Abstract
Myocardial ischemia followed by reperfusion (I/R) induces irreversible damage to cardiac muscle. Medical treatment that effectively prevents I/R injury would alleviate the consequent development of cardiac remodeling and failure. Mechanisms that extend lifespan often make organisms resistant to stress, and an accumulation of such mechanisms may prevent aging and susceptibility to age-associated diseases. Sirtuins are a group of molecules involved in longevity and stress resistance. Stimulation of silent information regulator 1 (Sirt1), the mammalian ortholog of yeast Sir2 and a member of the sirtuin family, extends the lifespan of mice fed a high fat diet and retards aging in the heart. Recent evidence suggests that stimulation of Sirt1 mimics ischemic preconditioning and protects the heart from I/R injury, suggesting an intriguing possibility of using longevity factors to treat cardiac disease. Here, we discuss the cardioprotective effects of Sirt1 and possible underlying mechanisms.
Introduction
Myocardial ischemia/reperfusion (I/R) induces irreversible myocardial damage despite relieving the myocardial ischemia, which in turn leads to cardiac remodeling characterized by dilation of the left ventricle (LV) and reduced contractility. Despite recent advancement in thrombolysis and primary percutaneous coronary interventions, which contribute to better preservation of a viable myocardium in post-myocardial infarction (MI) patients, I/R injury remains the major cause of heart failure. The mortality rate of post-MI patients after one year remains over 20%. Minimizing I/R injury would dramatically attenuate cardiac remodeling and improve the prognosis of patients. Although many interventions alleviating the extent of myocardial injury in animal models of I/R have been tested in patients, thus far none of them have exhibited definitive advantages over the control, suggesting that a novel mechanism of intervention is needed (Yellon and Hausenloy 2007).
Elucidating the mechanisms mediating aging and controlling the lifespan of organisms is an important theme in modern biology, and some interventions and molecular mechanisms affecting the lifespan of animals have already been identified. One such intervention is caloric restriction (CR), which led to the extension of lifespan and/or the delayed onset of age-related pathologies in a rodent model (McCay et al. 1989). Several key intracellular signaling mechanisms critically regulate the lifespan of various organisms (Guarente 2011). For example, sirtuins play an important role in mediating lifespan extension induced by nutrient restriction, and stimulation of sirtuins is sufficient to prolong the lifespan of many organisms, including mice fed a high fat diet (Guarente 2011). Although the underlying mechanisms through which the reported interventions eventually affect the lifespan of the organisms remain to be clarified, one well-received hypothesis, termed the “hormesis” hypothesis, proposes that the longevity mechanisms confer stress resistance, the accumulation of which extends the lifespan. This raises a possibility that activation of longevity mechanisms may protect the heart from I/R injury as well.
Sirtuins are mammalian homologs of the silent information regulator 2 protein (Sir2p), which was initially identified in yeast. Sirtuins consist of a class of proteins that possess NAD+-dependent deacetylase and mono-ADP-ribosyltransferase activity. While some prokaryotes lack sirtuins, all eukaryotes encode several sirtuins in their genomes (Imai et al. 2000). Mammals have seven sirtuins (Sirt1-7) distributed in different subcellular compartments, including the nucleus (Sirt1, −2, −6, and −7), cytoplasm (Sirt1 and Sirt2) and mitochondria (Sirt3, −4, and −5) (Haigis and Guarente 2006). Sirt1 deacetylates many transcription factors, including p53, Forkhead box O (FoxO) transcription factors, peroxisome proliferator-activated receptor γ (PPARγ), liver X receptor (LXR), NF-κB and BMAL1, as well as nuclear coactivators, including PPARγ coactivator 1α (PGC1α), c-AMP responsive element binding protein regulated transcription coactivator 2 (CTCR2, also known as TORC2) and period homolog 2 (PER2). Sirt1 also deacetylates endothelial nitric oxide synthase (eNOS), serine/threonine kinase 11 (STK11, also known as LKB1), and histones H1, H3 and H4 (Haigis and Guarente 2006). Sirt1 plays a protective role against aging and age-related diseases, such as neurodegenerative disease, cardiovascular disease, osteoporosis, chronic kidney disease and metabolic syndrome (Guarente 2011). Sirtuins have drawn much of the public’s attention because their activity is stimulated by resveratrol, a component of red wine seeds (Howitz et al. 2003), and because sirtuins may mediate longevity (Lombard et al. 2011), although these points are somewhat controversial. We have shown recently that stimulation of Sirt1 retards aging and inhibits I/R injury in the heart (Hsu et al. 2010). This raises the possibility that enhancing the activity of a longevity factor may represent a novel modality of reducing myocardial injury associated with I/R. Here, we discuss the protective role of Sirt1 in the heart during I/R and the underlying mechanisms involved.
Regulation of Sirt1 expression and activity
Expression of Sirt1 is increased by stress, such as pressure overload and nutrient starvation, in the heart (Alcendor et al. 2007; Shinmura et al. 2008). Sirt1 is also upregulated in response to a locally acting mIGF-1 isoform (Vinciguerra et al. 2009), and after exercise (Ferrara et al. 2008) and acute ischemic preconditioning (IPC) (Hsu et al. 2010). However, Sirt1 is downregulated 24 hours after I/R (Hsu et al. 2010). The expression of Sirt1 is positively and negatively regulated by transcription factors and cofactors. E2F1 is a crucial activator (Wang et al. 2006), whereas p53, hypermethylated in cancer (HIC), and C-terminal-binding protein (CtBP), which forms a complex with HIC, are repressors (Chen et al. 2005; Zhang et al. 2007). FoxO3 induces Sirt1 expression through an interaction with p53 on the Sirt1 promoter region (Nemoto et al. 2004), and FoxO1 is recruited to the Sirt1 promoter region in a positive feedback manner (Xiong et al. 2011). Sirt1 is also recruited to both coactivator and corepressor complexes as a cofactor, regulating its own expression by deacetylating or interacting with certain transcription factors. Hu antigen R (HuR) binds and stabilizes Sirt1 mRNA, whereas Sirt1 mRNA is dissociated from HuR and degraded when HuR is phosphorylated in response to oxidative stress (Abdelmohsen et al. 2007). In addition, several microRNAs, such as miR-34a, miR-134, miR-195, miR-199a, miR-217 and others, have been reported to regulate Sirt1 expression (Gao et al. 2010; Menghini et al. 2009; Rane et al. 2009; Yamakuchi et al. 2008).
Sirt1 activity is also regulated by post-translational mechanisms, including post-translational modification and protein-protein interactions. Sirt1 is phosphorylated by the c-Jun N-terminal kinase 1 (JNK1) and cyclin B/Cdk1, to enhance its deacetylation activity (Nasrin et al. 2009; Sasaki et al. 2008). On the other hand, serine-specific protease-1 (SENP1) represses the deacetylation activity of Sirt1 by detaching small ubiquitin-related modifier 1 (SUMO1) (Yang et al. 2007). Active regulator of Sirt1 (AROS) and deleted in breast cancer 1 (DBC1), which bind to Sirt1, are positive and negative regulators of Sirt1, respectively (Kim et al. 2007; Kim et al. 2008; Zhao et al. 2008). Sirt1 has both nuclear localization signals and nuclear export signals, suggesting that nucleocytoplasmic shuttling may regulate the function of Sirt1 (Tanno et al. 2007). Some caspases suppress the activity of Sirt1 through cleavage and degradation (Ohsawa and Miura 2006), while Sirt1 is sumoylated during IPC, which enhances the activity of Sirt1 in the mouse heart (Nadtochiy et al. 2011a). Whether the function of Sirt1 is affected by other forms of post-translational modification and/or protein-protein interaction during I/R is not known.
An enzymatic reaction depends upon the concentration of its substrates and end products. The intracellular level of nicotinamide adenine dinucleotide (NAD+), which is a substrate of Sirt1, or the NAD+/NADH ratio, is increased by CR, fasting and exercise. NAD+ is essential for the deacetylase and mono-ADP-ribosyltransferase activity of Sirt1 (Imai et al. 2000). Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the transfer of a phosphoribosyl residue from phosphoribosyl pyrophosphate (PRPP) to nicotinamide, to produce nicotinamide mononucleotide (NMN), thereby serving as a rate-limiting enzyme in the mammalian NAD+ salvage pathway (Imai 2011). Interestingly, the protein and mRNA expression levels of Nampt are reduced by aging and some stresses, including transverse aortic constriction (TAC) and ischemia/reperfusion (I/R) in the heart (Hsu et al. 2009b). Overexpression of Nampt in the heart reduces infarct size and apoptosis in response to I/R (Hsu et al. 2009a). Although downregulation of Nampt impairs glucose tolerance in mice, administration of NMN partially restores insulin secretion from pancreatic β cells (Revollo et al. 2007). These results suggest that NMN may be given exogenously to compensate for decreased NAD+ and Sirt1 activity in the heart. Nampt is also regulated with the circadian rhythm by clock genes, including aryl hydrocarbon receptor nuclear translocator-like 1 (ARNTL1, also known as BMAL1) and PER2, via Sirt1-mediated deacetylation (Asher et al. 2008; Nakahata et al. 2008; Ramsey et al. 2009). It is possible that the circadian oscillation in Nampt expression may contribute to the known circadian oscillation in ischemic susceptibility in coronary artery disease patients, through regulation of NAD+ and Sirt1 (Krantz et al. 1996).
The mechanism mediating the protective effect of Sirt1 during I/R
Mild to moderate myocyte-specific overexpression of Sirt1 inhibits, whereas cardiac-specific downregulation of Sirt1 enhances, I/R injury by regulating transcription of cardioprotective and/or apoptotic genes (Hsu et al. 2010). Sirt1 also mediates the protective effect of first window IPC (Nadtochiy et al. 2011a; Nadtochiy et al. 2011b). IPC rapidly activates Sirt1 through sumoylation (Nadtochiy et al. 2011a), which in turn induces lysine deacetylation of cytosolic proteins involved in cardioprotection (Nadtochiy et al. 2011a). NF-κB, isocitrate dehydrogenase, GAPDH and eNOS have been proposed as functional targets of IPC -induced Sirt1 (Nadtochiy et al. 2011b). Thus, Sirt1 protects the heart against I/R injury through both transcriptional and post-translational mechanisms, allowing Sirt1 to protect the heart against both early and late phases of I/R.
Oxidative stress
During reperfusion, generation of superoxide anions (O2−) from mitochondria is robustly increased. O2− is dismutated by superoxide dismutase (SOD) to produce hydrogen peroxide (H2O2) (Shao et al. 2011). O2− can also be a source of OH− and ONOO−, which are highly reactive molecules. Increased production of reactive oxygen species causes oxidation of mitochondrial proteins and induces mitochondrial dysfunction, which exacerbates oxidative stress. Increasing lines of evidence suggest that Sirt1 protects cardiomyocytes from oxidative stress (Alcendor et al. 2007) (Vinciguerra et al. 2011). Overexpression of Sirt1 upregulates expression of anti-oxidants, including MnSOD and thioredoxin, and attenuates oxidative stress in the heart in response to I/R (Hsu et al. 2010). Protein expression of catalase is also increased in cardiac-specific Sirt1 transgenic mice exposed to paraquat (Alcendor et al. 2007). Sirt1 deacetylates FoxO family transcription factors, induces nuclear translocation of FoxOs, and activates transcription of anti-oxidants and other cardioprotective molecules (Daitoku et al. 2004). It should be noted, however, that very high cardiac Sirt1 expression induces oxidative stress in transgenic mice, in association with dysregulation of the mitochondrial biogenesis program and mitochondrial function. These findings suggest that the cardiac effect of Sirt1 against oxidative stress is dose-dependent (Alcendor et al. 2007).
During I/R, both oxygen and energy depletion, and subsequent acute recovery of oxygen and energy supply, induce accumulation of unfolded proteins in the endoplasmic reticulum (ER), a phenomenon known as ER stress. Excessive ER stress induces apoptosis through upregulation of C/EBP homologous protein (CHOP) and caspase-12. Sirt1 overexpression and resveratrol decrease splicing of X-box binding protein 1 (XBP1), a marker of ER stress, in the murine liver, suggesting that Sirt1 can serve as a negative regulator of the unfolded protein response (UPR) in diabetes (Li et al. 2011). Whether the protective effect of Sirt1 during I/R is mediated through alleviation of ER stress remains to be elucidated.
Apoptosis and necrosis
Sirt 1 deacetylates known facilitators of apoptosis, including p53, and inhibitors of apoptosis, such as the DNA repair factor Ku70, thereby inhibiting apoptosis. Post-translational modification of signaling molecules rapidly affects the survival and death of cardiomyocytes during I/R. Downregulation of Sirt1 and Nampt during ischemia may contribute to rapid activation of p53 and consequent apoptosis in the heart (Hsu et al. 2010). In addition, Sirt1 positively affects transcription of protective molecules and negatively affects that of proapoptotic molecules through deacetylation of the FoxO family transcription factors. For example, Sirt1 induces deacetylation and nuclear translocation of FoxO1 and upregulates B cell lymphoma-2 (Bcl-2) and Bcl-like X (Bcl-xL), anti-apoptotic members of the Bcl-2 family, whereas it downregulates Bcl-2-associated X (Bax), a proapoptotic member of the Bcl-2 family of proteins, during I/R in Sirt1 transgenic mice (Hsu et al. 2010). FoxOs act as both independent transcription factors and transcription factor co-factors. The precise mechanisms by which Sirt1 controls gene expression in either direction through FoxO1 during cardiac stress remain to be elucidated.
Both mPTP opening and activation of a specific signaling pathway induce necrosis, which constitutes a significant portion of the cell death that occurs during I/R. Necrotic cells distribute noxious substances and debris in the vicinity, including ATP, uric acid, high mobility group box 1 (HMGB1) and soluble extracellular matrices, which in turn recruit macrophages and neutrophils (Medzhitov 2008). Sirt1 decreases the transcriptional activity of NF-κB through deacetylation of RelA/ p65, a subunit of NF-κB, which may, in turn, prevent inflammation and consequent cardiac remodeling (Nadtochiy et al. 2011b).
Autophagy
Autophagy is an important mechanism of bulk degradation occurring in three different forms: microautophagy, chaperone-mediated autophagy, and macroautophagy. Macroautophagy, characterized by the formation of an autophagosome, a double membrane vesicle, which fuses to lysosomes for degradation, is the most studied, and is often referred to simply as “autophagy” (Mizushima et al. 2010). Autophagy is typically activated by nutrient/energy starvation, where activation of the self-eating mechanism recycles amino acids and fatty acids recovered from the lysosomes to produce ATP for cell survival. Autophagy plays a salutary role in mediating cellular quality control, by eliminating misfolded or unfavorably modified proteins and dysfunctional organelles. Nutrient starvation induces activation of Sirt1, which in turn induces autophagy in cardiomyocytes. Sirt1 induces deacetylation of FoxO1, thereby upregulating proteins required for autophagy, such as Rab-protein 7 (Rab7), which stimulates autophagosome-lysosome fusion (Hariharan et al. 2010). Sirt1 also stimulates autophagy by deacetylating some Atg proteins (Lee et al. 2008), as well as through stimulation of AMPK (Fulco et al. 2008; Matsui et al. 2007). Protein expression of Nampt is reduced during ischemia, and Nampt supplementation enhances autophagy and reduces myocardial injury (Hsu et al. 2009b). These results suggest that the Nampt-Sirt1 pathway stimulates autophagy during myocardial ischemia, thereby protecting the heart from cell death. It should be noted, however, that excessive activation of autophagy at the time of reperfusion could exacerbate myocardial injury. Thus, whether stimulation of autophagy contributes to the overall protective mechanism of Sirt1 during I/R may be determined by the length of myocardial ischemia before reperfusion.
Other sirtuins
Mitochondria are the main source of ROS and are an important regulator of cell death during I/R (Yellon and Hausenloy 2007). Cardiac-specific Sirt3-overexpressing mice show an increased expression of MnSOD and catalase and attenuated ROS production in response to angiotensin II or isoproterenol stimulation (Sundaresan et al. 2009). Sirt3 has been reported to deacetylate cyclophilin D (CypD), the regulatory component of the mitochondrial permeability transition pore (mPTP), and mitochondrial function is impaired in cardiomyocytes obtained from Sirt3 knockout mice (Hafner et al. 2010). A recent report showed that CR induces a reduction in mitochondrial ROS production, as well as the deacetylation of NADH dehydrogenase (ubiquinone) Fe-S protein 1 (NDUFS1) and the cytochrome bc1 complex Rieske subunit (Shinmura et al. 2011). According to this report, Sirt3 is one of the leading candidates for the deacetylase responsible for these CR-induced deacetylations, although a significant increase in Sirt3 expression was not observed in CR hearts. Despite the accumulating reports on the protective effects of Sirt3 against stresses, there is presently no report on its protective effect against I/R injury. Sirt7, a nuclear form of sirtuin, also has a protective role in the heart (Vakhrusheva et al. 2008). However, the role of Sirt7 during I/R remains to be elucidated. On the other hand, Sirt2, another nuclear form of sirtuin, is upregulated by anoxia-reoxygenation in H9c2 cells, and downregulation of Sirt2 protects against anoxia-reoxygenation injury. Downregulation of Sirt2 causes upregulation of 14-3-3-ζ, and changes the subcellular localization of BAD from mitochondria to the cytosol (Lynn et al. 2008). Interestingly, small molecule inhibitors of Sirt2 are available and exhibit neuro-protection in a cellular model of Parkinson’s disease (Outeiro et al. 2007).
Sirt1-activating compounds (STACs)
Medical treatment to stimulate the activity of endogenous Sirt1 may represent an effective method for induction of pharmacological preconditioning and/or treatment of I/R injury. Resveratrol, a polyphenol found in red wine, is well known as a natural potent activator of Sirt1 (Howitz et al. 2003). Resveratrol is well known to be cardioprotective against I/R injury, but its cardioprotective effects have been attributed to its antioxidant properties and to upregulation of NO (Hung et al. 2000). Thus far, over 3,500 Sirt1-activating compounds (STACs) have been synthesized and analyzed (Lavu et al. 2008). Whether STACs and resveratrol really act through Sirt1 has been debated (Beher et al. 2009; Pacholec et al. 2010). However, a recent report showed that STACs directly interact with Sirt1 and stimulate its enzymatic activity through an allosteric mechanism (Dai et al. 2010). Several clinical trials on obesity, metabolic syndrome, diabetes and aging have been launched to evaluate the efficacies of resveratrol and STACs, including SRT2104, SRT2379 and SRT501 (Camins et al. 2010).
Concluding remarks
Increasing lines of evidence suggest that Sirt1 protects the heart from aging and I/R. Stimulating endogenous Sirt1 and/or sirtuins appears to be a promising modality to reduce the level of I/R injury. However, there are many unsolved issues regarding the function of Sirt1 in the heart. First, whether Sirt1 is protective or detrimental appears to depend upon the nature of the stress. For example, our recent study suggested that upregulation of Sirt1 during pressure overload is not necessarily protective (Oka et al. 2011). Thus, in order to use stimulation of Sirt1 as a modality to treat patients with heart diseases, care must be taken to determine in which conditions stimulation of Sirt1 is purely beneficial. At present, it is hard to generalize the function of Sirt1 in the heart. Activation of Sirt1 may be beneficial when the heart has to conserve energy, like during starvation and ischemia (Hariharan et al. 2010; Hsu et al. 2010), but not necessarily when the heart requires more energy to maintain contractility, such as during pressure overload (Oka et al. 2011). Further investigations are needed to elucidate the functionality of Sirt1 in the heart against different forms of stress. Second, the effect of Sirt1 is dose-dependent. Mild overexpression of Sirt1 failed to protect the heart from I/R injury (Nadtochiy et al. 2011b), whereas a high level of Sirt1 expression can either be harmful by itself (Alcendor et al. 2007) or can facilitate heart failure in response to pressure overload (Oka et al. 2011). The optimum level of Sirt1 must be identified in order to achieve the maximum treatment benefit against each stress condition. Third, although both CR and resveratrol protect the heart from I/R, whether Sirt1 mediates their effect remains to be shown (Shinmura et al. 2011). Fourth, how NAD+/NADH is regulated in the heart during stress is not fully understood. Coupling between sirtuins and Nampt, and interactions between Sirt1 and the cardiac mitochondrial metabolism and other NAD+-consuming enzymes, such as Poly [ADP-ribose] polymerase 1, remain to be elucidated. Fifth, Sirt1 affects metabolism through interaction/deacetylation of key transcription factors or co-factors, such as PPAR and PGC-1 (Oka et al. 2011). How Sirt1-mediated changes in glucose and fatty acid metabolism affect I/R injury remains to be elucidated. Finally, the cellular targets of Sirt1 and the role of protein deacetylation in mediating the protective effects of Sirt1 during I/R injury should be elucidated. Since several clinical trials have already been launched to evaluate STACs in the treatment of non-cardiac diseases, STACs may also become a new class of treatment for I/R injury in the near future (Baur 2010). Furthermore, elucidating the relevant downstream targets of Sirt1 may allow us to develop even more effective methods to protect the heart from I/R injury.
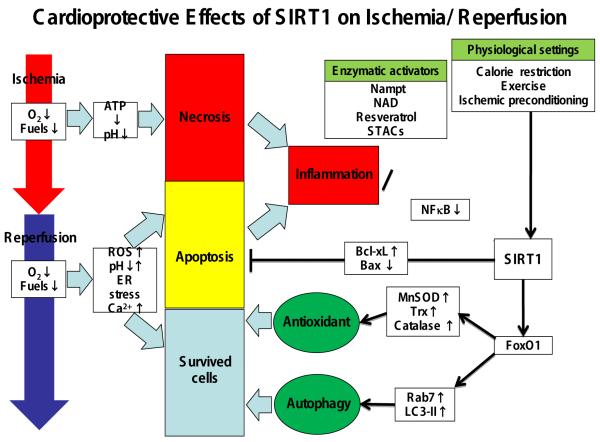
Figure 1. Cardioprotective Effects of Sirt1 on Ischemia/Reperfusion. Expression and activity of Sirt1 are regulated by multiple mechanisms.
Sirt1 protects the heart from I/R injury through multiple mechanisms. For example, Sirt1 suppresses ROS production, apoptosis and inflammation, whereas it also induces autophagy. The effect of Sirt1 is mediated through both protein deacetylation (post-translational modification) and transcription (such as modulation of FoxO).
ROS: reactive oxygen species; UPR: unfolded protein response; NF-κB: nuclear factor of κ light polypeptide gene enhancer in B-cells; XBP: X-box binding protein; Bcl-xL: B cell lymphoma like X; Bax: Bcl-2-associated X; MnSOD: manganese superoxide dismutase; Trx: thioredoxin; Rab7: Rab-protein 7; LC3-II: light chain 3-II; FoxO1: forkhead box O1; NAD: nicotinamide adenine dinucleotide; STACs: Sirt1-activating compounds.
Acknowledgments
The authors wish to thank Christopher D. Brady and Daniela Zablocki for critical revisions of the manuscript.
Funding sources
This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, AG27211 and the Foundation of Leducq Transatlantic Network of Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- An unSIRTain role in longevity. Nat Med. 17:1350–1351. doi: 10.1038/nm1111-1350. [DOI] [PubMed] [Google Scholar]
- Abdelmohsen K, Pullmann R, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804:1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Camins A, Sureda FX, Junyent F, Verdaguer E, Folch J, Pelegri C, Vilaplana J, Beas-Zarate C, Pallàs M. Sirtuin activators: designing molecules to extend life span. Biochim Biophys Acta. 2010;1799:740–749. doi: 10.1016/j.bbagrm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010:914–923. doi: 10.18632/aging.100252. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. others. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009a:481–491. doi: 10.1161/CIRCRESAHA.109.203703. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009b;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Imai S. Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585:1657–1662. doi: 10.1016/j.febslet.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Kop WJ, Gabbay FH, Rozanski A, Barnard M, Klein J, Pardo Y, Gottdiener JS. Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation. 1996;93:1364–1371. doi: 10.1161/01.cir.93.7.1364. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008:841–853. doi: 10.1038/nrd2665. England. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008:3374–3379. doi: 10.1073/pnas.0712145105. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Pletcher SD, Canto C, Auwerx J. Ageing: longevity hits a roadblock. Nature. 2011;477:410–411. doi: 10.1038/477410a. [DOI] [PubMed] [Google Scholar]
- Lynn EG, McLeod CJ, Gordon JP, Bao J, Sack MN. SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett. 2008;582:2857–2862. doi: 10.1016/j.febslet.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5:155–171. discussion 172. [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. others. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011a;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, Brookes PS. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol. 2011b;301:H1506–1512. doi: 10.1152/ajpheart.00587.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Miura M. Caspase-mediated changes in Sir2alpha during apoptosis. FEBS Lett. 2006;580:5875–5879. doi: 10.1016/j.febslet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J. PPARalpha-Sirt1 Complex Mediates Cardiac Hypertrophy and Failure through Suppression of the ERR Transcriptional Pathway. Cell Metab. 2011;14:598–611. doi: 10.1016/j.cmet.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. others. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007:363–375. doi: 10.1016/j.cmet.2007.09.003. others. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Oka SI, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, Ohta S, Katsumata Y, Fukuda K, Ishiwata K. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011;109:396–406. doi: 10.1161/CIRCRESAHA.111.243097. others. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2009;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M, Santini MP, Martinez C, Pazienza V, Claycomb WC, Giuliani A, Rosenthal N. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00766.x. in press. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. others. [DOI] [PubMed] [Google Scholar]
- Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang SY, Fleuriel C, Leprince D, Rocheleau JV, Piston DW, Goodman RH. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104:829–833. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]