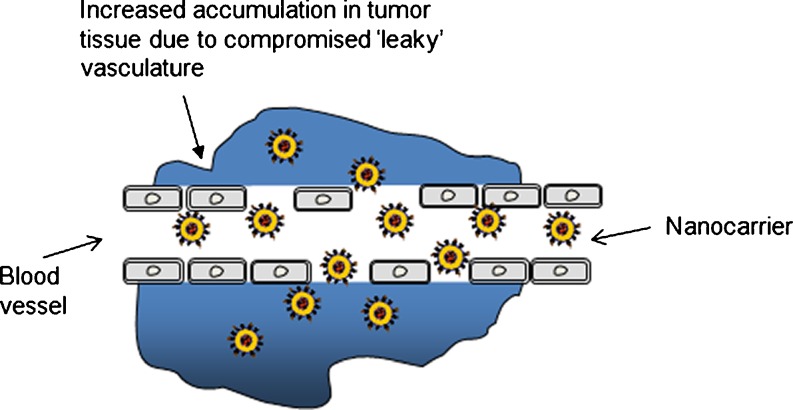
Some characteristic features of tumors that allow large molecules and nanocarriers such as liposomes to accumulate by the EPR effect form the basis for passive targeting (6). This has been proven in many tumors (19, 20) and in infarcted tissue areas (9). The increased permeability of the blood vessels in tumors is typical of rapid and defective angiogenesis. After being accumulated in the tumors, nontargeted liposomes become localized within tumor-residing macrophages in the interstitium surrounding the tumor cells (21, 22). The high interstitial pressure and large interstitial space compared with normal tissues result in the limited distribution of liposomes within the tumor interstitium (23). Further, the liposomes are retained within the tumor area due to the absence of functioning lymphatics (22). When loaded with drug, this result in a higher accumulation for liposomal drug than the free drug which also enters the tumor rapidly but is also cleared rapidly (24). Although, the threshold vesicle size of ∼400 nm was reported for extravasation into tumors (25), some studies have shown more effective extravasation with <200 nm particles (19, 26; Fig. 1).
Fig. 1.
Schematic illustration of passive targeting via the EPR effect
Longevity of Liposomes and the Need for Surface Modification of Liposomes
In the 1980s, it was shown that the liposomal delivery could improve the therapeutic index of encapsulated antitumor drugs, such as doxorubicin (27). The conventional doxorubicin liposomal formulations had an improved safety; however, it failed to demonstrate increased therapeutic activity against implanted tumors than the free drug (28). This lack of improved antitumor activity was ascribed to instability of liposomes in blood due to binding of plasma proteins and the release of up to 50% of their contents (29), rapid sequestration by macrophages in the liver and spleen (30), and liposomal lipid membrane digestion by intracellular enzymes. Later, during the 1970s and 1980s, considerable efforts were made in designing liposomes that can stably circulate in the blood for longer periods of time. The longevity allows maintenance of a higher level of drug in the blood for an extended period of time and can provide better accumulation in pathological sites via the EPR effect (6). Liposomes composed of high-phase transition lipids and cholesterol with small (<50 nm diameter) size were found to resist degradation in blood and to circulate for several hours in rodents (31) and in human cancer patients (32). Thus, it was understood that passive targeting depends on both, the size of the nanocarrier and the physicochemical properties that influence the circulation time. However, the use of saturated phospholipids and cholesterol does not fully overcome their binding to serum components.
The first approach was to prepare liposomes simulating the erythrocyte membrane. Thus, liposomal surface was modified with gangliosides and sialic acid derivatives such as monosialoganglioside (GM1; 33). The next approach was to “mask” the liposomal nanocarriers by surface modification with hydrophilic polymers with a well-solvated and flexible main chain, such as PEG (3, 34–37). PEG can be incorporated on the liposome surface by physical adsorption onto liposome surface or by covalent attachment onto the surface of preformed liposomes. The most widely used method is to anchor the polymer in the liposomal membrane via a cross-linked lipid (i.e., PEG–DSPE; 38). The molecular weight and structure of PEG can be easily controlled.
The mechanism of increasing longevity of liposomes in the circulation by steric stabilization has been extensively studied (39–42). PEG or GM1 occupies the space immediately adjacent to the liposome surface and sterically hinders interactions of blood components with the liposomal surface and reduces the binding of plasma proteins (39, 42–45) thus reducing their interactions with opsonins and capture by the RES (46).
The ability of PEG to increase the circulation time of the liposomes depends on both the amount of grafted PEG and the length, or molecular weight of the polymer (38). The greatest improvements in blood residence time were reported with longer-chain PEGs. The SM/PC/CHOL/DSPE-PEG liposomes with higher molecular weight PEG (i.e., PEG 1900 and PEG 5000) produced higher blood levels than for liposomes containing PEG-lipid with a shorter chain PEG (i.e., PEG 750 and PEG 120). The inclusion of PEG 2000 doubled the amount of lipid remaining in the plasma compared to formulations containing PEG 350–750 (38). Various long-circulating liposomal formulations have been developed containing anticancer drugs, such as doxorubicin, arabinofuranosylcytosine, adriamycin, cisplatin, and vincristin (47–51). Doxil® (PEGylated liposomal doxorubicin) has an increased circulation time compared to free doxorubicin and is up to six times more effective than free doxorubicin (52, 53). Additional attempts have been made to prepare long-circulating liposomes using poly[N-(2-hydroxypropyl)methacrylamide] (54), poly-N-vinylpyrrolidones (55), l-amino acid-based biodegradable polymer–lipid conjugates (56) and polyvinyl alcohol (57). However, PEGylated liposomes can still induce activation of complement systems and thus are not completely biologically inert (58).
However, sometimes, PEGylated liposomes after accumulation in the target area, such as tumor, are unable to easily release the drug and kill tumor cells. Similarly, after cellular delivery via endocytic pathway, the presence of the PEG coat on its surface can further prevent endosomal escape and delivery of the contents into the cytoplasm (59–61). Thus, to solve this problem, the chemistry was developed to promote the detachment of PEG from the lipid anchor under particular locally existing conditions (11, 12, 62). For example, linkages that would degrade only in the acidic environment characteristic of the endocytic vesicles or acidic tumor mass are based on the diortho esters (63, 64), hydrazones (65), vinyl ethers (62, 66), cysteine-cleavable lipopolymers (67). These linkages are stable at pH around 7.5 but are relatively fast hydrolysed at pH values of 6 and below. Upon the detachment of PEG coating, membrane destabilization should occur, delivering the liposomal contents to target.
Choice of Drug and Lipid Composition
Simply long circulation is of no importance if the drug is rapidly released from the formulation before reaching the tumor site. Thus, the choice of drug and lipid composition is very important to exploit the benefit of the EPR effect. Liposomes can be loaded with a variety drugs depending on their hydrophobic properties (68). Highly hydrophilic drugs may suffer from limited bioavailability at the tumor site due to their extremely low membrane permeability and thus eventually low drug release at the tumor site. Highly hydrophobic drugs tend to associate mainly with the liposomal membrane and show faster redistribution of the drug to plasma components and thus lower entrapment stability. Until now, amphipathic drugs (anthracyclines and Vinca alkaloids) appear to be the most suitable for liposomal carriers due to possibility to tune the drug-release rates to maintain the stability of the formulation in the plasma, and to promote the drug release at the tumor site.
The choice of lipid composition is also crucial for maintaining stability of liposomes while in the circulation. The correct choice of lipids can reduce the binding of serum proteins (69) or stabilize the drug formulation to reduce the rate of drug leakage. The presence of cholesterol in liposomes is responsible for maintenance of membrane bilayer stability and long circulation times in vivo (70, 71). For drug-loaded liposomes, cholesterol is necessary for maintenance of the drug in the liposomal interior. Liposomes composed of high-phase transition lipids formed more stable formulations, with better retention of entrapped drug and showed an apparent increase in drug circulation lifetimes. Liposome-coated polymers such as PEG have been shown to be less dependent with respect to clearance on size, membrane fluidity, and surface charge density (72). The liposomes of similar composition have shown more rapid RES uptake with increase in size (73). It was shown that in the case of DSPC/Chol (3:2) liposomes extruded through 400-nm filters the clearance was 7.5 times as fast as liposomes extruded through 200-nm filters, which in turn were cleared five times as fast as small unilamellar vesicles (74, 75). The addition of PEG–DSPE into the liposome composition resulted in clearance rates that were relatively insensitive to size in the range of 80–250 nm (37, 75). The effect of surface charge on liposome clearance was shown using eggPC/cholesterol liposomes with anionic lipids added in a 1:10:5 ratio (anionic lipid/eggPC/cholesterol) (76). It was found that liposomes containing phosphatidylglycerol (PG), phosphatidic acid (PA), and phosphatidylserine (PS; PS > PA > PG) were cleared more rapidly than neutral liposomes. Addition of ganglioside GM1 or phosphatidylinositol resulted in longer circulation. In addition, liposomes were also prepared using PEG-PE (36, 37). It was found that sterically stabilized liposomes with hidden charge were cleared more slowly. Liposomes without PEG–PE were cleared more rapidly than neutral liposomes of similar composition. With respect to liposome composition, it was shown that liposomes containing unsaturated lipids, such as eggPC, are cleared more rapidly than those containing high-phase transition phospholipids (DSPC/cholesterol). However, upon inclusion of PEG-DSPE, liposomes with either some charge or low-phase transition lipids were found in plasma after 24 h similar to those with neutral high-phase transition lipids. Thus, steric stabilization makes the rate of clearance relatively independent of the lipid composition for empty liposomes (37, 39).
Limitations of Passive Targeting
Although passive targeting has been the most preferred approach for clinical therapy, it suffers from several limitations. The porosity and pore size of tumor vessels varies with the type and status of tumors (19, 77). Thus, a passive targeting effect may not be achievable in all tumors. Some drugs cannot diffuse efficiently throughout the tumor and homogeneous targeting of tumor cells within a tumor is not always feasible. In most solid tumors, the elevated interstitial fluid pressure (78) can also inhibit the homogeneous distribution of nanocarriers within the tumor tissues (79). This may induce multiple-drug resistance (80).
ACTIVE TARGETING OF LIPOSOMES
Ultimately, “active targeting” via modification of liposomal surface with a targeting ligand is envisioned, and when optimized can result in increased accumulation at the target site or intracellular delivery to target cells. Certain ligands, upon binding, can release the liposomal contents intracellularly by induction of receptor-mediated endocytosis (72). This effect can reduce the diffusion of the drug from the tumor, thus increasing overall efficacy. In certain cases, liposomes targeted to internalizing receptors may be able to at least partially overcome drug resistance (53).
Selection of a Target Antigen
The targeted antigen is usually carefully selected based on its selective or overexpression on the tumor tissue or on the angiogenic blood vessels supporting the tumor. A number of targeting ligands have been studied for development of targeted liposomal formulations. These include proteins (antibodies or antibody fragments), nucleic acids (aptamers), and other receptor ligands (peptides, carbohydrates, and vitamins). There are several considerations for selecting target antigen such as relative degree of overexpression or selective expression on the target (81, 82), the ability to internalize the ligand-targeted formulation (83), and the degree of shedding of the target antigen (84).
For example, high levels of antigen expression on nontarget cells will result in nonspecific toxicity. Sometimes, the relative degree of overexpression also plays an important role. In the case of HER2-targeted immunoliposomes, the receptor-mediated endocytosis and toxicity was observed with SKBR-3 or BT-474 breast cancer cells, which have more than million HER2/neu growth factor receptors/cell density than for MCF-7 cells with ~10,000 receptors/cell (81). Thus, this receptor density threshold also protects healthy tissues which show low level of HER2/neu receptors expression. Similar receptor density-dependent targeting effect was also observed for CD19-targeted liposomes (85).
For the liposome-encapsulated drugs, liposome internalization into target cells compared to mere surface cell binding is important. It was shown that in the case of HER2-targeted doxorubicin-loaded liposomes, significant antitumor efficacy was observed when internalizing ligand was used (86) as opposed to targeting to HER2/neu that did not result in internalization when compared to nontargeted liposomes (87). The degree of shedding of the target antigen is also as important factor. Many cancer cells are known to shed their membrane antigens in conditions of high tumor load. Thus, in conditions with high degree of shedding, the liposomes could be cleared faster due to the binding with the solubilized antigen (84).
Development of Targeted Liposomes: Derivatization of PEGylated Liposomes
The particular characteristics of long-circulating liposomes mentioned earlier also apply to the development of targeted liposomal formulations. These include the choice of lipid components, surface-modification for long circulation, stability while in the circulation, and efficient drug delivery at the target site. Ligand-targeted liposomal formulations must stably encapsulate the drug till it reaches the desired target (such as tumor) in order to exploit the benefits provided by increased tumor accumulation or tumor cell internalization. Premature drug release will lead to an apparent increase in the rate of clearance of the liposomal drug from the circulation.
Initially, immunoglobulins of the IgG class and their fragments were attached by covalent binding to the liposome surface or by hydrophobic inclusion into the liposomal membrane (88). However, the majority of resultant immunoliposomes are accumulated in the liver. It was later shown that by combining long-circulating properties with targetability, certain drawbacks of immunoliposomes could be overcome (7, 9, 10). Conjugation of antibody fragments that lacked the Fc portion to the free PEG termini in liposomes could result in liposomal circulation times identical to PEGylated non-immunoliposomes (89).
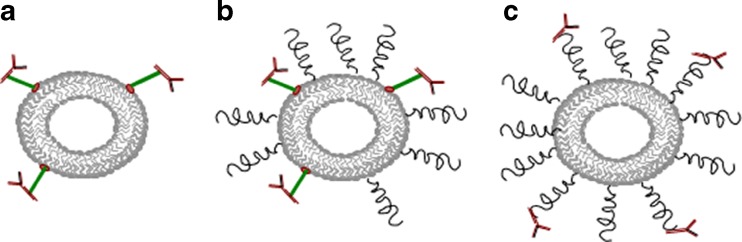
Earlier, targeting ligands were attached to liposomes in three different ways: In the first case, ligands are conjugated directly on the phospholipid head groups of non-PEGylated liposomes (type A liposomes); in the second case, ligands are conjugated directly on the phospholipid head groups of PEGylated liposomes (type B liposomes); and in the third case, ligands are conjugated on the free terminus of PEGylated chains (type C liposomes, Fig. 2; 90). Type A liposomes bound specifically to target cells but exhibited rapid blood clearance. Type B liposomes circulated longer but exhibited reduced targeting due to steric hindrance of the targeting ligand by the neighboring polymer chains. Type C liposomes were engineered to have minimal shielding of the ligand by the neighboring grafted PEG chains. However, in vivo, their circulation time was inversely proportional to their conjugated ligand-grafting density. For folate-targeted liposomes, it was shown that a PEG linker sufficient to distance the low molecular weight folate ligand from the liposome surface allowed receptor binding (91). Similarly for antiHER2 antibody fragments (Fab′) targeted liposomes, it was shown that Fab′ conjugated to the distal end of PEG demonstrated better binding and internalization than when conjugated directly to the membrane surface (81). Similar results were obtained by others (92, 93). Thus, to minimize the steric hindrances for ligand binding to the target, it is advantageous to attach the targeting ligand via a PEG spacer arm, so that the ligand is extended beyond the PEG coating. Various targeting moieties such as monoclonal antibodies (mAb) or fragments, peptides, growth factors, glycoproteins, carbohydrates, and receptor ligands have been attached to the activated water-exposed ends of liposome-grafted polymeric chains (94–96).
Fig. 2.
Schematics of antibody attachment to liposomes. Antibodies conjugated directly on the phospholipid head groups of non-PEGylated liposomes (a); antibodies conjugated directly on the phospholipid headgroups of PEGylated liposomes (b); and antibodies conjugated on the free terminus of PEGylated chains (c)
A lipophilic moiety inserted within the lipid portion of the bilayer is used typically to anchor the ligand. The linkage between the anchor and the ligand should be stable, non-immunogenic, and should not affect the reactivity of the ligand or the stability of the liposomal drug.
The PEG–lipid conjugates used for surface coating of liposomes are derived from mPEG, which has nonreactive methoxy terminal groups. To attach a targeting moiety to long-circulating liposome, various attempts have been made to functionalize PEG tips in PEG–lipid conjugates. Several types of lipopolymers of the general formula X-PEG-PE, where X represents a reactive functional group-containing moiety are now commercially available that can be used to for the conjugation (Table I).
Table I.
Examples of End-Group Functionalized Lipid–Polymer Conjugates of the General Formula X-PEG-PE Used for Preparation of Ligand-Modified Liposomes
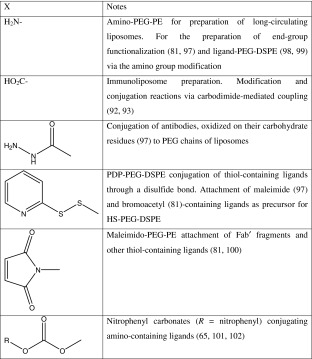
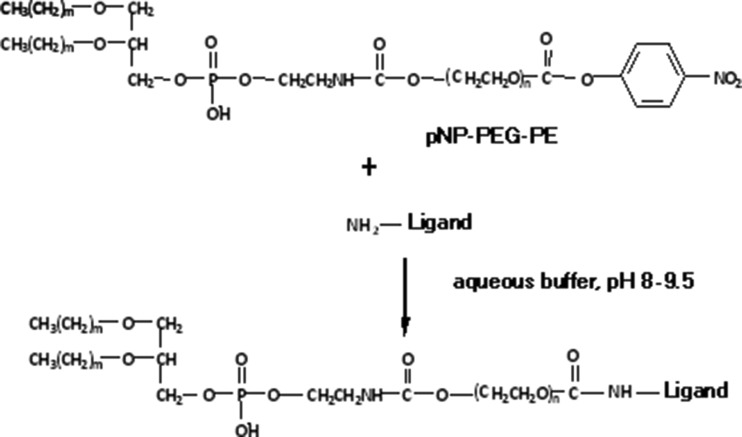
The heterobifunctional PEG derivatives containing hydroxyl and carboxyl or amino groups were used to synthesize most of the end-group functionalized PEG–lipids. Usually, the hydroxyl end-group of PEG was derivatized to form a urethane attachment with the hydrophobic lipid anchor, PE, while the amino or carboxyl groups were utilized for further functionalization. More recently, a simple one-step procedure was introduced to attach a large variety of amino group-containing ligands to the distal end of the liposome-attached polymeric chains, via liposome-incorporated amphiphilic PEG derivative, p-nitrophenylcarbonyl-PEG-PE (pNP-PEG-PE) (97, 98). The water-exposed pNP group is available for binding with any amino group-containing compound forming a stable and nontoxic urethane (carbamate) bond (Fig. 3).
Fig. 3.
Schematic of amino group-containing ligand, such as antibody attachment using pNP-PEG-PE
Other methods used for the coupling of ligands to the distal tips of PEG chains include PEG activation with a hydrazine group (in case of antibody attachment, hydrazine reacts with the oxidized carbohydrate groups in the oligosaccharide moiety of the antibody); pyridyldithiopropionate (PDP) group (after conversion of the PDP into the thiol, it reacts with maleimide groups of the premodified ligand); or maleimide group (reacts with thiol groups in prethiolated ligand). The ligand (antibody) binding to PEGylated liposomes has also been performed via the PEG terminus modified with cyanuric chloride. See reviews on various coupling techniques in (99).
Sometimes, the conjugation with a lipid anchor can result in decreased cell binding due to loss of activity due to reactivity with critical binding site residues or due to improper orientation of the ligand after conjugation. Use of naturally occurring reactive residues located distal to the antigen binding site such as the carbohydrate residue of IgG molecules (100) or reduced cysteines located in the hinge region of antibodies (81) will allow for correct orientation for targeting with minimal interference with the receptor binding site. Also, the coupling conditions must be optimized to prevent denaturation of the protein.
Three general protocols have been used to prepare ligand-bearing PEGylated liposomes. In the first protocol, the end-group functionalized PEG–lipids (Table I) are incorporated into the liposomes and then conjugated to specific ligands (generally used for macromolecular ligands, such as immunoglobulins). However, it is possible that some of the reactive end-groups on the surface can lead to crosslinking through multiple attachments to a single protein molecule. On the other hand, some unreacted reactive groups on the inner surface of liposomes can undergo some side reactions with drug molecules or other lipid components. Thus, sometimes the quenching of the unreacted end group is required (81).
In the second protocol, the ligand–PEG–PE conjugate is synthesized first and then mixed with other liposome-forming components. However, with this approach, it is possible that few conjugates are not available for interaction with target due to the inward facing towards the aqueous compartment of liposomes (81).
The third protocol, which is named the “postinsertion technique”, (101) involves co-incubation of ligand–PEG–PE conjugates with preformed plain or PEGylated liposomes (81). The advantage of this approach is that all the ligand moieties are positioned on the outer surface of the liposomes. High-insertion efficiencies (>80%) are obtained when co-incubation conditions (temperature and duration) are optimized. If insertion is performed at elevated temperatures (55–60°C) to incorporate high-phase transition lipids, protein ligand denaturation can take place. Similar insertion efficiencies can be attained if incubation is performed at 37°C overnight. In particular, this “postinsertion technique” was used to prepare immuno-Doxil by modifying it with p-nitrophenylcarbonyl-PEG-PE (pNP)-PEG-PE-modified anticancer 2C5 monoclonal antibody (97).
Antibody-Targeted Liposomes as an Example
Antibodies (mainly of the IgG class) are the most extensively studied ligands for experimental targeted chemotherapy of various tumors with drug-loaded liposomes (1, 102, 103). Antibodies can be attached to surface of PEGylated liposomes using chemistries and techniques mentioned earlier. The most widely used approach is the reaction of the thiol groups on an antibody with maleimide-containing phospholipid molecules (104). Various high- and low-molecular weight compounds have been attached to liposomes by using pyridyldithiopropionyl-PE or maleimide reagents (99). Free thiol groups located on immunoglobulin Fab fragments are also used for attachment, since these SH groups are positioned far from the antigen-binding sites. It is also important to note that in the case of PEGylated long-circulating liposomes, the surface should not be overmodified with antibodies so as to compromise the liposome longevity too much (105).
Initially, with active targeting, it was assumed that increased amounts of drug-loaded liposomes would enter in the desired target site compared to nontargeted liposomes. Recent studies with targeted liposomes have provided mixed results. Some studies suggested the role of antibody in enhancing accumulation of liposomes in tumors (106) while others contradicted these results (89, 107–109). Also, antibody-targeted liposomes have not always resulted in increased therapeutic efficacy of the drug (110–112). Possible reasons include increased clearance of targeted liposomes, lack of internalization, increased drug release, poor stability while in circulation, and reduced cancer cell penetration due to the binding site barrier effect or receptor downregulation. Thus, both a careful selection of the target receptor and proper engineering of the targeted liposomal construct are important.
We have shown that a nucleosome-specific monoclonal antibody (mAb 2C5) capable of recognition of various tumor cells via the tumor cell’s surface-bound nucleosomes significantly improved Doxil® targeting to tumor cells and increased its cytotoxicity (113) both in vitro and in vivo in different test systems including an intracranial human brain U-87 tumor xenograft in nude mice (114). Whole body gamma-scintigraphic imaging of these immunoliposomes showed two to three times more accumulation in the tumor than nonspecific IgG-conjugated or plain liposomes in a murine carcinoma model (106). Effective targeting of HER2-overexpressing tumors with anti-HER2 long-circulating liposomes has been observed (115, 116). PEGylated liposomes modified with antibody CC52 against rat colon adenocarcinoma CC531 provided specific accumulation of liposomes in a metastatic model (117).
Elevated levels of epidermal growth factor receptor (EGFR) are found on several types of solid tumors and consequently, strategies for targeting EGFR have been designed. In order to avoid Fc-mediated uptake of immunoliposomes both Fab′ and recombinant formats (e.g., scFv)1 have been employed for the preparation of anti-EGFR immunoliposomes (89, 118). Selective binding and internalization into EGFR-overexpressing tumor cells as well as selective cytotoxicity of drug-loaded anti-EGFR immunoliposomes was demonstrated. Superiority of immunoliposomal delivery of various drugs, e.g., doxorubicin, epirubicin, and vinorelbine, over free drug, and nontargeted liposomal formulations was confirmed in xenograft tumor models (89).
However, a number of studies found that the targeting ligands did not improve the tumor accumulation of liposomes (89, 107–109, 119). Recently Kirpotin et al. (107) provided experimental evidence for the mechanisms underlying increased antitumor efficacy by anti-HER2 immunoliposomes and concluded that it does not involve enhanced accumulation of anti-HER2 immunoliposomes in tumor tissue due to antigen binding. Both targeted and nontargeted liposomes achieved similarly high levels (7–8% of injected dose/g) of tumor tissue accumulation in HER2-overexpressing breast cancer xenografts (BT-474). Using colloidal gold-labeled liposomes they showed the anti-HER2 immunoliposomes accumulated within tumor cells, whereas nontargeted liposomes were predominantly located in the extracellular matrix. The authors concluded that the rate-limiting step for their tumor localization is extravasation from the tumor vasculature and that mAb-antigen interactions do not necessarily facilitate this process. Alternatively, designed anti-HER2 immunoliposomes also failed to demonstrate increased tumor accumulation when compared with nontargeted sterically stabilized liposomes (87). There was no therapeutic gain with antibody-modified liposomes reported in these studies possibly because of insufficient immunoliposome internalization. Some studies have also suggested that cell surface binding by itself may serve to limit the distribution of liposomes within the tumor (110). It was speculated that this binding site barrier effect could be avoided if antibody fragments with reduced avidity for their cell surface targets are used allowing a deeper penetration of the carrier within the tumor. This effect could be attributed to both the reduced size and reduced avidity of the antibody fragments to its target (120).
In general, liposomes targeted to internalizing receptors have shown greater cancer cell cytotoxicity both in vitro and in (85) vivo (82, 121, 122). This may be due to degradation of liposome in lysosomes and release of drug (123). The degradation rate was dependent on type of lipid (i.e., the phase transition temperature of lipid) but could also vary depending on cell type (85, 124, 125). Internalization of liposomes can also increase the efficacy by reducing the diffusion of drug away from cancer cells (126).
With this in mind, one can imagine that the targeting ligand’s affinity and surface density on the nanoparticle will determine cellular uptake. Recently, Zhou et al. clearly demonstrated that high-density, low-affinity antibody fragments can provide uptake into cancer cells that is not increased by greater affinity and thus proved the importance of multivalency of the targeted nanoparticles (127).
In some other cases, however, liposome internalization seems not to be important. The PEGylated liposomes loaded with vincristine or doxorubicin and modified with antibodies against internalizing CD19 antigen or non-internalizing CD20 antigen demonstrated therapeutic effects which depend more on the type of drug used than on its ability to be internalized. Thus, in this case, the cytotoxicity of targeted liposomes depended on the rate of drug release from the liposomes (128, 129).
Hosokawa et al. demonstrated that nontargeted doxorubicin-loaded liposomes were toxic to various cancer cells to the extent reflecting cell sensitivity to the drug. In the case of antibody-targeted liposomes, the cytotoxicity was proportional to the surface density of the targeted antigen (130) and the critical antigen surface concentration was about 4 × 104 sites per single cell. Similar observations were made by others (85, 86).
One should expect certain changes in the pharmacokinetics and biodistribution of plain and long-circulating liposomes after their modification with antibodies. Although some early studies did not reveal big differences in biodistribution between antibody-free and antibody-modified liposomes (131), in general, antibody modification can increase the liposome clearenace because of increased uptake of the modified liposomes via Fc receptors of circulating, or liver macrophages or opsonization of the liposome-tagged antibody molecules (132, 133). Whole antibodies can also trigger complement-mediated cytotoxicity and antibody-dependent cellular cytotoxicity (96). The use of antibody Fab fragments can minimize these effects (134). It is worth noting that in case of antibody-modified PEGylated liposomes, even after slight decrease in the circulation time, these liposomes still show sufficiently long circulation for good target accumulation.
The addition of surface-attached antibody to a liposomal preparation will certainly result in the cost increase of the final product because of the high cost of antibodies and additional preparation steps.
Other Targeted Liposomes
Other targeting ligands have also shown promising results when attached to long-circulating liposomes. Transferrin (Tf) receptor (TfR) is over-expressed on the surface of many tumor cells and antibodies against TfR as well as Tf itself have been used both for targeting liposomes to tumors and within tumor cells (135–137). The utility of Tf-coupled PEG liposomes for the intracellular targeting of liposomes to tumor cells via receptor-mediated endocytosis is already proven (138). The importance of size of the Tf-stealth liposomes for tissue targeting has been demonstrated by Hatakeyama et al. They found that small size, less than 80 nm, is an important factor for the tissue targeting of Tf-stealth liposomes based on receptor-mediated endocytosis, especially in the liver and brain (136). The heart is able to take up both small and relatively large liposomes in a Tf-dependent manner. These results suggest that Tf can serve as a ligand for the active targeting of stealth liposomes in vivo and that regulation of size can be used to confer tissue selectivity of Tf-stealth liposomes.
Since folate receptor (FR) is frequently over-expressed in many tumor cells (139), folate-modified liposomes are another well-investigated nanocarrier. FR-targeted liposomes potentially enhanced tumor cell uptake and antitumor efficacy of encapsulated drugs (140, 141). Some examples of targeted PEGylated liposomes are given in Table II.
Table II.
Examples of PEGylated Liposomes Used for Active Targeting
| Targeting ligand, drug | Results | Reference |
|---|---|---|
| Nucleosome-specific mAb 2C5, doxorubicin | • Enhanced toxicity to various cancer cell lines in vitro and in vivo | (113, 145, 146) |
| • More accumulation in tumors with targeted liposomes than nontargeted liposomes in mice bearing Lewis lung carcinoma or murine breast carcinoma | ||
| Anti-HER2 mAb fragments, doxorubicin | • Enhanced antitumor effect over nontargeted liposomes in vivo | (107) |
| • Similar accumulation of targeted and nontargeted liposomes in tumors in a nude mouse model with HER2-overexpressing BT-474 cells | ||
| Folate, doxorubicin | • Significantly higher antitumor effect in mice bearing murine lung carcinoma M109 with the folate ligand in PEGylated and masked folate-linked liposomes than with non-PEGylated liposomes independent of circulation time after i.v. injection | (140) |
| EGFR, various drugs (doxorubicin, epirubicin, or vinorelbine) | • Higher antitumor effects than nontargeted liposomes in vivo. | (89) |
| • No difference in tumor accumulation in nude mice with MDA-MB-468 overexpressing EGFR | ||
| Thiolated Herceptin, paclitaxel | • Enhanced cellular uptake in vitro and enhanced antitumor effect in vivo against BT-474 | (147) |
| RGD peptide, doxorubicin | • Enhanced anti-tumor effect over non-targeted liposomes | (108, 109) |
| • Similar accumulation in tumors in nude mice | ||
| Transferrin, doxorubicin | • Enhanced intracellular uptake of entrapped doxorubicin by HepG2 cells | (137) |
| • Enhanced doxorubicin concentration in tumors and decreased doxorubicin concentration in heart and kidneys in tumor-bearing mice | ||
| RGD peptide, paclitaxel | • Enhanced uptake and cytotoxicity in vitro | (148) |
| • Increased antitumor activity in vivo in mice bearing SKOV-3 solid tumors | ||
| Anti-MT1-MMP antibody, doxorubicin | • Increased uptake by MT1-MMP-over-expressing HT1080 fibrosarcoma cells in vitro and more effective tumor growth inhibition in vivo | (149) |
“SMART” LIPOSOMES
The ideal smart nanocarrier should first specifically accumulate in the required organ or tissue, and then deliver its load into target cells. Specific target (tumor, infarct) accumulation could be achieved by the passive targeting via the EPR effect (4, 5) or by antibody-mediated active targeting (1), while the intracellular delivery could be mediated by certain internalizable ligands (folate, transferrin) or by cell-penetrating peptides (CPPs; 142).
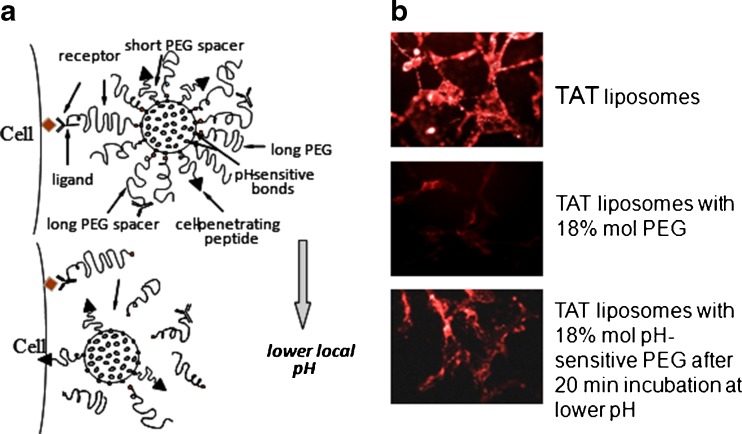
Keeping this in mind, it is necessary to build drug delivery system (DDS) carrying simultaneously on their surface various functional moieties. Moreover, these DDS should possess the ability to “switch on” certain functions under the action of a local stimuli characteristic of a targeted pathological zone such as an increased temperature or the lowered pH characteristic of an inflamed, ischemic, or neoplastic tissue. These “smart” DDS should be engineered in such a way that while in circulation the nonspecific cell-penetrating function is shielded by sterically protecting polymer or antibody and should be able to circulate for long time. Upon accumulation the target, the protecting polymer or antibody attached to the surface of the DDS via the stimulus-sensitive bond should detach under local pathological conditions and expose the previously hidden second function to allow the subsequent delivery of the carrier and its cargo inside cells (Fig. 4a). This type of shielding is especially important for CPP-bearing nanocarriers since it is well known that all CPPs are highly nonselective and could interact with nontarget organs.
Fig. 4.
Schematic structure of a multifunctional “smart” liposome with temporarily “hidden” function, for example CPP, and “shielding” polymeric coat with or without targeting antibody attached to it. Polymeric chains are attached to the carrier surface via low pH-degradable bonds. After the accumulation in the tumor due to PEG (longevity) and/or antibody (specific targeting), pH-dependent de-shielding of the temporarily hidden cell-penetrating function allows for carrier penetration inside tumor cells (a); interaction of “smart” TAT peptide-modified liposomes. Rhodamine-labeled TAT liposomes are effectively taken up by cells. The attachment of PEG-chains to the liposome surface (18% mol) sterically shields the TAT function and TAT-mediated liposome uptake is almost completely blocked. If, however, PEG is attached to the liposome surface via pH-sensitive bonds, a brief incubation at lowered pH results in the elimination of long PEG-chains from the liposome surface, de-shielding TAT function and TAT-mediated uptake of the liposomes by cells (b). Modified from (144)
We have recently prepared targeted long-circulating PEGylated liposomes possessing several functionalities (65, 143). The target recognition was provided by attachment of a monoclonal antibody (infarct-specific antimyosin antibody 2G4 or cancer-specific antinucleosome antibody 2C5) to their surface and intracellular penetration via TATp moieties attached to the liposomal surface via a shorter PEG spacer. The PEG–PE used for liposome surface modification was made degradable by inserting a pH-sensitive hydrazone bond between PEG and PE (PEG-Hz-PE). At normal physiological pH values, TATp on the surface of liposomes was “shielded” by the longer, protecting PEG-chains (pH-sensitivePEG2000-PE or PEG5000-PE) or by the long pNP-PEG-PE moieties used to attach antibodies to the nanocarrier (non-pH-degradable PEG3400-PE or PEG5000-PE). These liposomes demonstrated a high specific binding with antibody substrates at pH 7.5–8.0. However, upon brief incubation at lower pH values (pH 5.0–6.0), TATp function was exposed for subsequent internalization of liposomes due to the removal of protective PEG shell by acidic hydrolysis of PEG-Hz-PE (Fig. 4b).
CONCLUSIONS
Liposomes have certainly come a long way as pharmaceutical carriers of a choice for drug and gene delivery. In general, the properties of drug-loaded liposomes must be determined experimentally to ensure development of stable (i.e., the retention of drug in liposomes till accumulation at target site) liposomal systems and ability to circulate in blood for longer period of time. Current research focuses either on actively targeted liposomes or on use of stimuli-sensitive liposomes. Such a delivery system should clearly show benefit of fast and effective accumulation in target tumors, higher drug delivery in tumors than other drug delivery systems and ability to internalize by the target cells thus creating high intracellular drug concentration. In development of ligand-targeted liposomal therapeutics, the following considerations should be taken into account. The target antigen should be selectively expressed or overexpressed on the target tumor cells in sufficient quantity so as to ensure firm binding of liposomes with cancer cells. Overmodification of ligand (while attachment to liposomal surface) should be avoided and it should provide unhindered interaction with target cells. In the case of PEGylated long-circulating antibody-modified liposomes, the quantity of attached antibodies should not compromise the longevity too much. It is also desirable to use internalizable ligands to facilitate delivery of liposomal drug inside cells followed by release of drug to achieve the desired therapeutic effect for a reasonable period of time.
References
- 1.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11(Suppl 2):S81–91. doi: 10.1016/S0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 2.Lasic DD. Liposomes: from physics to applications. Amsterdam: Elsevier; 1993. [Google Scholar]
- 3.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–7. doi: 10.1016/0014-5793(90)81016-H. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84. doi: 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 5.Maeda H. Enhanced permeability and retention (EPR) effect: basis for drug targeting to tumors. In: Muzykantov V, Torchilin VP, editors. Biomedical aspects of drug targeting. Boston, MA: Kluwer; 2003. pp. 211–28. [Google Scholar]
- 6.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–5. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Blume G, Cevc G. Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta. 1993;1146(2):157–68. doi: 10.1016/0005-2736(93)90351-Y. [DOI] [PubMed] [Google Scholar]
- 8.Connor J, Huang L. pH-sensitive immunoliposomes as an efficient and target-specific carrier for antitumor drugs. Cancer Res. 1986;46(7):3431–5. [PubMed] [Google Scholar]
- 9.Torchilin VP, Klibanov AL, Huang L, O'Donnell S, Nossiff ND, Khaw BA. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. FASEB J. 1992;6(9):2716–9. doi: 10.1096/fasebj.6.9.1612296. [DOI] [PubMed] [Google Scholar]
- 10.Abra RM, Bankert RB, Chen F, Egilmez NK, Huang K, Saville R, et al. The next generation of liposome delivery systems: recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. J Liposome Res. 2002;12(1–2):1–3. doi: 10.1081/LPR-120004770. [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, Kirchmeier MJ, Moase EH, Zalipsky S, Allen TM. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515(2):144–58. doi: 10.1016/S0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- 12.Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using "Smart" pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007;15(7–8):538–45. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond DC, Zignani M, Leroux J. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39(5):409–60. doi: 10.1016/S0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 14.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int J Hyperthermia. 2006;22(3):205–13. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 15.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202(4374):1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 16.Sabate R, Barnadas-Rodriguez R, Callejas-Fernandez J, Hidalgo-Alvarez R, Estelrich J. Preparation and characterization of extruded magnetoliposomes. Int J Pharm. 2008;347(1–2):156–62. doi: 10.1016/j.ijpharm.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Fortin-Ripoche JP, Martina MS, Gazeau F, Menager C, Wilhelm C, Bacri JC, et al. Magnetic targeting of magnetoliposomes to solid tumors with MR imaging monitoring in mice: feasibility. Radiology. 2006;239(2):415–24. doi: 10.1148/radiol.2392042110. [DOI] [PubMed] [Google Scholar]
- 18.West KR, Otto S. Reversible covalent chemistry in drug delivery. Curr Drug Discov Technol. 2005;2(3):123–60. doi: 10.2174/1570163054866882. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 21.Huang SK, Mayhew E, Gilani S, Lasic DD, Martin FJ, Papahadjopoulos D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992;52(24):6774–81. [PubMed] [Google Scholar]
- 22.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–6. [PubMed] [Google Scholar]
- 23.Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81(8):570–6. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- 24.Gabizon A, Chemla M, Tzemach D, Horowitz AT, Goren D. Liposome longevity and stability in circulation: effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J Drug Target. 1996;3(5):391–8. doi: 10.3109/10611869608996830. [DOI] [PubMed] [Google Scholar]
- 25.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6. [PubMed] [Google Scholar]
- 26.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 27.Gabizon A, Dagan A, Goren D, Barenholz Y, Fuks Z. Liposomes as in vivo carriers of adriamycin: reduced cardiac uptake and preserved antitumor activity in mice. Cancer Res. 1982;42(11):4734–9. [PubMed] [Google Scholar]
- 28.Gabizon AA. Liposomal anthracyclines. Hematol Oncol Clin North Am. 1994;8(2):431–50. [PubMed] [Google Scholar]
- 29.Gabizon A, Chisin R, Amselem S, Druckmann S, Cohen R, Goren D, et al. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br J Cancer. 1991;64(6):1125–32. doi: 10.1038/bjc.1991.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Hu Q, Song YK. Liposome clearance from blood: different animal species have different mechanisms. Biochim Biophys Acta. 1995;1240(2):277–84. doi: 10.1016/0005-2736(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 31.Proffitt RT, Williams LE, Presant CA, Tin GW, Uliana JA, Gamble RC, et al. Tumor-imaging potential of liposomes loaded with In-111-NTA: biodistribution in mice. J Nucl Med. 1983;24(1):45–51. [PubMed] [Google Scholar]
- 32.Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, et al. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol. 1996;14(8):2353–64. doi: 10.1200/JCO.1996.14.8.2353. [DOI] [PubMed] [Google Scholar]
- 33.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA. 1988;85(18):6949–53. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev. 1995;16:141–55. doi: 10.1016/0169-409X(95)00022-Y. [DOI] [Google Scholar]
- 35.Allen TM, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987;223(1):42–6. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- 36.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88(24):11460–4. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113(2):171–99. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 38.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 39.Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991;1070(1):187–92. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- 40.Needham D, Hristova K, McIntosh TJ, Dewhirst MW, Lasic DD. Polymer grafted liposomes: physical basis for the "stealth" property. J Liposome Res. 1992;2:411–30. doi: 10.3109/08982109209010218. [DOI] [Google Scholar]
- 41.Lasic DD, Martin F. Stealth liposomes. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 42.Allen TM. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1994;13:285–309. doi: 10.1016/0169-409X(94)90016-7. [DOI] [Google Scholar]
- 43.Chonn A, Semple SC, Cullis PR. Separation of large unilamellar liposomes from blood components by a spin column procedure: towards identifying plasma proteins which mediate liposome clearance in vivo. Biochim Biophys Acta. 1991;1070(1):215–22. doi: 10.1016/0005-2736(91)90167-7. [DOI] [PubMed] [Google Scholar]
- 44.Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly(ethylene glycol)-coated vesicles. Biochim Biophys Acta. 1991;1062(1):77–82. doi: 10.1016/0005-2736(91)90337-8. [DOI] [PubMed] [Google Scholar]
- 45.Woodle MC. Surface-modified liposomes: assessment and characterization for increased stability and prolonged blood circulation. Chem Phys Lipids. 1993;64(1–3):249–62. doi: 10.1016/0009-3084(93)90069-F. [DOI] [PubMed] [Google Scholar]
- 46.Senior JH. Fate and behavior of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst. 1987;3(2):123–93. [PubMed] [Google Scholar]
- 47.Huang SK, Stauffer PR, Hong K, Guo JW, Phillips TL, Huang A, et al. Liposomes and hyperthermia in mice: increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994;54(8):2186–91. [PubMed] [Google Scholar]
- 48.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–92. [PubMed] [Google Scholar]
- 49.Boman NL, Masin D, Mayer LD, Cullis PR, Bally MB. Liposomal vincristine which exhibits increased drug retention and increased circulation longevity cures mice bearing P388 tumors. Cancer Res. 1994;54(11):2830–3. [PubMed] [Google Scholar]
- 50.Allen TM, Mehra T, Hansen C, Chin YC. Stealth liposomes: an improved sustained release system for 1-beta-d-arabinofuranosylcytosine. Cancer Res. 1992;52(9):2431–9. [PubMed] [Google Scholar]
- 51.Kim ES, Lu C, Khuri FR, Tonda M, Glisson BS, Liu D, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2001;34(3):427–32. doi: 10.1016/S0169-5002(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 52.Gabizon AA. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Adv Drug Deliv Rev. 1995;16:285–94. doi: 10.1016/0169-409X(95)00030-B. [DOI] [Google Scholar]
- 53.Gabizon AA. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19(4):424–36. doi: 10.1081/CNV-100103136. [DOI] [PubMed] [Google Scholar]
- 54.Whiteman KR, Subr V, Ulbrich K, Torchilin VP. Poly(Hpma)-coated liposomes demonstrate prolonged circulation in mice. J Liposome Res. 2001;11(2–3):153–64. doi: 10.1081/LPR-100108459. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP, Levchenko TS, Whiteman KR, Yaroslavov AA, Tsatsakis AM, Rizos AK, et al. Amphiphilic poly-N-vinylpyrrolidones: synthesis, properties and liposome surface modification. Biomaterials. 2001;22(22):3035–44. doi: 10.1016/S0142-9612(01)00050-3. [DOI] [PubMed] [Google Scholar]
- 56.Metselaar JM, Bruin P, de Boer LW, de Vringer T, Snel C, Oussoren C, et al. A novel family of l-amino acid-based biodegradable polymer-lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjug Chem. 2003;14(6):1156–64. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release. 2001;75(1–2):83–91. doi: 10.1016/S0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 58.Moein Moghimi S, Hamad I, Bunger R, Andresen TL, Jorgensen K, Hunter AC, et al. Activation of the human complement system by cholesterol-rich and PEGylated liposomes-modulation of cholesterol-rich liposome-mediated complement activation by elevated serum LDL and HDL levels. J Liposome Res. 2006;16(3):167–74. doi: 10.1080/08982100600848801. [DOI] [PubMed] [Google Scholar]
- 59.Holland JW, Hui C, Cullis PR, Madden TD. Poly(ethylene glycol)–lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 1996;35(8):2618–24. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 60.Hong RL, Huang CJ, Tseng YL, Pang VF, Chen ST, Liu JJ, et al. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: is surface coating with polyethylene glycol beneficial? Clin Cancer Res. 1999;5(11):3645–52. [PubMed] [Google Scholar]
- 61.Erbacher P, Bettinger T, Belguise-Valladier P, Zou S, Coll JL, Behr JP, et al. Transfection and physical properties of various saccharide, poly(ethylene glycol), and antibody-derivatized polyethylenimines (PEI) J Gene Med. 1999;1(3):210–22. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<210::AID-JGM30>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 62.Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, Patri VS, Kim JM, et al. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjug Chem. 2009;20(1):47–59. doi: 10.1021/bc800239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo X, Szoka FC., Jr Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG–diortho ester–lipid conjugate. Bioconjug Chem. 2001;12(2):291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- 64.Li W, Huang Z, MacKay JA, Grube S, Szoka FC., Jr Low-pH-sensitive poly(ethylene glycol) (PEG)-stabilized plasmid nanolipoparticles: effects of PEG chain length, lipid composition and assembly conditions on gene delivery. J Gene Med. 2005;7(1):67–79. doi: 10.1002/jgm.634. [DOI] [PubMed] [Google Scholar]
- 65.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, et al. "SMART" drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem. 2006;17(4):943–9. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boomer JA, Inerowicz HD, Zhang Z-Y, Bergstrand N, Edwards K, Kim J-M, et al. Acid triggered release from sterically-stabilized fusogenic vesicles via a hydrolytic dePEGylation strategy. Langmuir. 2003;19:6408–15. doi: 10.1021/la030104y. [DOI] [Google Scholar]
- 67.Zalipsky S, Qazen M, Walker JA, 2nd, Mullah N, Quinn YP, Huang SK. New detachable poly(ethylene glycol) conjugates: cysteine-cleavable lipopolymers regenerating natural phospholipid, diacyl phosphatidylethanolamine. Bioconjug Chem. 1999;10(5):703–7. doi: 10.1021/bc990031n. [DOI] [PubMed] [Google Scholar]
- 68.Barenholz Y. Design of liposome-based drug carriers: from basic research to application as approved drugs. In: Lasic DD, Papahadjopoulos D, editors. Medical applications of liposomes. New York: Elsevier; 1998. pp. 545–65. [Google Scholar]
- 69.Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973;311(3):330–48. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 70.Gregoriadis G, Davis C. Stability of liposomes in vivo and in vitro is promoted by their cholesterol content and the presence of blood cells. Biochem Biophys Res Commun. 1979;89(4):1287–93. doi: 10.1016/0006-291X(79)92148-X. [DOI] [PubMed] [Google Scholar]
- 71.Senior J, Gregoriadis G. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 1982;30(24):2123–36. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 72.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743. [PubMed] [Google Scholar]
- 73.Abra RM, Hunt CA. Liposome disposition in vivo. III. Dose and vesicle-size effects. Biochim Biophys Acta. 1981;666(3):493–503. doi: 10.1016/0005-2760(81)90311-8. [DOI] [PubMed] [Google Scholar]
- 74.Senior J, Crawley JC, Gregoriadis G. Tissue distribution of liposomes exhibiting long half-lives in the circulation after intravenous injection. Biochim Biophys Acta. 1985;839(1):1–8. doi: 10.1016/0304-4165(85)90174-6. [DOI] [PubMed] [Google Scholar]
- 75.Allen TM, Hansen C, Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989;981(1):27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- 76.Gabizon A, Papahadjopoulos D. The role of surface charge and hydrophilic groups on liposome clearance in vivo. Biochim Biophys Acta. 1992;1103(1):94–100. doi: 10.1016/0005-2736(92)90061-P. [DOI] [PubMed] [Google Scholar]
- 77.Bae YH. Drug targeting and tumor heterogeneity. J Control Release. 2009;133(1):2–3. doi: 10.1016/j.jconrel.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 79.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 80.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 81.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36(1):66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 82.Park JW, Hong K, Carter P, Asgari H, Guo LY, Keller GA, et al. Development of anti-p185HER2 immunoliposomes for cancer therapy. Proc Natl Acad Sci USA. 1995;92(5):1327–31. doi: 10.1073/pnas.92.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–4. [PubMed] [Google Scholar]
- 84.Liebert M, Wedemeyer GA, Stein JA, Washington RW, Jr, Flint A, Ren LQ, et al. Identification by monoclonal antibodies of an antigen shed by human bladder cancer cells. Cancer Res. 1989;49(23):6720–6. [PubMed] [Google Scholar]
- 85.Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58(15):3320–30. [PubMed] [Google Scholar]
- 86.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–81. [PubMed] [Google Scholar]
- 87.Goren D, Horowitz AT, Zalipsky S, Woodle MC, Yarden Y, Gabizon A. Targeting of stealth liposomes to erbB-2 (Her/2) receptor: in vitro and in vivo studies. Br J Cancer. 1996;74(11):1749–56. doi: 10.1038/bjc.1996.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torchilin VP. Liposomes as targetable drug carriers. Crit Rev Ther Drug Carrier Syst. 1985;2(1):65–115. [PubMed] [Google Scholar]
- 89.Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 90.Maruyama K. PEG-immunoliposome. Biosci Rep. 2002;22(2):251–66. doi: 10.1023/A:1020138622686. [DOI] [PubMed] [Google Scholar]
- 91.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269(5):3198–204. [PubMed] [Google Scholar]
- 92.Blume G, Cevc G, Crommelin MD, Bakker-Woudenberg IA, Kluft C, Storm G. Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim Biophys Acta. 1993;1149(1):180–4. doi: 10.1016/0005-2736(93)90039-3. [DOI] [PubMed] [Google Scholar]
- 93.Maruyama K, Takizawa T, Yuda T, Kennel SJ, Huang L, Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies. Biochim Biophys Acta. 1995;1234(1):74–80. doi: 10.1016/0005-2736(94)00263-O. [DOI] [PubMed] [Google Scholar]
- 94.Medina OP, Zhu Y, Kairemo K. Targeted liposomal drug delivery in cancer. Curr Pharm Des. 2004;10(24):2981–9. doi: 10.2174/1381612043383467. [DOI] [PubMed] [Google Scholar]
- 95.Lopes De Menezes DE, Kirchmeier MJ, Gagne J-F, Pilarski LM, Allen TM. Cellular trafficking and cytotoxicity of anti-Cd19-targeted liposomal doxorubicin in B lymphoma cells. J Liposome Res. 1999;9:199–228. doi: 10.3109/08982109909024786. [DOI] [Google Scholar]
- 96.Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42(5):439–62. doi: 10.1016/S0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 97.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, et al. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511(2):397–411. doi: 10.1016/S0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 98.Torchilin VP, Rammohan R, Weissig V, Khaw BA, Klibanov A, Samokhin GP, editors. PEG-Immunoliposomes: attachment of monoclonal antibody to distal ends of PEG chains via p-nitrophenylcarbonyl groups. 27th International Symposium on Controlled Release of Bioactive Materials; 2000; Paris: Controlled Release Society, Inc
- 99.Torchilin VP, Weissig V, Martin FJ, Heath TD, New RRC. Surface modifications of liposomes. In: Torchilin VP, Weissig V, editors. Liposomes: a practical approach. 2. Oxford: Oxford University Press; 2003. pp. 193–229. [Google Scholar]
- 100.Hansen CB, Kao GY, Moase EH, Zalipsky S, Allen TM. Attachment of antibodies to sterically stabilized liposomes: evaluation, comparison and optimization of coupling procedures. Biochim Biophys Acta. 1995;1239(2):133–44. doi: 10.1016/0005-2736(95)00138-S. [DOI] [PubMed] [Google Scholar]
- 101.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (stealth) immunoliposomal drugs. FEBS Lett. 1999;460(1):129–33. doi: 10.1016/S0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 102.Sofou S, Sgouros G. Antibody-targeted liposomes in cancer therapy and imaging. Expert Opin Drug Deliv. 2008;5(2):189–204. doi: 10.1517/17425247.5.2.189. [DOI] [PubMed] [Google Scholar]
- 103.Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv. 2008;5(9):1003–25. doi: 10.1517/17425247.5.9.1003. [DOI] [PubMed] [Google Scholar]
- 104.Martin FJ, Papahadjopoulos D. Irreversible coupling of immunoglobulin fragments to preformed vesicles. An improved method for liposome targeting. J Biol Chem. 1982;257(1):286–8. [PubMed] [Google Scholar]
- 105.Moreira JN, Ishida T, Gaspar R, Allen TM. Use of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicity. Pharm Res. 2002;19(3):265–9. doi: 10.1023/A:1014434732752. [DOI] [PubMed] [Google Scholar]
- 106.Elbayoumi TA, Torchilin VP. Tumor-specific anti-nucleosome antibody improves therapeutic efficacy of doxorubicin-loaded long-circulating liposomes against primary and metastatic tumor in mice. Mol Pharm. 2009;6(1):246–54. doi: 10.1021/mp8001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 108.Xiong XB, Huang Y, Lu WL, Zhang H, Zhang X, Zhang Q. Enhanced intracellular uptake of sterically stabilized liposomal doxorubicin in vitro resulting in improved antitumor activity in vivo. Pharm Res. 2005;22(6):933–9. doi: 10.1007/s11095-005-4588-x. [DOI] [PubMed] [Google Scholar]
- 109.Xiong XB, Huang Y, Lu WL, Zhang X, Zhang H, Nagai T, et al. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94(8):1782–93. doi: 10.1002/jps.20397. [DOI] [PubMed] [Google Scholar]
- 110.Allen TM, Ahmad I, Lopes de Menezes DE, Moase EH. Immunoliposome-mediated targeting of anti-cancer drugs in vivo. Biochem Soc Trans. 1995;23(4):1073–9. doi: 10.1042/bst0231073. [DOI] [PubMed] [Google Scholar]
- 111.Vingerhoeds MH, Steerenberg PA, Hendriks JJ, Dekker LC, Van Hoesel QG, Crommelin DJ, et al. Immunoliposome-mediated targeting of doxorubicin to human ovarian carcinoma in vitro and in vivo. Br J Cancer. 1996;74(7):1023–9. doi: 10.1038/bjc.1996.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moase EH, Qi W, Ishida T, Gabos Z, Longenecker BM, Zimmermann GL, et al. Anti-MUC-1 immunoliposomal doxorubicin in the treatment of murine models of metastatic breast cancer. Biochim Biophys Acta. 2001;1510(1–2):43–55. doi: 10.1016/s0005-2736(00)00334-5. [DOI] [PubMed] [Google Scholar]
- 113.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100(1):135–44. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 114.Gupta B, Torchilin VP. Monoclonal antibody 2 C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol Immunother. 2007;56(8):1215–23. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, et al. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74(1–3):95–113. doi: 10.1016/S0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 116.Shmeeda H, Tzemach D, Mak L, Gabizon A. Her2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J Control Release. 2009;136(2):155–60. doi: 10.1016/j.jconrel.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Kamps JA, Koning GA, Velinova MJ, Morselt HW, Wilkens M, Gorter A, et al. Uptake of long-circulating immunoliposomes, directed against colon adenocarcinoma cells, by liver metastases of colon cancer. J Drug Target. 2000;8(4):235–45. doi: 10.3109/10611860008997902. [DOI] [PubMed] [Google Scholar]
- 118.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63(12):3154–61. [PubMed] [Google Scholar]
- 119.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin Cancer Res. 2003;9(17):6551–9. [PubMed] [Google Scholar]
- 120.Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52(12):3402–8. [PubMed] [Google Scholar]
- 121.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233(2):134–44. doi: 10.1016/0005-2736(94)00235-H. [DOI] [PubMed] [Google Scholar]
- 122.Park JW, Hong K, Kirpotin DB, Papahadjopoulos D, Benz CC. Immunoliposomes for cancer treatment. Adv Pharmacol. 1997;40:399–435. doi: 10.1016/S1054-3589(08)60146-5. [DOI] [PubMed] [Google Scholar]
- 123.Storm G, Steerenberg PA, Emmen F, van Borssum Waalkes M, Crommelin DJ. Release of doxorubicin from peritoneal macrophages exposed in vivo to doxorubicin-containing liposomes. Biochim Biophys Acta. 1988;965(2–3):136–45. doi: 10.1016/0304-4165(88)90049-9. [DOI] [PubMed] [Google Scholar]
- 124.Chu CJ, Dijkstra J, Lai MZ, Hong K, Szoka FC. Efficiency of cytoplasmic delivery by pH-sensitive liposomes to cells in culture. Pharm Res. 1990;7(8):824–34. doi: 10.1023/A:1015908831507. [DOI] [PubMed] [Google Scholar]
- 125.Trubetskaya OV, Trubetskoy VS, Domogatsky SP, Rudin AV, Popov NV, Danilov SM, et al. Monoclonal antibody to human endothelial cell surface internalization and liposome delivery in cell culture. FEBS Lett. 1988;228(1):131–4. doi: 10.1016/0014-5793(88)80601-X. [DOI] [PubMed] [Google Scholar]
- 126.Allen TM, Hansen C, Stuart DD. Targeted stericalliy stabilized liposomal drug delivery. In: Lasic DD, Papahadjopoulos D, editors. Medical applications of liposomes. New York: Elsevier; 1998. pp. 545–65. [Google Scholar]
- 127.Zhou Y, Drummond DC, Zou H, Hayes ME, Adams GP, Kirpotin DB, et al. Impact of single-chain Fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor-expressing tumor cells. J Mol Biol. 2007;371(4):934–47. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sapra P, Allen TM. Improved outcome when B-cell lymphoma is treated with combinations of immunoliposomal anticancer drugs targeted to both the CD19 and CD20 epitopes. Clin Cancer Res. 2004;10(7):2530–7. doi: 10.1158/1078-0432.CCR-03-0376. [DOI] [PubMed] [Google Scholar]
- 129.Allen TM, Mumbengegwi DR, Charrois GJ. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin Cancer Res. 2005;11(9):3567–73. doi: 10.1158/1078-0432.CCR-04-2517. [DOI] [PubMed] [Google Scholar]
- 130.Hosokawa S, Tagawa T, Niki H, Hirakawa Y, Nohga K, Nagaike K. Efficacy of immunoliposomes on cancer models in a cell-surface-antigen-density-dependent manner. Br J Cancer. 2003;89(8):1545–51. doi: 10.1038/sj.bjc.6601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Emanuel N, Kedar E, Bolotin EM, Smorodinsky NI, Barenholz Y. Targeted delivery of doxorubicin via sterically stabilized immunoliposomes: pharmacokinetics and biodistribution in tumor-bearing mice. Pharm Res. 1996;13(6):861–8. doi: 10.1023/A:1016096910822. [DOI] [PubMed] [Google Scholar]
- 132.Allen TM, Brandeis E, Hansen CB, Kao GY, Zalipsky S. A new strategy for attachment of antibodies to sterically stabilized liposomes resulting in efficient targeting to cancer cells. Biochim Biophys Acta. 1995;1237(2):99–108. doi: 10.1016/0005-2736(95)00085-H. [DOI] [PubMed] [Google Scholar]
- 133.Kamps JA, Scherphof GL. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Deliv Rev. 1998;32(1–2):81–97. doi: 10.1016/s0169-409x(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 134.Flavell DJ, Noss A, Pulford KA, Ling N, Flavell SU. Systemic therapy with 3BIT, a triple combination cocktail of anti-CD19, -CD22, and -CD38-saporin immunotoxins, is curative of human B-cell lymphoma in severe combined immunodeficient mice. Cancer Res. 1997;57(21):4824–9. [PubMed] [Google Scholar]
- 135.Anabousi S, Bakowsky U, Schneider M, Huwer H, Lehr CM, Ehrhardt C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur J Pharm Sci. 2006;29(5):367–74. doi: 10.1016/j.ejps.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 136.Hatakeyama H, Akita H, Maruyama K, Suhara T, Harashima H. Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. Int J Pharm. 2004;281(1–2):25–33. doi: 10.1016/j.ijpharm.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 137.Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373(1–2):116–23. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 138.Ishida O, Maruyama K, Tanahashi H, Iwatsuru M, Sasaki K, Eriguchi M, et al. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm Res. 2001;18(7):1042–8. doi: 10.1023/A:1010960900254. [DOI] [PubMed] [Google Scholar]
- 139.Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci USA. 1991;88(13):5572–6. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamada A, Taniguchi Y, Kawano K, Honda T, Hattori Y, Maitani Y. Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clin Cancer Res. 2008;14(24):8161–8. doi: 10.1158/1078-0432.CCR-08-0159. [DOI] [PubMed] [Google Scholar]
- 141.Lu Y, Wu J, Wu J, Gonit M, Yang X, Lee A, et al. Role of formulation composition in folate receptor-targeted liposomal doxorubicin delivery to acute myelogenous leukemia cells. Mol Pharm. 2007;4(5):707–12. doi: 10.1021/mp070058l. [DOI] [PubMed] [Google Scholar]
- 142.Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57(4):637–51. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 143.Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug Chem. 2007;18(2):363–70. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71(3):431–44. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009;15(6):1973–80. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Elbayoumi TA, Torchilin VP. Enhanced cytotoxicity of monoclonal anticancer antibody 2 C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. Eur J Pharm Sci. 2007;32(3):159–68. doi: 10.1016/j.ejps.2007.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, et al. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 2007;24(12):2402–11. doi: 10.1007/s11095-007-9425-y. [DOI] [PubMed] [Google Scholar]
- 148.Zhao H, Wang JC, Sun QS, Luo CL, Zhang Q. RGD-based strategies for improving antitumor activity of paclitaxel-loaded liposomes in nude mice xenografted with human ovarian cancer. J Drug Target. 2009;17(1):10–8. doi: 10.1080/10611860802368966. [DOI] [PubMed] [Google Scholar]
- 149.Hatakeyama H, Akita H, Ishida E, Hashimoto K, Kobayashi H, Aoki T, et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int J Pharm. 2007;342(1–2):194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]