Abstract
Although cognitive neuroscience has made remarkable progress in understanding the involvement of the prefrontal cortex in executive control, the broader functional networks that support high-level cognition and give rise to general intelligence remain to be well characterized. Here, we investigated the neural substrates of the general factor of intelligence (g) and executive function in 182 patients with focal brain damage using voxel-based lesion–symptom mapping. The Wechsler Adult Intelligence Scale and Delis–Kaplan Executive Function System were used to derive measures of g and executive function, respectively. Impaired performance on these measures was associated with damage to a distributed network of left lateralized brain areas, including regions of frontal and parietal cortex and white matter association tracts, which bind these areas into a coordinated system. The observed findings support an integrative framework for understanding the architecture of general intelligence and executive function, supporting their reliance upon a shared fronto-parietal network for the integration and control of cognitive representations and making specific recommendations for the application of the Wechsler Adult Intelligence Scale and Delis–Kaplan Executive Function System to the study of high-level cognition in health and disease.
Keywords: fronto-parietal network, general intelligence, executive function, voxel-based lesion–symptom mapping
Introduction
The search for organizing principles that govern human intelligence represents a central and enduring aim of cognitive neuroscience, with emerging research providing new insight into the neural architecture of goal directed, intelligent behaviour (Barbey and Barsalou, 2009; Barbey et al., 2009a, b, 2011a, b; Miller and Phelps, 2010; Barbey and Grafman, 2011; Barbey and Patterson, 2011). Extensive functional neuroimaging evidence indicates that the prefrontal cortex plays a central role in executive control processes for human intelligence (Miller, 2000; Miller and Cohen, 2001). Fundamental questions, however, remain in the absence of definitive neuropsychological evidence to elucidate the functional organization of the prefrontal cortex and its role in higher cognitive functions. A central question concerns how the prefrontal cortex contributes to the information processing architecture of high-level cognition and, in particular, whether prefrontal networks are computationally necessary for core features of human intelligence (Colom et al., 2010; Deary et al., 2010; Colom and Thompson, 2011).
Historically, theories of intelligence have focused on the analysis of a general factor, referred to as psychometric g, which has been shown to underlie performance on a broad range of cognitive tests (Spearman, 1904, 1927; Jensen, 1998; Hunt, 2011). Neuroscience models deriving from Spearman's classic theory propose that the prefrontal cortex provides a unified neural architecture for human intelligence (Duncan et al., 2000; Duncan, 2010). Accumulating neuroscience data support this framework, demonstrating recruitment of prefrontal cortex networks for performance on tests of general intelligence (Prabhakaran et al., 1997; Esposito et al., 1999; Duncan et al., 2000; Bishop et al., 2008) and executive function (Duncan and Owen, 2000; Miller, 2000; Miller and Cohen, 2001; Duncan, 2006). Monkey electrophysiological data further indicate that cells within the prefrontal cortex adaptively code different kinds of task-relevant information in different behavioural contexts, supporting the involvement of the prefrontal cortex in a wide range of high-level cognitive processes (Duncan, 2010; Miller and Cohen, 2001).
The alternative to Spearman's general factor model proposes that tests of intelligence reflect the average or combined activity of many separate cognitive operations (Thomson, 1951; Barbey and Sloman, 2007; Bartholomew et al., 2009). According to this framework, general intelligence depends on a variety of different cognitive processes that are mediated by functionally specialized brain regions (Jung and Haier, 2007; Colom et al., 2009; Gläscher et al., 2009, 2010; Colom and Thompson, 2011; Karama et al., 2011). This model proposes that general intelligence incorporates (i) temporal and occipital specific areas for processing sensory information; (ii) parietal cortex for sensory integration and abstraction; (iii) frontal areas for reasoning and problem solving; and (iv) the anterior cingulate for response selection and the inhibition of automatic responses. Advocates of this framework propose that discrete regions within this distributed network are necessary for key competencies of general intelligence, with an emphasis on dorsolateral prefrontal cortex and parietal cortex (Jung and Haier, 2007). White matter association tracts, especially the superior longitudinal/arcuate fasciculus, are thought to play a critical role in the reliable communication of information across these regions. This framework, therefore, predicts that general intelligence is implemented within a distributed network of functionally specialized brain areas, emphasizing the importance of frontal and parietal regions and the white matter association tracts that bind these areas into a coordinated system (Botvinick et al., 2004; Gruber and Goshke 2004; Ramnani and Owen 2004; Dosenbach et al., 2007). Gläscher et al. (2010) provide key neuropsychological evidence to support this account, conducting a thorough lesion mapping analysis of general intelligence in a large sample of patients with focal brain lesions. These authors found that impairment on measures of general intelligence was associated with selective damage to frontal and parietal regions, as well as white matter association tracts connecting these sectors (e.g. the superior longitudinal/arcuate fasciculus; see also Jung and Haier, 2007; Chiang et al., 2009; Colom et al., 2009; Gläscher et al., 2009; Colom and Thompson, 2011).
The information processing architecture of general intelligence, therefore, remains the focus of ongoing research and debate, with investigators proposing that psychometric g embodies a single cognitive ability implemented within processing networks of the prefrontal cortex (Duncan et al., 2000; Duncan, 2010) or derives instead from multiple cognitive operations that engage a distributed system of functionally specialized cortical regions (Colom et al., 2006a, b, 2007, 2009; Jung and Haier, 2007; Gläscher et al., 2010, 2009; Colom and Thompson, 2011).
A parallel forum of debate examines the architecture of executive functions in the prefrontal cortex, with several decades of cognitive and neuroscientific research investigating the top-down control mechanisms that guide behaviour and the prefrontal cortex networks upon which these functions depend (Miller, 2000; Miller and Cohen, 2001; Barbey and Barsalou, 2009; Barbey et al., 2009a, b, 2011a, b; Barbey and Grafman, 2011; Barbey and Patterson, 2011). The central role of prefrontal cortex networks in general intelligence and executive function raises the question of how these high-level processes are related (Roca et al., 2010). Of particular interest is whether general intelligence and executive function (i) engage common or distinct neural systems; and (ii) operate on the basis of highly localized (Duncan et al., 2000; Duncan, 2010) or broadly distributed cortical networks (Jung and Haier, 2007; Colom et al., 2009; Gläscher et al., 2009, 2010; Colom and Thompson, 2011; Karama et al., 2011).
Neuropsychological patients with focal brain lesions provide a rare opportunity to study the mechanisms underlying general intelligence and executive function, supporting the investigation of lesion-deficit associations, which elucidate the necessity of specific brain structures and advance our understanding of the information processing architecture of high-level cognition. Of the neuropsychological patient studies that have examined the neural bases of general intelligence (Basso et al., 1973; Black, 1976; Eslinger and Damasio, 1985; Shallice and Burgess, 1991; Bechara et al., 1994; Duncan et al., 1995, 1996; Burgess and Shallice, 1996; Isingrini and Vazou, 1997; Parkin and Java, 1999; Blair and Cipolotti, 2000; Kane and Engle, 2002; Bugg et al., 2006; Tranel et al., 2008; Gläscher et al., 2009, 2010; Roca et al., 2010) and executive function (Ptito et al., 1995; D'Esposito and Postle,1999; Muller et al., 2002; Baldo and Dronkers, 2006; D'Esposito et al., 2006; Volle et al., 2008; Tsuchida and Fellows, 2009), all share one or more of the following features: diffuse (rather than focal) brain lesions, lack of comparison subjects carefully matched for pre- and post-injury performance measures, and exclusive use of general intelligence or executive function tests. As a consequence, there has been no comprehensive evaluation of psychometric g and executive function in a relatively large sample of patients with focal brain damage, and across a broad range of tasks and stimulus material. The absence of such data represents a substantial gap in the understanding of both the cognitive and neural architecture of high-level cognition.
The aim of the present investigation is therefore to characterize key competencies of general intelligence and executive function in a large sample of patients with focal brain lesions (n = 182). Studying their functional organization can help shed light on how general intelligence and executive function contribute to information processing, and set the stage for future research exploring how their cognitive and neural architecture emerges through evolution and development, and is altered through psychiatric illness and neurological disease.
Materials and methods
Participant data
Participants were drawn from the Phase 3 Vietnam Head Injury Study registry, which includes American male veterans who suffered brain damage from penetrating head injuries in the Vietnam War (n = 182). All subjects gave informed written consent. Phase 3 testing occurred between April 2003 and November 2006. Demographic and background data for the Vietnam Head Injury Study are reported in Table 1 (Koenigs et al., 2009; Raymont et al., 2010; Barbey et al., 2011a, b). No effects on test performance were observed in the Vietnam Head Injury Study sample on the basis of demographic variables (e.g. age, years of education, lesion size).
Table 1.
Demographic and background data
| Demographic data | Patient group |
|---|---|
| Age | 58.13 |
| Sex (male) (%) | 100.00 |
| Years of education | 15.00 |
| Total per cent volume loss (cm3) | 3.19 |
‘Age’ refers to age at the time of Phase 3 evaluation. ‘Sex’ refers to the percentage of male veterans. ‘Years of education’ refers to the total number of years of education the veterans completed.
Lesion analysis
CT data were acquired during the Phase 3 testing period. Axial CT scans without contrast were acquired at Bethesda Naval Hospital on a GE Medical Systems Light Speed Plus CT scanner in helical mode (150 slices per subject, field of view covering head only). Images were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, overlapping slice thickness of 2.5 mm and a 1-mm slice interval. Lesion location and volume were determined from CT images using the Analysis of Brain Lesion software (Makale et al., 2002; Solomon et al., 2007) contained in MEDx v3.44 (Medical Numerics) with enhancements to support the Automated Anatomical Labelling atlas (Tzourio-Mazoyer et al., 2002). Lesion volume was calculated by manual tracing of the lesion in all relevant slices of the CT image then summing the traced areas and multiplying by slice thickness. A trained neuropsychiatrist performed the manual tracing, which was then reviewed by J.G., who was blind to the results of the neuropsychological testing. As part of this process, the CT image of each subject's brain was spatially normalized to a CT template brain image. This template was created by spatial normalization of a neurologically healthy individual's CT brain scan to MNI space using the Automated Image Registration program (Woods et al., 1993). For each subject, a lesion mask image in MNI space was saved for voxel-based lesion–symptom mapping (see below; Bates et al., 2003). This method applies a t-test to compare, for each voxel, scores from patients with a lesion at that voxel contrasted against those without a lesion at that voxel. The reported findings were thresholded using a False Discovery Rate correction of q < 0.05. To ensure sufficient statistical power for detecting a lesion-deficit correlation, our analysis only included voxels for which four or more patients had a lesion. The lesion overlap map for the entire Vietnam Head Injury Study patient sample is illustrated in Supplementary Fig. 1 (n = 182). It is important to emphasize that conclusions drawn from the Vietnam Head Injury Study sample are restricted to the voxel space identified in Supplementary Fig. 1, which provides broad coverage of the cerebral hemispheres but does not include subcortical brain structures.
Neuropsychological tests
We administered the Wechsler Adult Intelligence Scale, 3rd Edition (WAIS; Wechsler, 1997) and subtests of the Delis–Kaplan Executive Function System (D–KEFS; Delis et al., 2001) to investigate the neural substrates underlying key competencies of general intelligence and executive function.
Wechsler Adult Intelligence Scale
The WAIS embodies a four-tier hierarchy, providing a Full Scale Intelligence Index (Tier 1) derived from Verbal and Performance Intelligence Indices (Tier 2), which each consist of component operations (Tier 3) measured by intelligence subtests (Tier 4). Verbal Intelligence examines general knowledge, vocabulary and the ability to reason using words and numbers and is assessed by Verbal Comprehension and Working Memory subtests. Performance Intelligence examines the ability to solve problems in novel situations, independent of acquired knowledge and is assessed by Perceptual Organization and Processing Speed subtests. Supplementary Table 1 provides a brief description of each test (for further details concerning their standardization, reliability and validity, see Wechsler, 1997).
Delis–Kaplan executive function system
The D–KEFS consists of multiple cognitive tests that are designed to assess executive control processes. Our analysis focused on five executive function measures that, in recent studies, have been found to be particularly sensitive to frontal lobe damage (Delis et al., 1992; Baldo et al., 2001; Cato et al., 2004; McDonald et al., 2005a, b, c). These tests include Trail Making, Verbal Fluency, Card Sorting, Twenty Questions and the Tower Test. Supplementary Table 2 provides a brief description of each test. Further details about these measures are provided in the D–KEFS manual (Delis et al., 2001).
Statistical analysis
Psychometricians have shown that intelligence tests can be classified according to the degree to which they involve the general factor of intelligence (g), computing robust and stable g-loadings based on the simultaneous consideration of several diverse intelligence measures (Carroll, 1993, 2003; Jensen and Weng, 1994; Hunt, 2011). Accumulating evidence further indicates that g-scores derived from multiple cognitive batteries are highly correlated, supporting the presence of g (as a common source of variance in cognitive performance) and demonstrating that ‘its measurement is not dependent on the use of specific mental ability tasks’ (Johnson et al., 2004).
Here, we present two types of analyses. The first applies voxel-based lesion–symptom mapping to standardized scores computed from the WAIS and D–KEFS to investigate the neural substrates of general intelligence (g) and executive function (cf. Colom et al., 2009; Glascher et al., 2009, 2010; Karama et al., 2011). The second explores and evaluates, for illustrative purposes, the factor structure underlying performance on the administered tests of general intelligence (g) and executive function (cf. Carroll, 1993, 2003; Colom et al., 2010; Colom and Thompson, 2011).
Voxel-based lesion-symptom mapping
Psychometric g and executive function scores were derived according to the following computational steps (Colom et al., 2009; Haier et al., 2009; Karama et al., 2011). First, g-scores for each participant were obtained using the first unrotated principal factor derived from the WAIS subtests. Principal axis factoring was the applied extraction method because only common variance is analysed. Participants’ scores on the first unrotated factor were computed using the regression method weighting raw scores according to the corresponding factor loadings. This method is computationally simple and generates scores that are almost perfectly correlated with g-scores derived from much more demanding computational approaches (Glascher et al., 2010) as demonstrated by Jensen and Weng (1994). Secondly, the same computational steps described for general intelligence (g) were followed for obtaining standardized executive scores. Psychometric g and executive scores were correlated with a value of 0.871 (P < 0.0001).
It is important to note that general intelligence (g) and executive function scores share 76% of the variance, but they also show 24% of the unique variance, thus leaving room for finding specific brain correlates for each psychological construct. These g and executive function scores for each subject were correlated to regional grey and white matter determined by voxel-based lesion–symptom mapping (Bates et al., 2003). This method compares, for every voxel, scores from patients with a lesion at that voxel contrasted against those without a lesion at that voxel (applying a false discovery rate correction of q < 0.05). Unlike functional neuroimaging studies, which rely on the metabolic demands of grey matter and provide a purely correlational association between brain regions and cognitive processes, voxel-based lesion–symptom mapping can identify regions playing a causal role in a particular cognitive domain by mapping where damage can interfere with performance.
Factor analysis and structural equation modelling
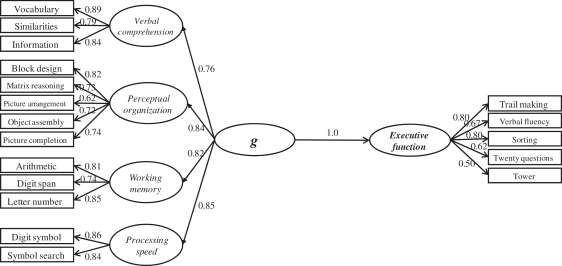
To assess the factor structure underlying performance on tests of general intelligence and executive function, we applied a Schmid–Leiman hierarchical factor analysis to all WAIS and D–KEFS measures (Schmid and Leiman, 1957; Carroll, 1993, 2003; Colom et al., 2006c). First-order factors were extracted from the correlation matrix defined by all measures (Supplementary Table 3). The three factors with eigenvalues >1 were retained for further analyses. Afterwards, the correlation matrix among these first-order factors was subjected to a new factor analysis. Principal axis factoring was employed to extract factors, because principal axis factoring analyses common variance only (removing error variance). Factor extraction was followed by an oblique Promax rotation. Table 2 reports the complete hierarchical factor matrix obtained from the analysis of WAIS and D–KEFS measures. Note that the higher order factor accounts for 36.3% of the common variance, whereas the three first-order factors account for much less common variance (from 5.5% to 8.3%). This is highly consistent with previous reports studying neurologically healthy samples (Johnson et al., 2004). As underscored by Colom and Thompson (2011) even when there is a positive and significant correlation among all these cognitive measures (Supplementary Table 3), this correlation is far from perfect. Table 2 also shows commonality (h2) and uniqueness (u2) values computed from the factor loadings. Although 57.4% of the common variance is accounted for by the obtained factors, the remaining variance is not. Importantly, the average loading for the WAIS measures on the higher order factor was 0.60, and the average loading for the D–KEFS measures on this same factor was 0.59, which is consistent with their equivalent psychometric behaviour on the factor representing the shared common variance (see Colom et al., 2006c for examples of re-analyses of several data sets using this hierarchical approach). The psychometric structure observed in the Vietnam Head Injury Study patient sample (Fig. 1) is similar to that of the WAIS standardization described by Taub (2001), providing evidence to further validate our assessment of psychometric g in this neuropsychological patient sample.
Table 2.
Schmid–Leiman hierarchical factor matrix of WAIS and D–KEFS measures
| WAIS and D-KEFS measures | Higher order factor | F1 | F2 | F3 | h2 | u2 |
|---|---|---|---|---|---|---|
| Vocabulary | 0.633 | 0.042 | 0.614 | 0.080 | 0.786 | 0.214 |
| Similarities | 0.597 | 0.203 | 0.410 | 0.068 | 0.570 | 0.430 |
| Information | 0.606 | 0.194 | 0.492 | 0.130 | 0.664 | 0.336 |
| Block design | 0.643 | 0.484 | 0.023 | 0.078 | 0.654 | 0.346 |
| Matrix reasoning | 0.602 | 0.413 | 0.135 | 0.002 | 0.551 | 0.449 |
| Picture arrangement | 0.493 | 0.397 | 0.060 | 0.004 | 0.404 | 0.596 |
| Object assembly | 0.538 | 0.473 | 0.042 | 0.060 | 0.519 | 0.481 |
| Picture completion | 0.581 | 0.393 | 0.086 | 0.216 | 0.546 | 0.454 |
| Arithmetic | 0.673 | 0.057 | 0.377 | 0.170 | 0.627 | 0.373 |
| Digit span | 0.526 | 0.113 | 0.390 | 0.191 | 0.478 | 0.522 |
| Letter number | 0.620 | 0.195 | 0.421 | 0.322 | 0.703 | 0.297 |
| Digit symbol | 0.659 | 0.182 | 0.069 | 0.468 | 0.691 | 0.309 |
| Symbol search | 0.638 | 0.211 | 0.071 | 0.425 | 0.637 | 0.363 |
| Trail making | 0.708 | 0.182 | 0.028 | 0.419 | 0.711 | 0.289 |
| Fluency | 0.592 | 0.099 | 0.294 | 0.328 | 0.554 | 0.446 |
| Sorting | 0.703 | 0.257 | 0.247 | 0.131 | 0.638 | 0.362 |
| Twenty questions | 0.526 | 0.127 | 0.162 | 0.182 | 0.352 | 0.648 |
| Tower test | 0.436 | 0.215 | 0.104 | 0.076 | 0.253 | 0.747 |
| Percentage of common variance | 36.3 | 7.3 | 8.3 | 5.5 | 57.4 | 42.6 |
F1, F2 and F3 represent first-order factors. h2 = commonality; u2 = uniqueness.
Figure 1.
SEM analysis of the administered WAIS and D–KEFS measures.
It is important to keep in mind that the factor structure obtained here is derived from the analysis of a clinical sample, raising the question of whether it resembles the obtained factor structure found for non-clinical samples. The available evidence supports the view that there is a close similarity. The factor structure of the Wechsler scales has been examined within clinical samples (Piedmont et al., 1992; Allen et al., 1998; Dickinson et al., 2006) including Alzheimer's disease (David et al., 2003), and lesion samples resembling those analysed here (Gläscher et al., 2010). The generalized finding shows that this factor structure is largely invariant, although some minor differences may emerge in the specific factor loadings for some measures. Dickinson et al. (2006) find ‘support for a hierarchical structure of cognition, similar in schizophrenia and control subjects, with a generalized cognitive ability factor strongly influencing a number of separable domains of cognitive performance’. Gläscher et al. (2010) demonstrated that the factor structure of the Wechsler battery is clearly preserved in their lesion patients. The same general picture is supported in the present study.
To further examine the simultaneous latent relationship between general intelligence (g) and executive measures, we conducted an SEM analysis (n = 182; Fig. 1). In this latent variable approach, specific task requirements have less influence on the estimates of the construct relations. It also partials out measurement error for each specific measure, and, therefore, latent variables provide a reliable estimate of the constructs of interest. Here, we computed SEM using the AMOS program (Arbuckle, 2006). Several fit indices were considered. First, the χ2/DF index is frequently considered as a rule of thumb, because it corrects the high sensitivity of the chi-square statistic for large sample sizes (Jöreskog, 1993). Values showing a good fit must be ∼2.0. Secondly, root mean square error of approximation (RMSEA) is usually recommended because it is sensitive to misspecification of the model. Values between 0 and 0.05 indicate good fit, values between 0.05 and 0.08 represent acceptable errors and values >0.10 are indicative of poor fit (Byrne, 1998). Finally, comparative fit index is also reported; acceptable values must be larger than 0.90 (Marsh et al., 1988). The fit for this model was appropriate: χ2 (131) = 283; CMIN/DF = 2.16; RMSEA = 0.08, comparative fit index = 0.921. Results indicate that within this model, psychometric variation in executive function is entirely explained by g, which is highly consistent with the correlation of 0.87 reported above between g and executive scores submitted to lesion analyses. This suggests that both psychological constructs depend largely on common cognitive operations and raises the intriguing possibility that these high-level processes may also recruit common neural machinery.
Results
General intelligence
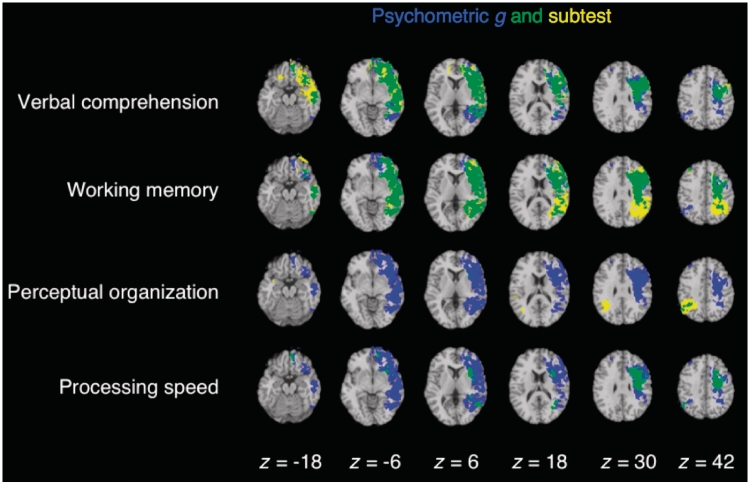
Psychometric g was associated with a distributed network of brain regions, sharing common anatomical substrates with Verbal Comprehension, Working Memory, Perceptual Organization and Processing Speed (Fig. 2). Significant effects encompassed locations for (i) language processing (e.g. Broca's area and left superior temporal gyrus); (ii) working memory (e.g. left dorsolateral prefrontal cortex, left inferior and superior parietal cortex, and left superior temporal gyrus); (iii) spatial processing (e.g. right inferior and superior parietal cortex); (iv) motor processing (e.g. left somatosensory and primary motor cortex), in addition to expected locations of major white matter fibre tracts; including (v) the anterior and dorsal bundle of the superior longitudinal/arcuate fasciculus connecting temporal, parietal and inferior frontal regions; (vi) the superior fronto-occipital fasciculus connecting dorsolateral prefrontal cortex and the frontal pole with the superior parietal lobule; and (vii) the uncinate fasciculus that connects anterior temporal cortex and amygdala with orbitofrontal and frontopolar regions. These findings suggest that g reflects the ability to effectively integrate verbal, spatial, motor and executive processes via a circumscribed set of cortical connections.
Figure 2.
Overlap (in green) of individual WAIS subtests (in yellow) with g (in blue). Each statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader's left.
Executive function
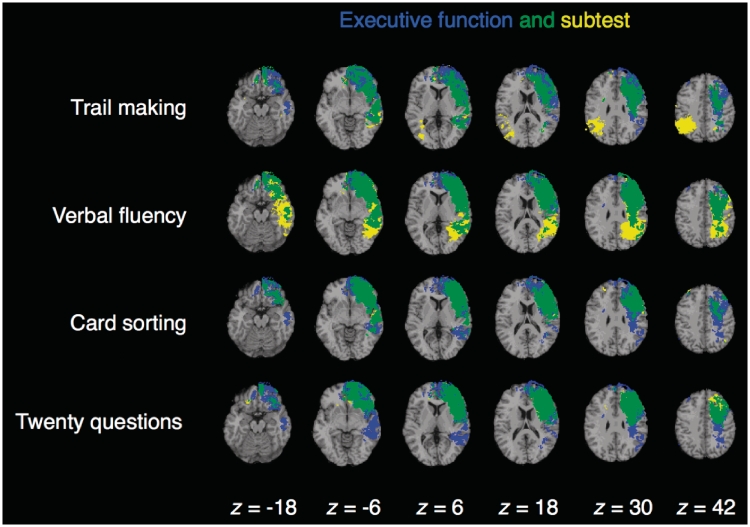
Executive function deficits were associated with damage to a distributed network of left lateralized brain areas, including regions that are necessary for executive control processes in the Trail Making, Verbal Fluency, Card Sorting and Twenty Questions tests (Fig. 3). The Tower Test failed to demonstrate significant lesion–symptom mapping results, consistent with the relatively low loading of this subtest on the higher order factor (0.44; Table 2). Prior research supports this interpretation, indicating that subtests with low g-loadings are associated with a smaller number of brain clusters (relative to subtests with high g-loadings; Colom et al., 2006a, b). Significant effects for the Trail Making, Verbal Fluency, Card Sorting and Twenty Questions tests encompassed major locations within the frontal lobe (e.g. lateral frontopolar cortex, anterior prefrontal cortex, dorsolateral prefrontal cortex, anterior cingulate/medial prefrontal cortex) and superior and inferior parietal cortex, in addition to white matter association tracts that connect these sectors (e.g. superior longitudinal/arcuate fasciculus, superior fronto-occipital fasciculus and uncinate fasciculus). These findings suggest that the frontal and parietal lobes are computationally necessary for executive control processes, and that the communication between these regions is of critical importance.
Figure 3.
Overlap (in green) of individual D–KEFS subtests (in yellow) with executive function (in blue). Each statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader's left.
Relationship between general intelligence and executive function
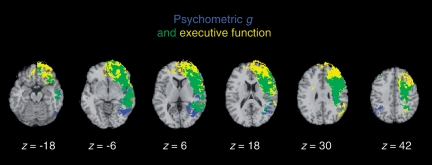
These lesion–symptom mapping results show that psychometric g and executive function are associated with lesions to a shared network of left lateralized brain areas, including regions of frontal and parietal cortex and white matter fibre tracts that bind these areas into a coordinated system (Fig. 4). This pattern of findings indicates that psychometric g and executive function largely depend on shared neural substrates, and suggests that high-level cognitive processes fundamentally depend on the interregional communication between frontal and parietal cortex.
Figure 4.
Overlap (in green) of psychometric g (in blue) with executive function (in yellow). Each statistical map is thresholded at 5% false discovery rate. In each axial slice, the right hemisphere is on the reader's left.
Although the neural substrates of psychometric g and executive function were largely shared, our analysis revealed areas that were related to psychometric g, and which may not be involved with executive function, consistent with the observed pattern of shared and unique variance in the behavioural data (Table 2). These regions included the left inferior occipital gyrus and the right superior and inferior parietal lobe (Fig. 4). The engagement of visual–spatial processing areas in psychometric g further emphasizes the broadly distributed cortical regions underlying general intelligence—extending well beyond prefrontal cortex regions typically associated with goal-directed, intelligent behaviour. We also observed areas that were related to executive function, but may not be involved with psychometric g, within the left anterior frontal pole, consistent with the functional selectivity of anterior prefrontal cortex regions in the executive control of behaviour (Fig. 4; Ramnani and Owen, 2004).
Discussion
In this study, we investigated the neural architecture of high-level cognition, examining the neural substrates of general intelligence (g) and executive function. We measured psychometric g and executive function in 182 patients with brain injury and applied voxel-based lesion–symptom mapping to identify the underlying neural substrates. Although the Vietnam Head Injury Study patient sample provided extensive brain coverage in both cerebral hemispheres (Supplementary Fig. 1), it did not include patients with damage to subcortical structures and therefore does not permit conclusions about the involvement of these brain regions.
We observed a significant effect on g and executive function with lesions in left hemispheric white matter sectors, including the superior longitudinal/arcuate fasciculus, which connect frontal and parietal cortices. Despite its distributed nature, the neural substrates of g and executive function were remarkably circumscribed, concentrated in the core of white matter and comprising a narrow subset of regions associated with performance on individual WAIS and D–KEFS subtests. The largest overlap between WAIS subtests and g was found for Verbal Comprehension and Working Memory, and for executive function measures of the D–KEFS (i.e. Trail Making, Verbal Fluency, Card Sorting and Twenty Questions). Collectively, these subtests assess verbal knowledge about the world, verbal reasoning, working memory capacity, as well as cognitive flexibility and executive control, and are associated with a distributed fronto-parietal network. This suggests that g and executive function draw on the combination of conceptual knowledge and executive processes, and that the communication between areas associated with these capacities is of critical importance.
The observed findings contribute to a growing body of neuropsychological patient evidence indicating that damage to a distributed network of frontal and parietal regions is associated with impaired performance on tests of general intelligence (Jung and Haier, 2007; Chiang et al., 2009; Colom et al., 2009; Gläscher et al., 2009, 2010; Colom and Thompson, 2011). A recent study by Gläscher et al. (2010) applied voxel-based lesion–symptom mapping to elucidate the neural substrates of psychometric g, reporting a left lateralized fronto-parietal network that converges with the observed pattern of findings and further supports the role of this network in components of general intelligence that draw upon conceptual knowledge and working memory. Our findings advance this line of research by elucidating the relationship between general intelligence and executive function—demonstrating that these domains recruit a highly overlapping and broadly distributed network of frontal and parietal regions (Fig. 4).
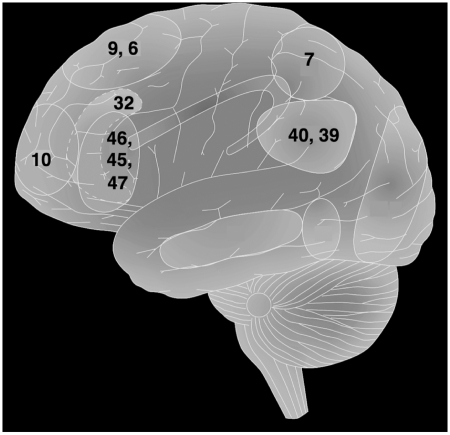
The fronto-parietal network identified by the present analysis includes lateral frontopolar cortex, anterior prefrontal cortex, dorsolateral prefrontal cortex, anterior cingulate/medial prefrontal cortex and the inferior and superior parietal lobe (Fig. 5). This constellation of regions is commonly engaged by tasks that require executive control processes (Botvinick et al., 2004; Gruber and Goshke 2004; Ramnani and Owen 2004; Dosenbach et al., 2007). The fronto-parietal network is recruited by paradigms that elicit controlled processing related to the simultaneous consideration of multiple interdependent contingencies (Kroger et al., 2002), conflicting stimulus–response mappings (Crone et al., 2006), and integrating working memory with attentional resource allocation (Koechlin et al., 1999). In addition, many of the regions in the fronto-parietal network show sustained activity over the duration of a task block (Velanova et al., 2003; Yarkoni et al., 2005; Dosenbach et al., 2006), supporting the maintenance and integration of items for goal-directed behaviour.
Figure 5.
An integrative architecture for general intelligence and executive function. Illustration of Brodmann areas (numbers on figure) involved in general intelligence and executive function, as well as the arcuate fasciculus and the major white matter association tract that connects the involved brain regions.
The observed pattern of findings is consistent with the proposal that the fronto-parietal network provides a unified architecture for the integration and control of cognitive representations. According to this framework, processes for integration and control are critical for the optimal recruitment of internal resources to exhibit goal-directed behaviour—supporting conceptual representations and executive processes that provide the basis for high-level cognition. We propose that mechanisms for integration and control are carried out by a central system, which has extensive access to sensory and motor representations (cf. Miller and Cohen, 2001) and that the fronto-parietal network is at an ideal site in the brain to support these functions. Nodes of this network are thoroughly and reciprocally connected with each other, as well as with other association cortices and subcortical areas, a property that allows widespread access to perceptual and motor representations at multiple levels. With this unique connectivity pattern, and specialization in a wide variety of high-level processes, the fronto-parietal network can function as a source of integration and top-down control in the brain. This framework, therefore, complements existing neuroscience models by highlighting the importance of white matter association tracts (e.g. the arcuate fasciculus) for the integration of cognitive representations in general intelligence (Jung and Haier, 2007), while also emphasizing the central role of top-down mechanisms within frontal and parietal cortices for the executive control of behaviour (Miller and Cohen, 2001). According to this framework, the fronto-parietal network is a core system that supports the integration and control of distributed patterns of neural activity throughout the brain, providing a unified architecture for general intelligence and executive function (Fig. 5).
In addition to identifying fronto-parietal contributions to general intelligence and executive function, our analysis uncovered areas that responded to each high-level domain (Fig. 4), without evidence of response to the other. Psychometric g was associated with lesions of the left inferior occipital gyrus and the right superior and inferior parietal lobe, areas that are widely implicated in visual–spatial processing (Zeki, 1993) and support the involvement of cortical regions for sensory processing in general intelligence. The analysis further revealed that the left anterior frontal pole was related to executive function, consistent with accumulating evidence to support the functional selectivity of anterior prefrontal cortex regions in executive control (Fig. 4; Ramnani and Owen, 2004). The observed pattern of association with common and potentially distinct brain regions provides key evidence for the neural architecture of general intelligence and executive function, identifying the shared neural circuitry that supports high-level cognition while also identifying regions recruited by each domain, which may not be involved in the other.
The importance of the fronto-parietal network in high-level cognition is further supported by recent evidence to suggest that this network represents a central feature in the evolution of the human brain. Previous cytoarchitectonic and histological studies have shown that the prefrontal cortex, particularly Brodmann area 10, is greatly expanded in both absolute size and percentage of whole-brain volume in humans relative to macaques and apes (Semendeferi et al., 2001). Van Essen and Dierker (2007) applied anatomical MRI to generate a map of estimated cortical expansion between macaque and human, identifying the anterior lateral prefrontal cortex and anterior inferior parietal lobule as regions of greatest cortical expansion in humans. Collectively, these studies indicate that the human fronto-parietal network may be especially important in the evolution of the human brain and the development of key competencies for general intelligence and executive control.
From a clinical perspective, understanding general intelligence and executive control deficits in patients with fronto-parietal damage may greatly facilitate the design of appropriate assessment tools and rehabilitation strategies, with potential improvement in patients’ cognitive abilities and daily living. Our findings identify specific tests of the WAIS and D–KEFS that may be targeted in clinical investigations to assess the functioning of the fronto-parietal network, particularly, Verbal Comprehension and Working Memory subtests of the WAIS and Twenty Questions, Trail Making, Verbal Fluency and Card Sorting subtests of the D–KEFS. The observed findings elucidate brain structures that are engaged by both general intelligence and executive function, as well as identifying some regions involved in one that may not be recruited by the other. These findings support predictions about the nature and significance of cognitive impairments that may result from damage to specific brain regions (Fig. 4).
It is important to emphasize in closing that the abilities measured by the WAIS and D–KEFS do not provide a comprehensive assessment of all human cognitive abilities. There are other aspects of human cognitive ability in addition to those capacities measured by the WAIS and D–KEFS, which contribute to mental life, notably those related to social and emotional functioning (Barbey et al., 2009a; Krueger et al., 2009). Understanding the neural architecture of general intelligence and executive function will ultimately require a broader assessment that examines the functional organization of cognitive, social and affective systems and their interactive role in high-level processes. The reported findings contribute to this emerging research programme by elucidating the role of the fronto-parietal network in psychometric g and executive function, demonstrating that this system provides an integrative neural architecture for key competencies of human intelligence.
Funding
U.S. National Institute of Neurological Disorders and Stroke intramural research programme; United States Army Medical Research (project grant); Material Command administered by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: a 30-year post-injury follow-up study, grant number DAMD17-01-1-0675); Ministerio de Ciencia e Innovación [Ministry of Science and Innovation, Spain] (grant PSI2010-20364 to R.C.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We are grateful to S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, V. Raymont, K. Reding and G. Tasick for their invaluable help with the testing of participants and organization of this study.
Glossary
Abbreviations
- D–KEFS
Delis–Kaplan Executive Function System
- WAIS
Wechsler Adult Intelligence Scale
References
- Allen DN, Huegel SG, Seaton BE, Goldstein G, Gurklis JA, van Kammen DP. Confirmatory factor analysis of the WAIS–R in patients with schizophrenia. Schizophr Res. 1998;34:87–94. doi: 10.1016/s0920-9964(98)00090-5. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL. Chicago: SPSS; 2006. Amos (Version 7.0) [Computer Program] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:529–38. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Intl Neuropsychol Soc. 2001;7:586–96. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Grafman J. The prefrontal cortex and goal-directed social behavior. In: Decety J, Cacioppo J, editors. The handbook of social neuroscience. New York: Oxford University Press; 2012. [Google Scholar]
- Barbey AK, Grafman J. An integrative cognitive neuroscience theory for social reasoning and moral judgment. Wiley Interdiscip Rev Cogn Sci. 2011;2:55–67. doi: 10.1002/wcs.84. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Orbitofrontal contributions to human working memory. Cerebral Cortex. 2011a;21:789–95. doi: 10.1093/cercor/bhq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. Architecture of counterfactual thought in the prefrontal cortex. In: Bar M, editor. Predictions in the brain. New York: Oxford University Press Inc; 2011b. pp. 40–57. [Google Scholar]
- Barbey AK, Barsalou LW. Models of reasoning and problem solving. In: Squire L, editor. Encyclopedia of neuroscience, Vol. 8. Oxford: Academic Press; 2009. pp. 35–43. [Google Scholar]
- Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social reasoning. Trends Neurosci. 2009a;32:603–10. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. Structured event complexes in the prefrontal cortex support counterfactual representations for future planning. Phil Trans R Soc Lond Biol Sci. 2009b;364:1291–300. doi: 10.1098/rstb.2008.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Patterson R. Architecture of explanatory inference in the human prefrontal cortex. Front Psychol. 2011;2:162. doi: 10.3389/fpsyg.2011.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Sloman SA. Base-rate respect: from ecological rationality to dual processes. Behav Brain Sci. 2007;30:241–54. doi: 10.1017/S0140525X07001653. [DOI] [PubMed] [Google Scholar]
- Bartholomew DJ, Deary IJ, Lawn M. A new lease of life for Thomson's bonds model of intelligence. Psychol Rev. 2009;116:567–79. doi: 10.1037/a0016262. [DOI] [PubMed] [Google Scholar]
- Basso A, De Renzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain. 1973;96:715–28. doi: 10.1093/brain/96.4.715. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Fossella J, Croucher CJ, Duncan J. COMT val158met genotype affects neural mechanisms supporting fluid intelligence. Cerebral Cortex. 2008;18:2132–40. doi: 10.1093/cercor/bhm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black FW. Cognitive deficits in patients with unilateral war-related frontal lobe lesions. J Clin Psychol. 1976;32:366–72. doi: 10.1002/1097-4679(197604)32:2<366::aid-jclp2270320234>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal: a case of “acquired sociopathy”. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Zook NA, DeLosh EL, Davalos DB, Davis HP. Age differences in fluid intelligence: Contributions of general slowing and frontal decline. Brain Cogn. 2006;62:9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–72. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Mahwah, NJ: Erlbaum; 1998. Structural equation modelling with LISREL, PRELIS, and SIMPLIS: basic concepts, applications, and programming. [Google Scholar]
- Carroll JB. Cambridge: Cambridge University Press; 1993. Human cognitive abilities. [Google Scholar]
- Carroll JB. The higher-stratum structure of cognitive abilities: current evidence supports g and about ten broad factors. In: Nyborg H, editor. The scientific study of general intelligence: tribute to Arthur R. Jensen. Oxford: Elsevier/Pergamon; 2003. pp. 5–21. [Google Scholar]
- Cato MA, Delis DC, Abildskov TJ, Bigler E. Assessing the elusive cognitive deficits associated with ventromedial prefrontal damage: a case of a modern-day Phineas Cage. J Int Neuropsychol Soc. 2004;10:453–65. doi: 10.1017/S1355617704103123. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Leow AD, Klunder AD, Dutton RA, Barysheva M, Rose SE, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–24. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. Neuroimage. 2006a;31:1359–65. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ. Finding the g-factor in brain structure using the method of correlated vectors. Intelligence. 2006b;34:561–70. [Google Scholar]
- Colom R, Jung RE, Haier RJ. General Intelligence and Memory Span: evidence for a common neuro-anatomic framework. Cogn Neuropsychol. 2007;24:867–78. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- Colom R, Haier RJ, Head K, Álvarez-Linera J, Quiroga M, Shih PC, et al. Gray matter correlates of fluid, crystallized, and spatial intelligence: testing the P-FIT model. Intelligence. 2009;37:124–35. [Google Scholar]
- Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues Clin Neurosci. 2010;12:489–501. doi: 10.31887/DCNS.2010.12.4/rcolom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Rebollo I, Abad FJ, Shih PC. Complex Span Tasks, Simple Span Tasks, and Cognitive Abilities: A Re-Analysis of Key Studies. Mem Cogn. 2006c;34:158–71. doi: 10.3758/bf03193395. [DOI] [PubMed] [Google Scholar]
- Colom R, Thompson PM. Understanding human intelligence by imaging the brain. In: Chamorro-Premuzic T, von Stumm S, Furnham A, editors. The Wiley-Blackwell handbook of individual differences. London: Wiley-Blackwell; 2011. pp. 330–52. [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–86. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Davis RN, Massman PJ, Doody RS. WAIS–R factor structure in Alzheimer's disease patients: a comparison of alternative models and an assessment of their generalizability. Psychol Aging. 2003;4:836–43. doi: 10.1037/0882-7974.18.4.836. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:89–101. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SEB, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and fMRI data. J Int Neuropsychol Soc. 2006;12:248–60. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–11. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. San Antonio TX: The Psychological Corporation; 2001. Delis Kaplan Executive Function System. [Google Scholar]
- Delis DC, Squire LR, Bihrle AM, Massman PJ. Componential analysis of problem-solving ability: performance of patients with frontal lobe damage and amnesic patients on a new sorting test. Neuropsychologia. 1992;30:683–97. doi: 10.1016/0028-3932(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Calkins E, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr Res. 2006;85:20–9. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligence behaviour. Trends Cogn Sci. 2010;14:172–9. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J. Brain mechanisms of attention. Q J Exp Psychol. 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–8. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, et al. A neural basis for general intelligence. Science. 2000;289:457–60. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bifrontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122:963–79. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, et al. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–91. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, et al. Distributed neural systems for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA. 2010;107:4705–9. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber O, Goschke T. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychol. 2004;115:105–21. doi: 10.1016/j.actpsy.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Colom R, Schroeder D, Condon C, Tang C, Eaves E, et al. Gray matter and intelligence factors: is there a neuro-g? Intelligence. 2009;37:136–44. [Google Scholar]
- Hunt EB. Cambridge: Cambridge University Press; 2011. Human intelligence. [Google Scholar]
- Isingrini M, Vazou F. Relation between fluid intelligence and frontal lobe in older adults. Int J Aging Human Dev. 1997;45:99–109. doi: 10.2190/WHWX-YNVB-079V-2L74. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: the science of mental ability . New York: Praeger; 1998. [Google Scholar]
- Jensen A, Weng L. What is a good g? Intelligence. 1994;18:231–58. [Google Scholar]
- Johnson W, Bouchard TJ, Krueger RF, McGue M, Gottesman II. Just one g: consistent results from three test batteries. Intelligence. 2004;32:95–107. [Google Scholar]
- Jöreskog KG. Testing structural equation models. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Beh Brain Sci. 2007;30:135–54. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier RJ, Waber DP, et al. The Brain Development Cooperative Group. Cortical thickness correlates of cognitive performance accounted for by the general factor of intelligence in health children aged 6 to 18. NeuroImage. 2011;55:1443–53. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Acheson D, Barbey AK, Solomon J, Postle BR, Grafman J. Areas of left perisylvian cortex mediate auditory-verbal short-term memory. Neuropsychologia. 2011;49:3612–9. doi: 10.1016/j.neuropsychologia.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle B, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;47:14980–6. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–85. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, McCabe K, Strenziok M, Zamboni G, Solomon J, et al. The neural bases of key competencies of emotional intelligence: Brain lesion evidence. Proc Natl Acad Sci USA. 2009;106:22486–91. doi: 10.1073/pnas.0912568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav Res Meth Ins C. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Marsh HW, Balla JR, McDonald RP. Goodness of fit indexes in confirmatory factor analysis: the effect of sample size. Psychol Bull. 1988;103:391–410. [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui VJ. Discriminating patients with frontal lobe epilepsy and temporal lobe epilepsy: utility of a multi-level design fluency test. Neuropsychology. 2005a;19:806–13. doi: 10.1037/0894-4105.19.6.806. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madozi VI. Is impairment in set-shifting specific to frontal-lobe dysfunction? Evidence from patients with frontal-lobe or temporal-lobe epilepsy. J Int Neuropsychol Soc. 2005b;11:477–81. [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Wetter SR, Tecoma ES, Iragui VJ. Response inhibition and set-shifting in patients with frontal-lobe epilepsy or temporal-lobe epilepsy. Epilepsy Behav. 2005c;7:438–46. doi: 10.1016/j.yebeh.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Phelps EA. Current Opinion in Neurobiology–Cognitive Neuroscience 2010. Current Opinion in Neurobiology. 2010;20:141–2. doi: 10.1016/j.conb.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Muller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J Cogn Neurosci. 2002;14:673–86. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Java RI. Deterioration in frontal lobe function in normal aging: influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539–45. doi: 10.1037//0894-4105.13.4.539. [DOI] [PubMed] [Google Scholar]
- Piedmont RL, Sokolove RL, Fleming MZ. An evaluation of various WAIS–R factor structures in a psychiatric sample. J Clin Psychol. 1992;48:658–66. doi: 10.1002/1097-4679(199209)48:5<658::aid-jclp2270480513>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Ptito A, Crane J, Leonard G, Amsel R, Caramanos Z. Visual-spatial localization by patients with frontal lobe lesions invading or sparing area 46. Neuroreport. 1995;6:45–8. doi: 10.1097/00001756-199509000-00018. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, Grafman J. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010;75:224–9. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–47. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J, Leiman JM. The development of hierarchical factor solutions. Psychometrika. 1957;22:53–61. [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–41. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–41. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions ABLe. Comput Methods Programs Biomed. 2007;86:245–54. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. General intelligence, objectively determined and measured. Am J Psychol. 1904;15:201–93. [Google Scholar]
- Spearman C. The abilities of man: their nature and measurement . New York: Macmillan; 1927. [Google Scholar]
- Taub E. A confirmatory analysis of the Wechsler Adult Intelligence Scale third edition: Is the verbal/performance discrepancy justified? Pract Assess Res Eval. 201;7 [Google Scholar]
- Thomson GH. The factorial analysis of human ability. 5th edn. London: University of London Press; 1951. [Google Scholar]
- Tranel D, Manzel K, Anderson SW. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using matrix reasoning. Clin Neuropsychol. 2008;22:242–61. doi: 10.1080/13854040701218410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci. 2009;12:2263–75. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–25. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional–anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–70. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle E, Kinkingnehun S, Pochon J, Mondon K, de Schotten MT, Seassau M, et al. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cerebral Cortex. 2008;10:1093–103. doi: 10.1093/cercor/bhn010. [DOI] [PubMed] [Google Scholar]
- Wechsler D. San Antonio TX: The Psychological Corporation; 1997. Wechsler adult intelligence test administration and scoring manual. [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with au- tomated algorithm. J Comput Assist Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS. Sustained neural activity associated with cognitive control during temporally extended decision making. Brain Res Cogn Brain Res. 2005;23:71–84. doi: 10.1016/j.cogbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Zeki S. Cambridge, MA: Blackwell Scientific Publications. Inc.; 1993. A vision of the brain. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.