Abstract
Lichens are symbiotic associations between fungi and photosynthetic algae or cyanobacteria. Microcystins are potent toxins that are responsible for the poisoning of both humans and animals. These toxins are mainly associated with aquatic cyanobacterial blooms, but here we show that the cyanobacterial symbionts of terrestrial lichens from all over the world commonly produce microcystins. We screened 803 lichen specimens from five different continents for cyanobacterial toxins by amplifying a part of the gene cluster encoding the enzyme complex responsible for microcystin production and detecting toxins directly from lichen thalli. We found either the biosynthetic genes for making microcystins or the toxin itself in 12% of all analyzed lichen specimens. A plethora of different microcystins was found with over 50 chemical variants, and many of the variants detected have only rarely been reported from free-living cyanobacteria. In addition, high amounts of nodularin, up to 60 μg g−1, were detected from some lichen thalli. This microcystin analog and potent hepatotoxin has previously been known only from the aquatic bloom-forming genus Nodularia. Our results demonstrate that the production of cyanobacterial hepatotoxins in lichen symbiosis is a global phenomenon and occurs in many different lichen lineages. The very high genetic diversity of the mcyE gene and the chemical diversity of microcystins suggest that lichen symbioses may have been an important environment for diversification of these cyanobacteria.
Keywords: Nostoc, Peltigera, cyanolichen, secondary metabolites, chemical defence
Lichens are symbiotic associations between a lichen-forming fungus and a photosynthetic partner that may be a green alga or cyanobacterium. Many extant lichen species have cyanobacteria as primary or accessory photosynthetic partners and are therefore collectively referred to as “cyanolichens.” Cyanolichens are found in most terrestrial habitats, from humid tropical and boreal forests to hot and cold deserts. They are important in the nitrogen cycle of many ecosystems through their ability to fix atmospheric nitrogen (1).
Nostoc is by far the most common genus of cyanobacterial symbionts in lichens (1). These filamentous cyanobacteria are able to photosynthesize and provide sugars but also to fix atmospheric nitrogen into ammonia, nitrates, or nitrites that can be absorbed by the fungal partner. Nostoc can be found growing on rocks and soil independent of lichen symbiosis. However, the known diversity of symbiotic Nostoc in lichen symbiosis far exceeds that of free-living Nostoc (2, 3). Extensive sampling of lichen communities has shown that most lichenized fungi tend to associate with restricted groups of Nostoc genotypes (4, 5). Nevertheless, the identity of the cyanobacterial symbiont has been found for just a small percentage of all cyanolichen species, and tropical cyanolichens, in particular, have received very little attention.
Microcystins and nodularins are small cyclic peptides that have caused animal poisonings around the world (6). They are potent inhibitors of eukaryotic protein phosphatases and are highly toxic (7, 8). Microcystins are suspected to act as tumor promoters (9), and the use of water contaminated with the toxin in renal dialysis is held responsible for the deaths of 60 patients in Brazil (10). Nodularins and microcystins are produced by bloom-forming cyanobacteria in freshwater ecosystems. We have previously shown that the Nostoc symbionts of the tripartite cyanolichen species Peltigera leucophlebia can produce hepatotoxic microcystins in lichen symbiosis (11). However, it was unclear whether the production of these potent hepatotoxins in lichen symbiosis is a frequent phenomenon. Here we report that microcystins and nodularins are produced in many different cyanolichen lineages and climatic regions all over the world.
Results
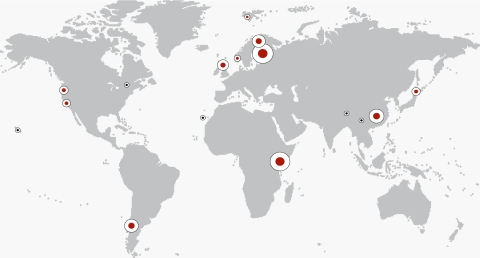
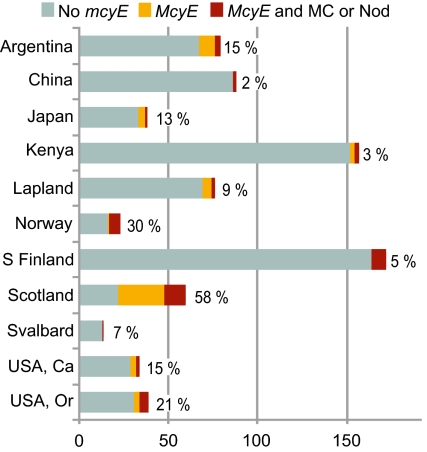
A total of 803 lichen thalli representing 23 different cyanolichen genera from different parts of the world were analyzed (Fig. 1 and Table 1). The mcyE gene was detected from 98 cyanolichen specimens (Table S1), and LC-MS/MS analysis confirmed the presence of microcystins in 42 of these lichens (Table 2). In addition, nodularin was detected from three specimens (Table 2). Although the quantity and taxonomic diversity of the lichens varied, toxin-containing lichen thalli were found from all geographical regions where more than 10 cyanolichen specimens were analyzed (Fig. 2). The highest percentages of cyanolichen thalli containing toxins and/or the mcyE gene were recorded for Scotland (58%), Norway (30%), and Oregon (USA; 21%).
Fig. 1.
Specimen collection locations shown on a world map. The size of the white circle reflects the number of specimens collected from that location. A red center indicates that also lichens containing microcystin or nodularin have been detected from the region.
Table 1.
Number of different lichen genera and specimens collected from each region
| No. of |
||
| Collection site | Genera | Specimens |
| Bariloche, Argentina | 7 | 79 |
| Quebec, Canada | 1 | 3 |
| Canary Islands | 2 | 9 |
| Hunan, China | 4 | 88 |
| Lijiang, China | 1 | 3 |
| Tibet, China | 1 | 1 |
| Southern Finland | 9 | 174 |
| Hawaii | 1 | 1 |
| Japan | 3 | 38 |
| Taita Hills, Kenya | 8 | 155 |
| Lapland | 5 | 76 |
| Norway | 8 | 23 |
| Scotland | 8 | 66 |
| Svalbard | 3 | 14 |
| California, United States | 10 | 34 |
| Oregon, United States | 7 | 39 |
Table 2.
Microcystin- or nodularin-containing lichen specimens and isolated Nostoc strains used in this study
| Genotype ID |
||||||
| No. | Species | 16S | mcyE | MH+(m/z) | MC/Nod main variant | cMC(μg g−1) |
| 2 | Nephroma laevigatum | B1 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 967 | [Asp3, DMAdda5]MC-LR | NQ |
| 3 | Sticta fuliginosa | B1 | B1, D2, I1, J1 | 1,024 | [Asp3]MC-RR | NQ |
| 4 | Sticta fuliginosa | B1 | B1, D2, I1, J1 | 1,038 | MC-RR | 170 |
| 5 | Peltigera dolichorhiza | — | B1, D2, I1, J1 | 1,023 | [ADMAdda5]MC-LR | NQ |
| 9 | Nephroma cellulosum | B2 | B2, B3, E2 | 1,065 | [Leu1, ADMAdda5]MC-LR | <10 |
| 11 | Nephroma cellulosum | B2 | B2, B3, E2 | 1,065 | [Leu1, ADMAdda5]MC-LR | <10 |
| 13 | Sticta sp. | B4 | B4 | 825 | Nod-R | 60 |
| 14 | Sticta sp. | — | B4 | 825 | Nod-R | 40 |
| 21 | Lobaria scrobiculata | D2 | B1, D2, I1, J1 | 967 | [Asp3, DMAdda5]MC-LR | 50 |
| 29 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 30 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 31 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | 60 |
| 32 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | 230 |
| 33 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 34 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 35 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 36 | Nephroma parile | F2 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | 210 |
| 38 | Peltigera degenii | G1 | G1 | 1,023 | [Leu1, Asp3]MC-LR | NQ |
| 47 | Peltigera dolichorhiza | I3 | I3 | 825 | Nod-R | <10 |
| 48 | Peltigera membranacea | I4 | I4-a | 1,052 | [Asp3, ADMAdda5]MC-RR | <10 |
| 49 | Peltigera membranacea | I4 | I4-a | 1,052 | [Asp3, ADMAdda5]MC-RR | <10 |
| 50 | Peltigera hymenina | I4 | I4-b | 1,052 | [Asp3, ADMAdda5]MC-RR | NQ |
| 51 | Peltigera collina | J1 | B1, B5, D1, F2, H3, H4, J1, K3, K4 | 1,066 | [ADMAdda5]MC-RR | NQ |
| 61 | Peltigera leucophlebia | J2 | B1, H1, J2, L4, L5 | 967 | [Asp3, DMAdda5]MC-LR | NQ |
| 62 | Peltigera evansiana | J3 | J1, J3, K3 | 1,037 | [Leu1]MC-LR | <10 |
| 75 | Peltigera praetextata | K3 | Ambiguous | 1,066 | [ADMAdda5]MC-RR | NQ |
| 80 | Peltigera degenii | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | 170 |
| 81 | Peltigera degenii | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | 200 |
| 82 | Peltigera degenii | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | <10 |
| Nostoc sp. strain UK222.Ib | 1,037 | [Leu1]MC-LR | 190 | |||
| 83 | Peltigera degenii | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | 40 |
| 84 | Peltigera neopolydactyla agg. | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | <10 |
| 85 | Peltigera neopolydactyla agg. | L4 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | NQ |
| 86 | Peltigera neopolydactyla agg. | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | <10 |
| Nostoc sp. strain UK89.II | 1,037 | [Leu1]MC-LR | 4,600 | |||
| 87 | Peltigera membranacea | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | <10 |
| Nostoc sp. strain UK92.II | 1,037 | [Leu1]MC-LR | 2,700 | |||
| 88 | Peltigera degenii | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | 50 |
| 89 | Peltigera degenii | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | 20 |
| 90 | Peltigera sp. | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | <10 |
| 91 | Peltigera sp. | L4 | L4, L6 | 1,037 | [Leu1]MC-LR | <10 |
| 92 | Peltigera degenii | L5 | B1, H1, J2, L4, L5 | 1,037 | [Leu1]MC-LR | 60 |
| 93 | Peltigera membranacea | L6 | L4, L6 | 1,037 | [Leu1]MC-LR | 70 |
| 94 | Peltigera membranacea | L6 | L4, L6 | 1,037 | [Leu1]MC-LR | 100 |
| 95 | Peltigera membranacea | L6 | L4, L6 | 1,037 | [Leu1]MC-LR | 30 |
| 96 | Peltigera membranacea | L6 | L6 | 1,037 | [Leu1]MC-LR | <10 |
| 97 | Peltigera neopolydactyla agg. | L6 | L6 | 1,037 | [Leu1]MC-LR | 20 |
| 98 | Peltigera sp. | — | X1 | 1,065 | [Leu1, ADMAdda5]MC-LR | NQ |
The numbering follows the numbers in Table S1. m/z is the mass-to-charge ratio of the protonated microcystin/nodularin ion MH+. The main toxin variant and the total toxin concentration (c) are shown when analyzed. NQ, not quantified. —, the sequence is missing.
Fig. 2.
Presence of the mcyE gene and microcystins or nodularins in different regions. The number of specimens is presented on the x axis. Specimens with the mcyE gene and microcystins or nodularins detected are in red, samples with only the mcyE gene are in orange, and without the gene or toxins are in gray. Percentages at the end of the bars are the proportions of specimens having the mcyE gene and/or toxins. Only regions with a specimen number over 10 are included in the chart. S Finland refers to southern Finland, and Lapland contains areas in Finland and Sweden north of latitude 65°.
A total of 52 different microcystin (MC) variants was identified, from which 20 variants had a relative intensity over 10% at least in one lichen sample (Table S2). The most common microcystin variant in lichens of the Nephroma guild was [ADMAdda5]MC-RR (Arg in positions two and four, and ADMAdda in position five), whereas the most common main variant in other cyanolichen species was [Leu1]MC-LR (Leu in positions one and two, and Arg in position four). Both main variants are infrequently found among free-living cyanobacteria. The microcystin concentration in lichen thalli varied from trace amounts to over 0.2 mg g−1 (Table 2). In culture, the amounts of microcystin produced by Nostoc sp. strains isolated from lichens varied between 0.2 and 5 mg g−1.
Nodularins were unexpectedly detected from two Kenyan and an Argentinian lichen specimen. Three different nodularin variants were identified, with nodularin-R being the main variant in all cases (Table S2). The nodularin concentration varied from trace amounts to 0.06 mg g−1, with the highest concentrations found in the two lichen specimens from Africa (Table 2).
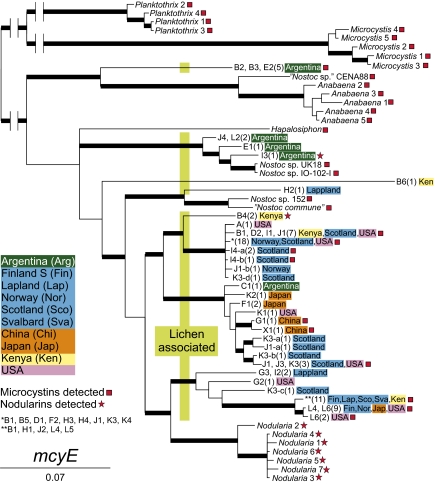
The 98 mcyE sequences consisted of 30 different and highly variable genotypes. Fifteen mcyE sequences were ambiguous and were not included in the phylogenetic analyses. The mcyE genes from lichens grouped with the mcyE genes of other heterocyst-forming cyanobacteria, but were not confined to any single lineage within this group (Fig. 3). Neither were they limited to certain cyanolichen hosts or geographical regions. Interestingly, the mcyE tree identifies the monophyletic genera Planktothrix, Microcystis, Anabaena, and Nodularia, but placed Nodularia well inside the robust crown group of sequences from lichen-associated Nostoc (Fig. 3). Furthermore, one cyanobacterial mcyE genotype amplified from five lichen specimens from Argentina grouped together with Anabaena and “Nostoc sp.” CENA88 sequences.
Fig. 3.
Bayesian trees compiled from mcyE gene sequences. Thick branches have a posterior probability value of 0.95 or higher. The genotype codes (e.g., F1) are coupled with specimens in Table S1. The number of lichen specimens in which the genotype has been found is in parentheses. Regions with color coding are the collection sites of the specimens. In the mcyE tree, the genotype code refers to the 16S genotype obtained from the same lichen specimen; for example, mcyE genotype “J4, L2 (2) Argentina” has been obtained from two individual lichen specimens both collected from Argentina, from which one gave 16S genotype J4 and the other L2. Red boxes and stars show genotypes acquired from samples that contained microcystins or nodularins.
Forty-one different cyanobacterial 16S rRNA genotypes were identified from 94 lichen specimens with an mcyE-containing cyanobacterium. Only one 16S rRNA genotype was identified from each sample from which an mcyE gene sequence had been amplified. The phylogenetic trees constructed using mcyE (Fig. 3) and 16S rRNA (Fig. S1) gene sequences were not congruent, but display a similar sporadic distribution of toxin-producing Nostoc symbionts among different lichen groups and geographical origins. The 16S rRNA tree clearly identifies the monophyletic genera Planktothrix, Microcystis, Anabaena, and Nodularia, but lichen-associated Nostoc sequences form a well-supported sister group to the aquatic Nostoc sp. strain 152. The lichen-symbiotic Nostoc form several lineages, including those associated with host fungi of the Nephroma guild (A–F) and the Peltigera guild (G–L) (4, 12). This shows that the geographical origin of a lichen specimen does not predict the identity of the Nostoc symbiont, and that the production of detectable amounts of toxins is not restricted to well-defined groups within Nostoc.
Discussion
Microcystins are best-known from blooms in aquatic water bodies, formed by a variety of different cyanobacterial genera (6). Nodularin has previously been reported only from the genus Nodularia, most commonly from brackish water ecosystems (6). Microcystins and nodularins have caused animal poisonings (6). Previously, a small amount of microcystin was detected from one lichen specimen (11), and some cultured cyanobacterial strains isolated from terrestrial environments have been shown to be toxic (13, 14). Our results demonstrate that large amounts of microcystin and nodularin are produced in numerous lichen species in many terrestrial environments all over the world. Thus, hepatotoxic lichens are a potential health risk for a variety of lichen-consuming herbivores and humans.
Our results provide several interesting insights into the biochemistry and molecular biology of microcystins and related peptides. The common structure of microcystins is cyclo(d-Ala–l-X–d-MeAsp–l-Z-Adda–d-Glu–Mdha), abbreviated as MC-XZ, where X and Z are variable amino acids, Adda is 3-amino-9-methoxy-10-phenyl-2,6,8-trimethyl-deca-4(E),6(E)-dienoic acid, and Mdha is N-methyl-dehydroalanine. There are over 100 previously published microcystin structures varying in the type of amino acids incorporated into the peptide, demethylation of MeAsp and Mdha, and modification to the Adda side chain (15). We detected over 50 chemical variants of microcystins, and 20 of these variants had a relative intensity of over 10% in at least one of the analyzed samples (Table S2). However, the most interesting aspect is how the structure varied in lichens: d-Ala in position 1 is usually highly conserved, but in lichens it is often replaced by d-Leu. [Leu1]MC-LR was reported only on a few occasions (16, 17). Furthermore, the amino acid Adda, unique to microcystin and nodularin, is often replaced by ADMAdda (O-acetyl-O-demethylAdda) in microcystins detected from lichens. In fact, only two lichen specimens had microcystins lacking both of these modifications.
Nodularin was detected from three lichen specimens collected from Argentina and Kenya. This was surprising, as nodularin has only been reported from the cyanobacterial genus Nodularia (18). An analogous compound motuporin has been isolated from the marine sponge Theonella swinhoei (19), but Nodularia or nodularin has never been reported to occur in lichens or in other terrestrial symbioses. The phylogenetic trees show that cyanobacterial 16S rRNA or mcyE gene sequences from nodularin-producing lichens did not group with those from Nodularia but were scattered among lichen-symbiotic Nostoc sequences.
The mcyE sequences obtained from lichen specimens were highly variable, and even if some of the variability detected in the mcyE gene were to originate from more loosely associated epiphytic cyanobacteria and not from the main Nostoc symbiont, the remaining diversity is considerable. The mcyE gene was studied partly because it is involved in the synthesis of the peculiar amino acid Adda and in the formation of the bond between Adda and d-glutamate (20, 21). Adda and d-glutamate are essential for the toxicity of microcystins and vary very little between microcystin variants (22). The mcyE gene is also thought to be unaffected by horizontal gene transfer (21, 23). Consequently, it varies less than genes coding for other more variable amino acids in the molecule, and does not reflect the chemical variability of microcystins the strain produces. This explains why specimens with identical mcyE genotypes can have different microcystin compositions, but it makes the variability of the mcyE gene itself even more intriguing because it is at least partially independent from the variation of the chemical structures.
From an evolutionary perspective, the present high diversity of mcyE genes in lichen-symbiotic cyanobacteria may be partly explained by the effects of their symbiotic way of life and dispersal. When packaged into the propagules of symbiotically dispersing lichens, the Nostoc symbionts invariably experience a genetic bottleneck and their population is reduced to only a few trichomes (24–26). At the same time, the close association with a symbiotic host may promote the evolution of different traits from in free-living cyanobacteria (27). The recurrent bottlenecks and other lichen symbiont populations shaping effects (4, 5, 28) may explain the surprising genetic and chemical diversity we now observe.
From a physiological perspective, environmental variables such as temperature and light are known to affect the quantity of microcystins produced (6, 29), and Hrouzek and coworkers (14) recently suggested that the cytotoxicity of terrestrial Nostoc strains could vary between different climate regimes. In our present data, evidence of toxin production was most commonly found in lichen specimens from humid, temperate regions such as Argentina, Norway, Scotland, and Oregon. Fewer specimens with the mcyE gene and/or toxins were found from regions representing other climate types. For example, in cool temperate or boreal and arctic climates, the percentages of mcyE-positive specimens were only 9% and 8%, respectively, and in the tropical montane cloud forests of Kenya it was only 3%. However, more detailed studies with well-balanced taxon sampling are needed before any definite links between toxin production and geography can be proposed.
From an ecological point of view, microcystins and nodularin are known to have caused deaths of wild and domestic animals (6). In terrestrial ecosystems, cyanolichens represent a potential source of hepatotoxins for grazers, which might also introduce toxins into food chains. Many molluscs and arthropods, but also mammals such as voles, squirrels, snub-nosed monkeys, and ruminants, are known to feed on lichens (30–34). Mollusc grazing has been suggested to be the limiting ecological factor for some cyanolichens in boreal rain forests in Norway (31). Lichens are important winter feed for reindeer and caribou, which graze heavily on Cladonia species with green algal symbionts (35). Even though the relatively high nitrogen content of cyanolichens suggests a better nutritional value (36, 37), reindeer distinctly avoid eating cyanolichens even during starvation. Many lichen-forming fungi produce toxic secondary substances that play a role in defense against herbivores. Our results suggest that the cyanobacterial symbionts may also contribute to this chemical defense.
Even though microcystins should inhibit the protein phosphatases of all eukaryotes, no effects on the eukaryotic partners of cyanolichens have been identified. Possible effects on fungi in general are not known, and only a few studies have demonstrated negative effects on green algae at environmentally relevant concentrations (<50 μg L−1) (7, 38). On the other hand, if microcystin were to have detrimental effects on fungi, selection pressures toward host benefit in lichen symbioses might have played a role in directing evolution toward low toxicity, particularly if toxicity per se is only a secondary effect of the compounds produced by the cyanobacterial symbionts.
Finally, from a human perspective, cyanolichen species are still used in traditional medicine and eaten by humans in some parts of Asia (39). One of the Peltigera specimens analyzed in our study was obtained directly from a traditional medicine man in Hunan Province, China. Although we did not detect any microcystins from that specimen, a morphologically identical specimen from the same region did contain microcystins. Cyanobacteria are known to produce a diversity of biologically active peptides such as microcystins and nodularin (18), and perhaps the traditional use of cyanolichens is partly based on the effects caused by cyanobacterial secondary metabolites.
We conclude that lichens containing toxins of cyanobacterial origin are a global phenomenon. Lichens seem to have provided an important environment for the diversification of symbiotic cyanobacteria—presumably by compartmentalizing the symbionts into small vertically transmitted populations that are relatively susceptible to random events, disruptive selection, and genetic drift. Further studies will, we hope, help us to clarify reasons why toxin-producing Nostoc symbionts appear to be most common in lichens from humid, maritime climates, as well as many other interesting issues that lay hidden behind the vast genetic and chemical variation of lichen-associated hepatotoxins.
Materials and Methods
Lichen specimens (803) from different parts of the world and representing many different lineages of lichen-forming fungi were obtained to achieve a wide coverage of different cyanolichen groups, ecosystems, and geographical areas; 552 lichen specimens were collected in the field by the authors and 251 specimens were acquired from the personal herbaria of colleagues.
DNA Extraction, PCR, and Sequencing.
Lichen fragments were selected under a microscope to avoid contamination with epiphytic cyanobacteria. Lichen DNA was extracted from a minute thallus fragment or in the case of tripartite species from cephalodia. Cyanobacterial symbionts were cultured and the cyanobacterial and lichen DNA was extracted as previously described (26).
Amplification of the cyanobacterial mcyE gene from lichen samples was performed with the primers mcyEF dgn and mcyER dgn (40). Initial screening was performed in a 30-μL volume containing 1 μL genomic DNA, 200 μM deoxynucleoside triphosphate (Finnzymes), 0.5 μM each primer, 0.5 U of Phusion high-fidelity DNA polymerase (Finnzymes), and 3% (vol/vol) DMSO (Finnzymes). The thermocycling parameters were as follows: An initial denaturation of 1 min at 98 °C was followed by 35 cycles of 10 s at 98 °C, 30 s at 59 °C, and 1 min 45 s at 72 °C, with a final extension of 10 min at 72 °C. The PCR was repeated in a 60-μL volume containing 2 μL genomic DNA and 1.0 U of enzyme for those samples judged to contain an mcyE gene after the initial PCR screening. The cyanobacterial 16S rRNA gene was amplified, all PCR products were purified and sequenced, and the chromatograms were edited as previously described (11, 26).
A total of 174 sequences was submitted to GenBank. This included all obtained 16S rRNA and mcyE sequences, with the exception of 15 ambiguous mcyE sequences. The accession numbers with other specimen information are presented in Table S1.
Microcystin Extraction and Analysis with LC-MS.
Dry lichen thallus (10–120 mg) or freeze-dried, cultured cyanobacteria (∼10 mg) was used for microcystin extraction. All specimens with the mcyE gene were analyzed by LC-MS, with the exception of five lichen specimens that were too small for analysis (three specimens collected from Japan, one from Scotland, and one from Oregon). In addition, some especially interesting and/or old specimens without the mcyE gene were analyzed, so that altogether 140 specimens were analyzed by LC-MS. The protocols for microcystin extraction and LC-MS analyses were done as previously described (11). The total microcystin concentration of the selected samples and strains was approximated with MC-RR (Alexis), MC-LR, and Nodularin-R standards (gifts from Z. Grzonka, University of Gdansk, Gdansk, Poland).
Phylogenetic Analyses.
Alignment of the sequence data was performed manually in PhyDE version 0.995 (41). In addition to the sequences generated during the course of this study, 27 additional pairs of 16S rRNA and mcyE gene sequences were obtained from GenBank (Table S3). Bayesian analyses were performed with MRBAYES version 3.1.2 (42) for the mcyE gene and 16S rRNA gene separately. For the mcyE and 16S genes the GTR+Γ and GTR+I+Γ model was applied, respectively. The best-fitting nucleotide substitution models were selected, analyses were performed, and figures were drawn and edited as previously described (43).
Supplementary Material
Acknowledgments
We thank Katja Fedrowitz for providing lichen specimens from Argentina, Scotland, and Sweden; Andreas Frisch and Göran Thor for lichens from Japan; Per Larsson for some specimens from Norway; and Heather Coffey for specimens from Canada. Lichen specimens from East Africa were obtained during field work at the Taita Research Station of the University of Helsinki in Wundanyi, Kenya. Dr. Geoffrey Mwachala from the National Museums of Kenya helped with permit issues and the transport of lichen specimens. Many lichen specimens from Hunan Province and western North America were collected during the course of the previous Academy of Finland Projects 153706, 10134229, and 168332. The study was funded by Academy of Finland Grants 122288 and 118637.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JQ007729–JQ007902).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200279109/-/DCSupplemental.
References
- 1.Rikkinen J. In: Cyanobacteria in Symbiosis. Rai AN, Bergman B, Rasmussen U, editors. Dordrecht, The Netherlands: Kluwer Academic; 2002. pp. 31–72. [Google Scholar]
- 2.Möllenhauer D, Bengtsson R, Lindstrom EA. Macroscopic cyanobacteria of the genus Nostoc: A neglected and endangered constituent of European inland aquatic biodiversity. Eur J Phycol. 1999;34:349–360. [Google Scholar]
- 3.Wright D, Prickett T, Helm RF, Potts M. Form species Nostoc commune (Cyanobacteria) Int J Syst Evol Microbiol. 2001;51:1839–1852. doi: 10.1099/00207713-51-5-1839. [DOI] [PubMed] [Google Scholar]
- 4.Rikkinen J, Oksanen I, Lohtander K. Lichen guilds share related cyanobacterial symbionts. Science. 2002;297:357. doi: 10.1126/science.1072961. [DOI] [PubMed] [Google Scholar]
- 5.Fedrowitz K. Insights into the ecology and genetics of lichens with cyanobacterial photobiont. Uppsala: Swedish Univ of Agric Sci; 2011. PhD thesis. [Google Scholar]
- 6.Sivonen K. In: The Encyclopedia of Microbiology. 3rd Ed. Schaechter M, editor. Oxford: Academic; 2009. pp. 290–307. [Google Scholar]
- 7.MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 8.Honkanen RE, et al. Cyanobacterial nodularin is a potent inhibitor of type 1 and type 2A protein phosphatases. Mol Pharmacol. 1991;40:577–583. [PubMed] [Google Scholar]
- 9.Nishiwaki-Matsushima R, et al. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin Oncol. 1992;118:420–424. doi: 10.1007/BF01629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jochimsen EM, et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- 11.Kaasalainen U, Jokela J, Fewer DP, Sivonen K, Rikkinen J. Microcystin production in the tripartite cyanolichen Peltigera leucophlebia. Mol Plant Microbe Interact. 2009;22:695–702. doi: 10.1094/MPMI-22-6-0695. [DOI] [PubMed] [Google Scholar]
- 12.Myllys L, Stenroos S, Thell A, Kuusinen M. High cyanobiont selectivity of epiphytic lichens in old growth boreal forest of Finland. New Phytol. 2007;173:621–629. doi: 10.1111/j.1469-8137.2006.01944.x. [DOI] [PubMed] [Google Scholar]
- 13.Oksanen I, et al. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. strain IO-102-I. Appl Environ Microbiol. 2004;70:5756–5763. doi: 10.1128/AEM.70.10.5756-5763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hrouzek P, et al. Cytotoxicity and secondary metabolites production in terrestrial Nostoc strains, originating from different climatic/geographic regions and habitats: Is their cytotoxicity environmentally dependent? Environ Toxicol. 2011;26:345–358. doi: 10.1002/tox.20561. [DOI] [PubMed] [Google Scholar]
- 15.Neffling MR. Fast LC-MS detection of cyanobacterial peptide hepatotoxins. Finland: Åbo Akademi Univ, Turku; 2010. PhD thesis. [DOI] [PubMed] [Google Scholar]
- 16.Matthiensen A, Beattie KA, Yunes JS, Kaya K, Codd GA. [D-Leu1]microcystin-LR, from the cyanobacterium Microcystis RST9501 and from a Microcystis bloom in the Patos Lagoon estuary, Brazil. Phytochemistry. 2000;55:383–387. doi: 10.1016/s0031-9422(00)00335-6. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Namikoshi M, Brittain SM, Carmichael WW, Murphy T. [D-Leu(1)] microcystin-LR, a new microcystin isolated from waterbloom in a Canadian prairie lake. Toxicon. 2001;39:855–862. doi: 10.1016/s0041-0101(00)00224-5. [DOI] [PubMed] [Google Scholar]
- 18.Sivonen K, Börner T. In: The Cyanobacteria: Molecular Biology, Genomics and Evolution. Herraro A, Flores E, editors. Norfolk, UK: Academic; 2008. pp. 159–197. [Google Scholar]
- 19.de Silva ED, et al. Motuporin, a potent protein phosphatase inhibitor isolated from the Papua New Guinea sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992;33:1561–1564. [Google Scholar]
- 20.Rouhiainen L, et al. Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl Environ Microbiol. 2004;70:686–692. doi: 10.1128/AEM.70.2.686-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tillett D, et al. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide-polyketide synthetase system. Chem Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 22.Sivonen K, Jones G. In: Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management. Chorus I, Bartram J, editors. London: E & FN Spon; 1999. pp. 41–111. [Google Scholar]
- 23.Rantala A, et al. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc Natl Acad Sci USA. 2004;101:568–573. doi: 10.1073/pnas.0304489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bright M, Bulgheresi S. A complex journey: Transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandel MJ. Models and approaches to dissect host-symbiont specificity. Trends Microbiol. 2010;18:504–511. doi: 10.1016/j.tim.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Fedrowitz K, Kaasalainen U, Rikkinen J. Genotype variability of Nostoc symbionts in three epiphytic Nephroma species in a boreal forest landscape. Bryologist. 2011;114:220–230. [Google Scholar]
- 27.Ran L, et al. Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLoS One. 2010;5:e11486. doi: 10.1371/journal.pone.0011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikkinen J. Ecological and evolutionary role of photobiont-mediated guilds in lichens. Symbiosis. 2003;34(2):99–110. [Google Scholar]
- 29.Kurmayer R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J Phycol. 2011;47:200–207. doi: 10.1111/j.1529-8817.2010.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y. Terrestriality and tree stratum use in a group of Sichuan snub-nosed monkeys. Primates. 2007;48(3):197–207. doi: 10.1007/s10329-006-0035-9. [DOI] [PubMed] [Google Scholar]
- 31.Gauslaa Y. Mollusc grazing may constrain the ecological niche of the old forest lichen Pseudocyphellaria crocata. Plant Biol (Stuttg) 2008;10:711–717. doi: 10.1111/j.1438-8677.2008.00074.x. [DOI] [PubMed] [Google Scholar]
- 32.Fischer BM, Schatz H, Maraun M. Community structure, trophic position and reproductive mode of soil and bark-living oribatid mites in an alpine grassland ecosystem. Exp Appl Acarol. 2010;52:221–237. doi: 10.1007/s10493-010-9366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nybakken L, Helmersen AM, Gauslaa Y, Selås V. Lichen compounds restrain lichen feeding by bank voles (Myodes glareolus) J Chem Ecol. 2010;36:298–304. doi: 10.1007/s10886-010-9761-y. [DOI] [PubMed] [Google Scholar]
- 34.Asplund J, Gauslaa Y. Mollusc grazing limits growth and early development of the old forest lichen Lobaria pulmonaria in broadleaved deciduous forests. Oecologia. 2008;155(1):93–99. doi: 10.1007/s00442-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 35.Danell K, Utsi P, Palo R, Eriksson O. Food plant selection by reindeer during winter in relation to plant quality. Ecography. 1994;17(2):153–158. [Google Scholar]
- 36.Storeheier PV, Mathiesen S, Tyler N, Olsen M. Nutritive value of terricolous lichens for reindeer in winter. Lichenologist. 2002;34:247–257. [Google Scholar]
- 37.Rai AN. In: Cyanobacteria in Symbiosis. Rai AN, Bergman B, Rasmussen U, editors. Dordrecht, The Netherlands: Kluwer Academic; 2002. pp. 97–116. [Google Scholar]
- 38.Babica P, Blaha L, Marsalek B. Exploring the natural role of microcystins—A review of effects on photoautotrophic organisms. J Phycol. 2006;42(1):9–20. [Google Scholar]
- 39.Wang L, Nahuri T, Harada H, Culberson C, Culberson W. Ethnic uses of lichens in Yunnan, China. Bryologist. 2001;104:345–349. [Google Scholar]
- 40.Fewer DP, et al. Recurrent adenylation domain replacement in the microcystin synthetase gene cluster. BMC Evol Biol. 2007;7:183. doi: 10.1186/1471-2148-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller K, Quandt D, Müller J, Neinhuis C. PhyDE 0.995: Phylogenetic data editor. 2005. ( http://www.phyde.de)
- 42.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 43.Olsson S, Kaasalainen U, Rikkinen J. Reconstruction of structural evolution in the trnL intron P6b loop of symbiotic Nostoc (Cyanobacteria) Curr Genet. 2012;58(1):49–58. doi: 10.1007/s00294-011-0364-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.