Abstract
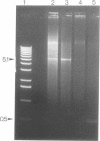
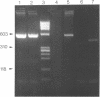
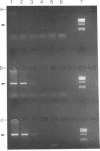
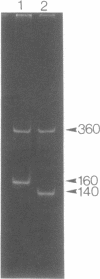
Using PCR, we found that normal heart muscle and brain from adult human individuals contain low levels of a specific mitochondrial DNA deletion, previously found only in patients affected with certain types of neuromuscular disease. This deletion was not observed in fetal heart or brain. Experimental tests support the idea that the deletion exists in vivo in adult mitochondria and is not an in vitro artifact of PCR. Our data provide direct experimental support for the idea that accumulation of mitochondrial DNA deletions may be important in aging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. A., Coombs M. M. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature. 1980 Sep 18;287(5779):244–245. doi: 10.1038/287244a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980 Jul 11;209(4453):297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Cui X. F., Li H. H., Goradia T. M., Lange K., Kazazian H. H., Jr, Galas D., Arnheim N. Single-sperm typing: determination of genetic distance between the G gamma-globin and parathyroid hormone loci by using the polymerase chain reaction and allele-specific oligomers. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9389–9393. doi: 10.1073/pnas.86.23.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L. I. Mitochondrial DNA in sickness and in health. Am J Hum Genet. 1990 Mar;46(3):415–417. [PMC free article] [PubMed] [Google Scholar]
- Gupta K. P., van Golen K. L., Randerath E., Randerath K. Age-dependent covalent DNA alterations (I-compounds) in rat liver mitochondrial DNA. Mutat Res. 1990 Jan;237(1):17–27. doi: 10.1016/0921-8734(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972 Apr;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in mitochondrial myopathies: sequence analysis and possible mechanisms. Nucleic Acids Res. 1989 Jun 26;17(12):4465–4469. doi: 10.1093/nar/17.12.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Hurko O. Preferential amplification and molecular characterization of junction sequences of a pathogenetic deletion in human mitochondrial DNA. Genomics. 1989 Oct;5(3):623–628. doi: 10.1016/0888-7543(89)90032-3. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Rutledge S. L., Stine O. C., Hurko O. Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8059–8062. doi: 10.1073/pnas.86.20.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne P., Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet. 1988 Apr 16;1(8590):885–885. doi: 10.1016/s0140-6736(88)91632-7. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Li H., Cui X., Arnheim N. Direct electrophoretic detection of the allelic state of single DNA molecules in human sperm by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4580–4584. doi: 10.1073/pnas.87.12.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Marzuki S., Ozawa T., Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Müller-Höcker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989 May;134(5):1167–1173. [PMC free article] [PubMed] [Google Scholar]
- Nakase H., Moraes C. T., Rizzuto R., Lombes A., DiMauro S., Schon E. A. Transcription and translation of deleted mitochondrial genomes in Kearns-Sayre syndrome: implications for pathogenesis. Am J Hum Genet. 1990 Mar;46(3):418–427. [PMC free article] [PubMed] [Google Scholar]
- Nelson I., Degoul F., Obermaier-Kusser B., Romero N., Borrone C., Marsac C., Vayssiere J. L., Gerbitz K., Fardeau M., Ponsot G. Mapping of heteroplasmic mitochondrial DNA deletions in Kearns-Sayre syndrome. Nucleic Acids Res. 1989 Oct 25;17(20):8117–8124. doi: 10.1093/nar/17.20.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Hougham A. J., Bulpitt K. J. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: evidence for an increased frequency of deletions/additions with aging. Mech Ageing Dev. 1988 Jun;43(3):279–293. doi: 10.1016/0047-6374(88)90037-1. [DOI] [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Ye J. H., Neckelmann S. N., Singh G., Webster K. A., Greenberg B. D. Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: mitochondrial DNA genes sustain seventeen times more mutations. Curr Genet. 1987;12(2):81–90. doi: 10.1007/BF00434661. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]