Abstract
Heart failure (HF) is associated with an overall stroke rate that is too low to justify anticoagulation in all patients. This study was conducted to determine if vascular risk factors can identify a subgroup of individuals with heart failure with a stroke rate high enough to warrant anticoagulation. The REGARDS study is a population-based cohort of US adults aged ≥45 years. Participants are contacted every six months by telephone for self- or proxy-reported stroke and medical records are retrieved and adjudicated by physicians. Participants were characterized into three groups: HF without atrial fibrillation (AF), AF with or without HF, and neither HF nor AF. Cardiovascular risk factors at baseline were compared between participants with and without incident stroke in HF and AF. Stroke incidence was assessed in risk factor subgroups in HF participants. Of the 30,239 participants, those with missing/anomalous data were excluded. Of the remaining 28,832, 1,360 (5%) had HF without AF, 2,528 (9%) had AF, and 24,944 (86%) had neither. Prior stroke/TIA (p=0.0004), diabetes (DM) (p=0.03) and higher systolic blood pressure (p=0.046), were associated with increased stroke risk in participants with HF without AF. In participants with HF without AF, stroke incidence was highest in those with prior stroke/TIA and DM (2.4 [1.1, 4.0] per hundred personyears). The combination of prior stroke/TIA and DM increases the incidence of stroke in participants with HF without AF. No analyzed subgroup had a stroke rate high enough to make it likely that the benefits of warfarin would outweigh the risks.
INTRODUCTION
Heart failure (HF) ranks second as a cause of cardiogenic stroke after atrial fibrillation (AF) with about 60,000 strokes per year(1), compared with about 105,000 strokes per year in AF.(2) In the US, HF affects about twice as many people (5 million) as AF (2.3 million)(3). Two community studies have shown the risk of incident stroke in HF to be 2 to 3 times that of the general population. In the Framingham study, the adjusted risk of stroke associated with HF was 2.1 in women and 2.7 in men.(4) In a recent Olmsted County, Minnesota study, stroke risk among those with HF was 2.9 times that of the control population risk over 5 years.(5)
The dilated left ventricle in HF is thought to be a source of cerebral embolism.(6) The stroke rate in HF has been assessed at 47.4 per 1000 persons over 5 years in a meta-analysis(7) and clinical studies put the rate at about 1.6% per year.(1) This is however much lower than the stroke rate in AF (76.0 per 1000 persons over 2 years).(8) Stroke risk stratification tools can identify individuals with AF in whom the risk of stroke is sufficiently high (stroke rate 3% to 5% per year)(9) to justify treatment with warfarin. The Congestive heart failure, Hypertension, Age greater than 75 years, Diabetes and Stroke (CHADS2) scheme identifies about 60% of individuals with AF having this stroke rate.(10) For HF without AF there are no clinical risk stratification tools for identifying a subgroup with a stroke rate high enough to justify anticoagulation. Up to 28% of HF individuals in sinus rhythm are currently treated with warfarin.(11;12) Many individuals with HF on warfarin may not be benefiting from it or may be suffering needless hemorrhagic complications. The aims of this study were firstly to examine the annual stroke rate in participants of the REGARDS study with HF without AF and also in subgroups of participants with vascular risk factors to determine if a subgroup with a stroke rate high enough to warrant anticoagulation can be identified. A second aim was to compare stroke risk factors between participants with HF and participants with AF. A third aim was to develop a stroke risk stratification scheme, similar to CHADS2 for HF.
METHODS
Design
The REasons for Geographic And Racial Differences in Stroke (REGARDS) study is a population-based study of black and white adults aged 45 years and older drawn as a stratified random sample of the general population living in the stroke belt, stroke buckle and the rest of the nation.(13;14) The stroke belt region with the highest rates of stroke mortality compared to the rest of the nation is defined as the eight southern states of North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas and the coastal plain of North Carolina, South Carolina, and Georgia is referred to as the “stroke buckle” due to even higher stroke mortality than the rest of the stroke belt.(13) Enrollment was in January 2003 through October 2007. The cohort consists of 30,239 participants, 45% men, 55% women; 58% white, 42% black ; 56% residing in the stroke belt and 44% in the remaining 40 contiguous states, being followed biannually by telephone to ascertain cognitive function, stroke and other outcomes
Procedures
REGARDS is approved by the Institutional Review Boards of institutions participating in the data collection activities. Participants were recruited from commercially-available lists of US residents using mail and telephone contact methods. Those who agreed to participate answered demographic, quality of life, health behavior, and medical history information; reported HF symptoms (two questions); and reported stroke history and symptoms using the Questionnaire for Verifying Stroke-free Status (QVSFS) (15) all via a computer-assisted telephone interview. During a subsequent home visit, written informed consent was obtained, as well as blood and urine samples, resting electrocardiogram (ECG), and blood pressure and body mass index measures. Medication audits also were performed. Further methodological details are available elsewhere(13).
Measures
Heart failure
For the diagnosis of HF, we included participants who self-reported both orthopnea and paroxysmal nocturnal dyspnea (PND), and also included participants without AF who had current use of digoxin at baseline. Orthopnea and PND are both major components of the Framingham HF criteria and we use this definition of HF. Used together they give a sensitivity for a diagnosis of HF of 75% (95%CI: 72%, 78%), a specificity of 47% (41%, 53%), and a positive predictive value of 83% (80%, 85%).(16) Digoxin use in persons without AF has a specificity of about 99% and a sensitivity of about 28% for the diagnosis of HF.(17)
Stroke/transient ischemic attack (TIA)
Presence of prior stroke/TIA was determined by using the QVSFS questionnaire(15;18) The first two items of this questionnaire elicit history of physician-verified stroke, mini-stroke, or TIA; a positive response on either of these items at study baseline indicates presence of prior stroke/TIA history.
Demographics
Age, sex, race, and region were ascertained by self-report.
Socioeconomic status
(SES) was represented by income and education levels. Annual income was categorized into three levels: less than $20K, $20–34K, $35–74K and ≥74K and refused. Education was categorized into four levels: below high school, high school, some college education and college graduate.
Health behavior
Alcohol and smoking histories were defined as follows: alcohol consumption— current, previous or no (lifelong abstinence) use; smoking—current vs. never or past smoker. The Short-Form 12-Item (SF-12)(19) health survey was administered to evaluate health-related quality of life mental and physical component scores.
Co-morbidities
The following definitions were used: diabetes (DM)—fasting glucose ≥126 ml/dL, non-fasting glucose ≥200 ml/dL, or self-reported use of DM medications; hypertension—systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure≥90 mmHg (average of two BP measurements), or self-reported use of hypertension medications; AF—based on ECG diagnosis, or a positive response to the question “Has a doctor or other health professional ever told you that you had atrial fibrillation?”;(20) ischemic heart disease-based on self-reported history of myocardial infarction, coronary bypass, angioplasty, or stenting, or based on electrocardiographic evidence of myocardial infarction; high sensitivity C-reactive protein; estimated glomerular filtration rate (based on the CKD-EPI equation);(21) warfarin use at baseline.
Incident stroke
Participants and/or proxies were contacted biennially by telephone for suspected stroke events.(14) For proxy reported deaths, interview was conducted with next of kin. Once a potential event was identified, the medical records were retrieved and available information was centrally adjudicated by at least two physician members of a committee of stroke experts. Stroke events were confirmed following the World Health Organization (WHO) definition as “rapidly developing clinical signs of focal, at times global, disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin.”(22) Events not meeting the WHO definition but characterized by symptoms lasting <24 hours with computed tomographic or magnetic resonance brain imaging consistent with acute ischemia or hemorrhage were classified as “clinical strokes.” Strokes were further classified into ischemic or hemorrhagic. This analysis included WHO and clinical ischemic stroke.
Statistical Methods
After excluding participants with missing data on medications or AF, and without any follow-up, participants were characterized as having HF without AF, AF with or without HF, and neither HF nor AF. Descriptive statistics both overall and by exposure (AF, HF without AF, neither HF nor AF) were computed, using either means/standard deviations, or frequencies and percents, and analysis of variance (ANOVA), t-tests or chi-square tests of association were used to test for differences across exposure groups. Distributions of continuous variables were inspected for normality. Stroke incidence rates were computed by exposure group, and a log-rank test was used to determine whether there were differences in the time until stroke between the groups. A Kaplan-Meier plot was generated to show the univariate associations between exposure and stroke. Cox’s Proportional Hazards model was fitted to assess the relationship between AF, HF without AF, or neither and stroke, both univariately (Model 0) and after adjustment for demographic factors (Model 1: age, sex, race, region, education, income, age*race), lifestyle factors (Model 2: Model 1 + alcohol use, smoking), SF-12 components (Model 3: Model 2 +Mental health component summary score of SF12, physical component summary score of SF12), and comorbidities (Model 4: Model 3 +hypertension, SBP, DM, prior stroke/TIA, ischemic heart disease, estimated glomerular filtration rate, C-reactive protein, warfarin use) and also to compare the stroke risk among HF participants, for different levels of significant risk factors. Interactions were assessed between the main exposure and hypertension and DM. Among participants with HF, age, sex, SBP, hypertension, DM and history of prior stroke/TIA at baseline were compared between those with and without incident stroke using t-tests or chi-square tests. Cox models were used to compare the stroke risk among HF participants, for different levels of risk factors. All models that include both age and race also included the interaction between the two, given the known existence of that interaction, to adjust for the differential relationship between race and stroke by age. In addition, we performed a sensitivity analysis excluding participants on warfarin, in order to assess the impact they have on the results (n=564).
RESULTS
Of 30,239 participants in the REGARDS study, 56 were excluded due to anomalous data values, 26 were excluded because they did not have a medications form completed and another 699 because they did not have data regarding their AF status. Another 552 participants were excluded due to lack of information regarding follow-up time, and another 74 were excluded due to SBP out of range or missing. Thus, 28,832 were included in the cohort for analysis. Among those, a total of 2528 were categorized as having AF (9%), 1360 with HF (5%), and the remainder (24,944) with neither. 677 (2%) had both AF and HF. Of the 1360 with HF, 1028 were classified based on their orthopnea/PND status, 299 were classified based only on digoxin use, and the remaining 33 met both criteria.
Table 1 shows the baseline demographic factors, lifestyle factors, SF-12 components, and comorbidities, overall and by HF/AF group. In addition, Table 1 contains the number and frequency of strokes in each group, as well as the average length of follow-up in each exposure group. With the exception of region, all factors assessed differ by HF/AF group.
Table 1.
Characteristics by diagnostic group
| Variable | All Participants (n=28,832) |
Neither HF or AF (n=24,944) |
AF with or without HF (n= 2528) |
HF w/o AF (n= 1360) |
p-value |
|---|---|---|---|---|---|
| Age (mean, STD) | 64.9 (9.4) | 64.7 (9.3) | 67.6 (9.7) | 63.7 (9.6) | <0.0001 |
| Male sex | 12981 (45%) | 11288 (45%) | 1157 (46%) | 536 (39%) | <0.0001 |
| Black race | 11778 (41%) | 10207 (41%) | 915 (36%) | 656 (48%) | <0.0001 |
| Region | 0.003 | ||||
| Belt | 9961 (35%) | 8574 (34%) | 8712 (35%) | 515 (38%) | |
| Buckle | 6051 (21%) | 5170 (21%) | 571 (23%) | 310 (23%) | |
| Non-belt | 12820 (44%) | 11200 (45%) | 1085 (43%) | 535 (45%) | |
| Education | <0.0001 | ||||
| <High School | 3561 (12%) | 2914 (12%) | 347 (14%) | 300 (22%) | |
| High School | 7439 (26%) | 6357 (26%) | 704 (28%) | 378 (28%) | |
| Graduate | |||||
| Some College | 7716 (27%) | 6674 (27%) | 664 (26%) | 378 (28%) | |
| College Grad | 10095 (35%) | 8985 (36%) | 809 (32%) | 301 (22%) | |
| Income | <0.0001 | ||||
| <$20,000 | 5142 (18%) | 4183 (17%) | 562 (22%) | 397 (29%) | |
| $20,000– $34,000 | 6969 (24%) | 5969 (24%) | 652 (26%) | 348 (26%) | |
| $35,000– $74,000 | 8607 (30%) | 7634 (31%) | 675 (27%) | 298 (22%) | |
| >= $74,000 | 4593 (16%) | 4172 (17%) | 297 (12%) | 124 (9%) | |
| Refused | 3521 (12%) | 2986 (12%) | 342 (14%) | 193 (14%) | |
| Alcohol Use | <0.0001 | ||||
| Current | 14922 (52%) | 13184 (53%) | 1164 (46%) | 574 (42%) | |
| Past | 5225 (18%) | 4336 (17%) | 564 (22%) | 325 (24%) | |
| Never | 8685 (30%) | 7424 (30%) | 800 (32%) | 461 (34%) | |
| Smoking | <0.0001 | ||||
| Current | 4145 (14%) | 3568 (14%) | 324 (13%) | 253 (19%) | |
| Past | 11567 (40%) | 9868 (40%) | 1147 (46%) | 552 (41%) | |
| Never | 13013 (45%) | 11414 (46%) | 1048 (42%) | 551 (41%) | |
| SBP (mean, STD) | 128 (17) | 127 (17) | 128 (18) | 129 (18) | 0.004 |
| Hypertensi ve (n, %) | 17022 (59%) | 14300 (57%) | 1740 (69%) | 982 (72%) | <0.0001 |
| Diabetic (n, %) | 6058 (22%) | 4936 (20%) | 639 (26%) | 483 (37%) | <0.0001 |
| MCS (mean, STD) | 54 (8) | 54 (8) | 53 (10) | 50 (11) | <0.0001 |
| PCS (mean, STD) | 47 (11) | 47 (10) | 42 (12) | 38 (12) | <0.0001 |
| Warfarin Use | 564 (22%) | 401 (2%) | 564 (22%) | 91 (7%) | <0.0001 |
| Stroke/TI A reported (@)baseline | 2860 (10%) | 2163 (9%) | 456 (18%) | 241 (18%) | <0.0001 |
STD: standard deviation
SBP: systolic blood pressure (millimeters of mercury)
MCS: mental health component summary score of Short-Form 12-Item survey
PCS: physical component summary score of Short-Form 12-Item survey
HF: heart failure
AF: atrial fibrillation
p-values from ANOVA for continuous variables, and χ2 tests of association for categorical variables
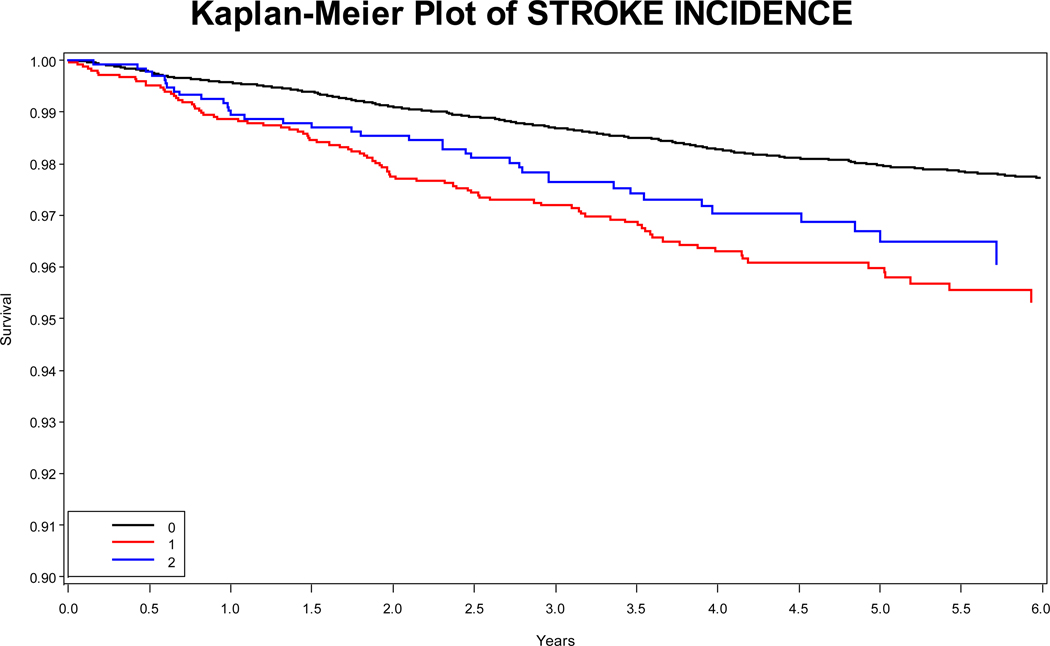
With respect to stroke rates, among those without either HF or AF, the incidence rate is 0.40 per 100 person-years (95% CI: 0.37, 0.44), while for those with AF, the incidence rate is 0.85 per 100 person-years (95% CI: 0.69, 1.0), and for those with HF alone, the incidence rate is 0.69 per 100 person-years (95% CI: 0.49, 0.93). Among those with both AF and HF, the incidence rate is 1.04 per 100 patient years (95% CI: 0.69, 1.45). Figure 1 depicts the Kaplan-Meier curves for the three groups.
Figure 1.
Legend: Red: atrial fibrillation; Blue: heart failure without atrial fibrillation; black: neither heart failure nor atrial fibrillation.
The Cox models, assessing the hazard of stroke for those in the AF and HF groups, compared to those with neither AF nor HF showed that even after multivariable adjustment, there is a significant relationship between HF/AF and stroke, with those with AF 1.5 times (1.2, 2.0) more likely to experience a stroke, and those with HF 1.2 times (0.81, 1.8) more likely to experience a stroke. Neither of the interactions assessed was statistically significant (HF/AF with hypertension: p=0.092, HF/AF with DM: p=0.43).
Table 2 compares the specific risk factors (age, sex, SBP, hypertension, DM and prevalent stroke/TIA at baseline) between those with and without incident stroke, among participants in the AF group, while Table 3 does the same, among those in the HF group. Among those in the AF group, only older age and prior stroke/TIA are significantly associated with incident stroke, while higher SBP is marginally associated with incident stroke. Among those in the HF alone group, SBP, DM, and prior stroke/TIA all differ between those with and without incident stroke. Table 4 shows that there is an increasing hazard ratio for stroke in participants with HF with additional risk factors and participants with prior stroke/TIA and DM were more likely to have stroke than those with diabetes alone. Sensitivity analyses excluding those on warfarin yielded similar results.
Table 2.
Relationship between stroke and risk factors, among those with atrial fibrillation.
| All AF (n=2528) |
AF, w/incident stroke (n=90) |
AF, w/o incident stroke (n=2438) |
p-value* | |
|---|---|---|---|---|
| Age | 67.6 (9.7) | 72.9 (8.5) | 67.4 (9.6) | <0.0001 |
| Male Sex | 1157 (46%) | 44 (49%) | 1113 (46%) | 0.55 |
| SBP | 128 (18) | 132 (18) | 128 (18) | 0.06 |
| HTN (n, %) | 1740 (69%) | 68 (74%) | 1673 (69%) | 0.25 |
| Diabetes (n, %) | 639 (26%) | 30 (34%) | 609 (26%) | 0.11 |
|
Prior Stroke/TIA |
456 (18%) | 32 (36%) | 424 (17%) | <0.0001 |
SBP: systolic blood pressure (millimeters of mercury)
HTN: hypertension
AF: atrial fibrillation
p-value for comparison between AF with incident stroke vs. AF without incident stroke, from t-tests for continuous variables, and χ2 tests of association for categorical variables
Table 3.
Relationship between stroke and risk factors, among those with heart failure without atrial fibrillation
| All HF (n=1360) |
HF, w/incident stroke (n=38) |
HF, w/o incident stroke (n=1322) |
p-value* | |
|---|---|---|---|---|
| Age | 63.7 (9.6) | 65.1 (10.3) | 63.7 (9.6) | 0.42 |
| Male Sex | 536 (39%) | 18 (47%) | 518 (39%) | 0.31 |
| SBP | 129 (18) | 135 (17) | 129 (18) | 0.046 |
| HTN (n, %) | 982 (72%) | 29 (76%) | 953 (72%) | 0.56 |
| Diabetes (n, %) | 483 (37%) | 20 (54%) | 463 (36%) | 0.03 |
|
Prior Stroke/TIA |
241 (17%) | 16 (42%) | 225 (17%) | 0.0004 |
SBP: systolic blood pressure (millimeters of mercury)
HTN: hypertension
p-value for comparison between AF with incident stroke vs. AF without incident stroke, from t-tests for continuous variables, and χ2 tests of association for categorical variables
Table 4.
Hazard ratio and stroke incidence among those with heart failure without atrial fibrillation
| Hazard ratio for stroke (ref=HF alone) |
Stroke incidence per 100 patient years |
|
|---|---|---|
| All HF | 0.69 (0.49,0.93) | |
| HF+DM | 1.9 (0.82,4.5) | 0.72 (0.24,1.2) |
| HF+prior stroke/TIA | 3.5 (1.3,9.5) | 1.3 (0.47,2.5) |
| HF+prior stroke/TIA+DM | 6.4 (2.7,15.1) | 2.4 (1.1,4.0) |
HF: heart failure
DM: diabetes
DISCUSSION
Our stroke incidence of 0.69 per 100 person years in participants with HF in the absence of AF is at the lower end of stroke incidence observed in recent HF trials (0.7 to 1.5% per year).(12;23) Our incidence was lower than in previous population studies(5;24) that found HF stroke rates between 0.8% and 3.2% per year. The Olmsted County study(5) which started in 1979 had a mortality rate (85% over 4.3 years), which is much higher than modern HF mortality rates. HF mortality has been falling due to improved medical management such as the widespread use of angiotensin converting enzyme inhibitors, and this is likely also to have reduced stroke rates in HF. Falling incidence of stroke has been noted in AF, with modern risk factor management(10;25) which probably explains our low AF stroke rates as well.
In participants with HF without AF, the combination of prior stroke/TIA and DM is associated with an incidence of stroke (2.4; 95%CI: 1.1,4.0) per hundred-person years, similar to that of the “moderate” stroke risk group by AF stratification schemes (2.2; 95%CI:1.1,3.5 per hundred person-years).(26) This incidence is still below the stroke rates that would be predicted by CHADS2 for the same risk factor combination in persons with AF (3.89 per hundred patient years),(25) and below commonly accepted rates (3% to 5%(9)) for warfarin anticoagulation There is however, increasing evidence that stroke risk during the 30 days after HF onset, may be almost double (hazard ratio 5.79, 95% CI 2.15–15.62)(27) the five year HF stroke risk (standardized morbidity ratio 2.9, 95% CI 2.2–3.8)(5), with a persisting smaller effect up to six months. This may also be true for the acute period after a stroke.(28;29) This suggests that during these acute periods, the stroke rates in the subgroup with prior stroke and diabetes may reach 4 to 5% percent per year and would justify anticoagulation. Further data are needed, however, on stroke incidence in these situations.
The most commonly used indicator for anticoagulation in HF is a decreased cardiac ejection fraction (EF).(30) EF does not appear to be a strong risk factor for stroke however(1) and reduced EF by itself is not a reliable method of identifying a high stroke risk population in HF as recent trials have shown.(12;23) In HF additional risk factors of DM and prior stroke/TIA increase the risk of stroke, however the number and risk weighting of risk factors for stroke is smaller in HF than in AF.(31) For example, in AF, prior stroke/TIA is a very strong risk factor imparting an adjusted risk of 9.79 (9.33–10.24)(31) for stroke compared with our finding of a hazard ratio of 3.5 (1.3–9.5) for stroke in participants with prior stroke/TIA among those with HF. This difference between HF and AF may reflect the much higher propensity for recurrent left atrial appendage thrombus in AF than left ventricular thrombus formation in HF.
There is only one other cohort study to investigate risk factors for stroke in persons with HF,(5) which found prior stroke, age and diabetes to be independent risk factors. No attempt was made to define subgroups with an increased stroke rate. Our findings support their data(5) that prior stroke/TIA and DM are risk factors for incident stroke in HF. Unlike that study, we did not find age to be a risk factor for stroke in HF, but those data showed only a very small increased (×1.04) risk with age. Framingham data showed that the effects of HF on stroke become progressively weaker with age,(32) which is the opposite to that seen in AF and age is unlikely to be an important HF stroke risk factor. We did not find hypertension to be a significant risk factor for stroke, but mean systolic blood pressure was higher in HF individuals with new stroke than in those without. We have previously shown a relationship between the lowest tertile of SBP and the risk of prevalent stroke/TIA in HF, which we could not confirm with this study using incident stroke.(33) It is possible that this may reflect more aggressive blood pressure treatment post stroke.
A limitation of this study is that we based the diagnosis of HF principally on the presence of orthopnea and PND. These are two of the major Framingham clinical HF criteria, and a diagnosis of HF requires the presence of two major criteria. Used together, orthopnea and PND, have a very good positive predictive value for the diagnosis of HF.(16;34;35) and have been used together to diagnose HF in previous studies.(36) In order to include asymptomatic participants with chronic HF, we added digoxin use as a second criterion for HF. Both our criteria have a high specificity (75%–99%) for the diagnosis of HF(16;17) but relatively low sensitivity. This lack of sensitivity likely resulted in a small admixture of HF cases in the “Neither HF nor AF” group. This may have biased our results towards the null, but is unlikely to have qualitatively affected our conclusions, because our HF cases represent a small percentage of the total cohort (5.5%). Participants with both HF and AF were all assigned to our AF group, but since the stroke risk of AF is higher than that of HF this would not have affected our results. The high rate of AF in this study (9%) may be due to using self-report of AF to diagnose AF. This enables detection of past episodes of paroxysmal AF that would not be detected by only using ECGs. Major strengths of this study are the large cohort size, which has allowed for a statistically robust analysis in the HF subgroup and the large proportion of African American participants in whom HF is frequent.
ACKNOWLEDGEMENTS
SOURCES OF FUNDING:
The authors acknowledge the participating investigators and institutions for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Grant Support: The REGARDS research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pullicino P, Homma S, Thompson JL, et al. Oral anticoagulation in patients with cardiomyopathy or heart failure in sinus rhythm. Cerebrovasc Dis. 2008;26:322–327. doi: 10.1159/000149581. [DOI] [PubMed] [Google Scholar]
- 2.Han SW, Nam HS, Kim SH, Lee, et al. Frequency and significance of cardiac sources of embolism in the TOAST classification. Cerebrovasc Dis. 2007;24:463–468. doi: 10.1159/000108438. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 9;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Verter J. Manifestations of coronary disease predisposing to stroke. The Framingham study. JAMA. 1983;250:2942–2946. [PubMed] [Google Scholar]
- 5.Witt BJ, Brown RD, Jr, Jacobsen SJ, et al. Ischemic stroke after heart failure: a community-based study. Am Heart J. 2006;152:102–109. doi: 10.1016/j.ahj.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Pullicino P, Homma S. Stroke in heart failure. Atrial Fibrillation revisited? J Stroke Cerebrovasc Dis. 2010;19:1–2. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Witt BJ, Gami AS, Ballman KV, et al. The incidence of ischemic stroke in chronic heart failure: a meta-analysis. J Card Fail. 2007 Aug;13:489–496. doi: 10.1016/j.cardfail.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: A major contributor to stroke in the elderly. The Framingham study. Arch Int Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 9.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline) J Am Coll Cardiol. 2011;57:223–242. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Baruch L, Gage BF, Horrow J, et al. Can patients at elevated risk of stroke treated with anticoagulants be further risk stratified? Stroke. 2007;38:2459–2463. doi: 10.1161/STROKEAHA.106.477133. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khadra AS, Salem DN, Rand WM, et al. Warfarin anticoagulation and survival: A cohort analysis from the Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;31:749–753. doi: 10.1016/s0735-1097(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberger RS, Hellkamp AS, Halperin JL, et al. the SCD-HeFT Investigators. Risk factors for thromboembolism in the SCD-Heft Study. Circulation. 2007;115:2637–2641. doi: 10.1161/CIRCULATIONAHA.106.661397. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011 Apr;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Allman RM, Aronow WS, et al. Diagnosis of heart failure in older adults: predictive value of dyspnea at rest. Archives of Gerontology and Geriatrics. 2004;38:297–307. doi: 10.1016/j.archger.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca C, Oliveira AG, Mota T, et al. Evaluation of the performance and concordance of clinical questionnaires for the diagnosis of heart failure in primary care. Eur J Heart Fail. 2004;6:813–812. doi: 10.1016/j.ejheart.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey) J Health Serv Res Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- 20.Meschia JF, Merrill P, Soliman EZ, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–587. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 23.Massie BM, Collins JF, Ammon SE, et al. The WATCH Trial Investigators. The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 24.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gage BF, Van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation - Stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 27.Alberts VP, Bos MJ, Koudstaal PJ, et al. Heart failure and the risk of stroke: the Rotterdam Study. Eur J Epidemiol. 2010;25:807–812. doi: 10.1007/s10654-010-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petty GW, Brown RD, Jr., Whisnant JP, et al. Survival and recurrence after first cerebral infarction - A population-based study in Rochester, Minnesota 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 29.Sacco RL, Shi T, Zamanillo MC, et al. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: The Northern Manhattan stroke study. Neurology. 1994;44:626–634. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 30.Heart Failure Society of America. Heart failure in patients with left ventricular systolic dysfunction. J Card Fail. 2006;12:e38–e57. doi: 10.1016/j.cardfail.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Rietbrock S, Heeley E, Plumb J, et al. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156:57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 33.Pullicino PM, McClure LA, Wadley VG, et al. Blood pressure and stroke in heart failure in the REGARDS study. Stroke. 2009;40:3706–3710. doi: 10.1161/STROKEAHA.109.561670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CS, Fitzgerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 35.Ekundayo OJ, Howard VJ, Safford MM, et al. Value of orthopnea, paroxysmal nocturnal dyspnea, and medications in prospective population studies of incident heart failure. Am J Cardiol. 2009;15(104):259–264. doi: 10.1016/j.amjcard.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: A study of all incident cases in Olmsted County Minnesota in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]