Abstract
We report the site-selective bromination of vancomycin to produce, with substantial efficiency, previously unknown mono-bromo-vancomycins, a di-bromo-vancomycin and a tri-bromo-vancomycin. We document the inherent reactivity of native vancomycin towards N-bromophthalimide. We then demonstrate significant rate acceleration, and perturbation of the inherent product distribution in the presence of a rationally designed peptide-based promoter. Alternative site-selectivity is observed as a function of solvent and replacement of the peptide with guanidine.
The selective chemical modification of complex molecules represents a long-standing challenge for chemical synthesis, due in part to the presence of multiple functional groups that often exhibit comparable reactivity. The application of catalysts to natural product derivatization provides a powerful way forward, provided that substantial selectivity can be achieved for different products. In recent years, both enzymes,1 small molecule-based catalysts and reagents2,3 have been studied in this context. A critical challenge in the field is the identification of new strategies that alter the inherent selectivity profiles of complex, polyfunctional scaffolds.3
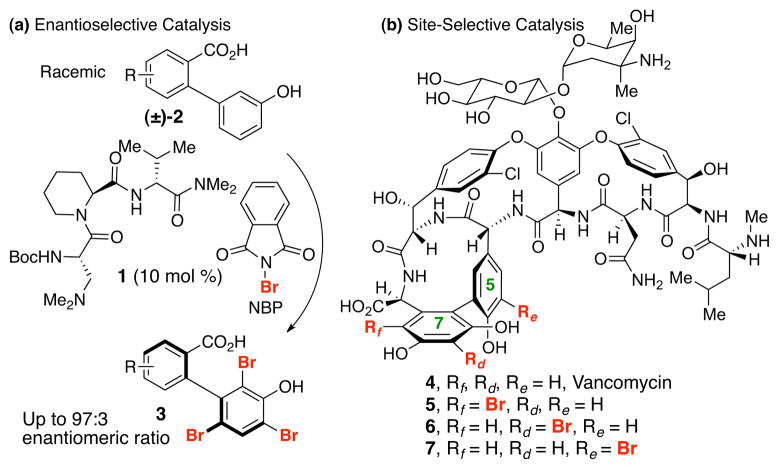
In this context, we wondered about the possible connection between recently described enantioselective arene functionalization reactions4 and selective derivatization of complex molecules such as vancomycin. For example, peptide-based catalyst 1 can effect enantioselective bromination reactions of racemic biaryl compounds such as 2 to deliver stereodefined biaryl compounds like 3 (Figure 1a). These observations prompted us to assess if catalysts might also be found that could deliver selectively the various regioisomers of mono-brominated vancomycin (4, Figure 1b). Vancomycin is a critical tool in the treatment of bacterial infections. Yet, vancomycin resistance has stimulated numerous studies of vancomycin analogs as alternatives.5 Total synthesis,6 biosynthetic manipulation7 and chemical derivatization8 have all contributed to the epic study of 4 as a tool in the fight against antibiotic resistance.
Figure 1.
(a) Atroposelective tribromination of biaryls. (b) Three possible sites (in red) for site selective bromination of 4.
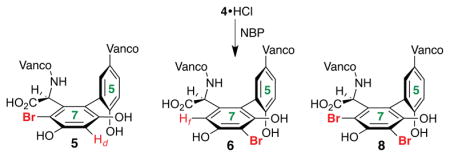
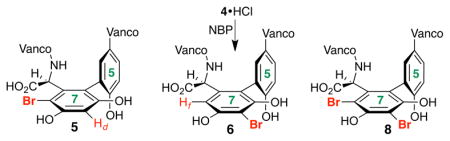
Vancomycin represents an intriguing scaffold for the study of site-selective modifications. Our goal was to establish if catalysis could enable the synthesis of alternative isomers of bromo-vancomycins, which themselves could prove candidates for further functionalization to yield new analogs.9 While there are many candidate sites for bromination of 4, we speculated that the most likely sites would be the Rf position of arene ring 7 (5), the Rd position of the same ring (6) and the Re position of ring 5 (7). In addition, multiple brominated derivatives of 4 might result if the reactive sites are of comparable reactivity. Indeed, at the outset of this study, the inherent reactivity of 4 to generic brominating agents was not described to our knowledge.
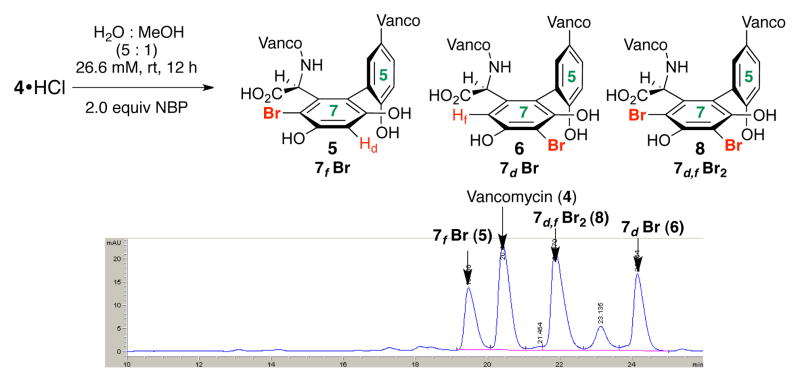
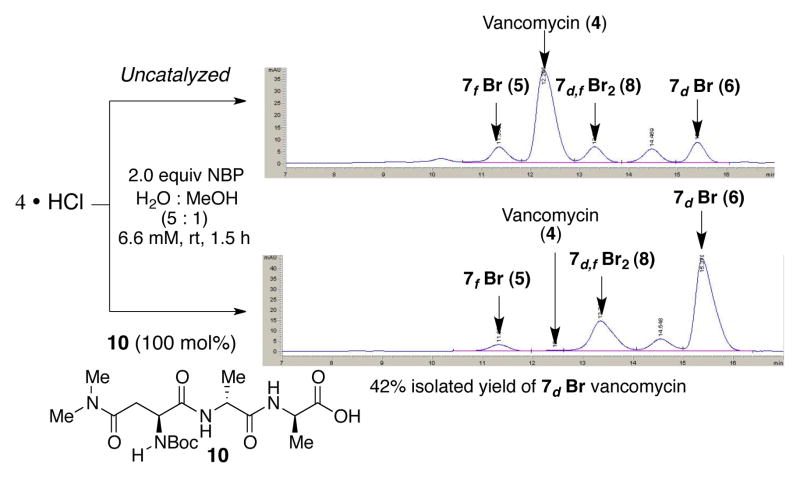
Our studies began with a delineation of the inherent reactivity of 4 toward N-bromophthalimide (NBP). Initially, our studies were undertaken with the guiding principle that reactions would be conducted in H2O and on native 4, without use of protecting groups. These decisions offered the potential to heighten utility. As shown in Figure 2, exposure of 4 to 2.0 equiv of NBP produces a mixture of products, with unreacted 4 as a major component. LCMS analysis and extensive, preparative HPLC purification allowed for isolation of the major constituents, albeit in modest quantities.
Figure 2.
HPLC trace (at 280 nm) for uncatalyzed reaction of 4
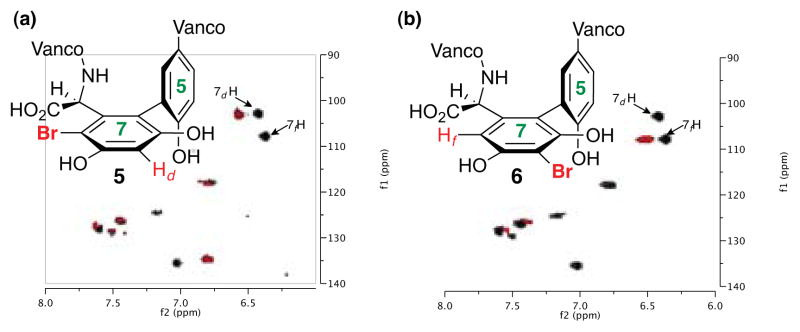
LCMS and 2D NMR methods enabled structural assignments. When spectra were recorded in D2O (500 MHz), chemical shift resolution of diagnostic signals proved excellent, and comparisons of the brominated derivatives to native 4 were particularly instructive. Figure 3 shows overlays of the decisive region of HSQC spectra for compounds 5 and 6 (cross peaks in red) with that obtained for 4 (cross peaks in gray). In each case, we noted the absence of the indicated Ar-H cross peak where Br had putatively been installed. Perturbation of chemical shifts elsewhere in the structures proved to be minimal.10a Of critical significance, the uncatalyzed bromination of vancomycin delivers an essentially 1:1 ratio of the monobrominated vancomycins 5 and 6, along with a very similar quantity of the dibrominated vancomycin 8 (71% conv., Figure 2).
Figure 3.
Overlay of characteristic changes in HSQC spectrum for brominated vancomycins (4 in gray, 5 and 6 are in red; For overlay of 4 and 8, see SI).
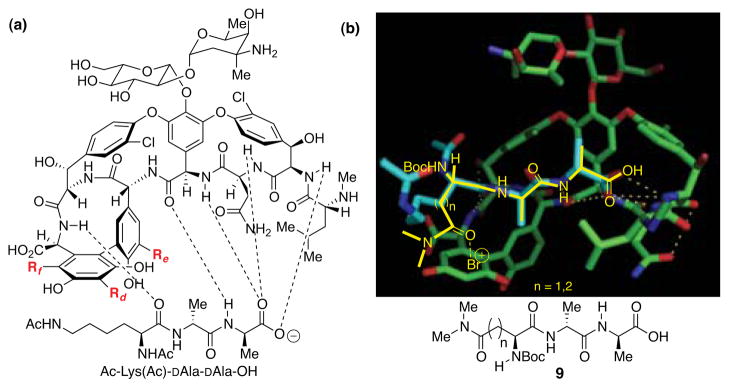
We then considered how catalysts might perturb the intrinsic product distribution exhibited by 4 (Figure 2). Given the well-known binding of 4 to DAla-DAla segments of the bacterial cell wall as part of its biological mode of action (Figure 4a),11 we chose to design catalysts based on this molecular recognition motif. For example, we targeted catalysts that would retain the DXaa-DXaa dipeptide motif as part of a binding domain between the catalyst and the substrate. In addition, we incorporated N,N-dimethyl amide functionality which we suspected would be responsible for rate acceleration of bromination reactions.12 Thus, peptides related to 9 (Figure 4b) emerged as our leads for study.
Figure 4.
a) Binding of vancomycin to Ac-Lys(Ac)-DAla-DAla-OH. b) Overlay of proposed peptide scaffold (yellow) with that of Ac-Lys(Ac)-DAla-DAla-OH (light blue) in vancomycin binding pocket. Adapted from ref 11c, PDB # 1FVM.
The peptides we evaluated are presented in Table 1. As in our assessment of the intrinsic reactivity of 4, we first conducted all reactions in H2O. In addition, we adopted a first order analysis of HPLC traces of the reactions, assessing relative peak areas without a rigorous determination of response factors for individual components of a given reaction. Even with these approximations, we were able to observe immediately that the impact of the peptide-based promoterscould be quite significant.
Table 1.
Optimization of peptide scaffold and reaction conditionsa
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | mol% | t (h) | % conv. | 5 6 8 | |
| 7f Br : 7d Br : 7d,f Br2 | ||||||
| 1 | No catalyst | NA | 12 | 71 | 1.0 : 1.0 : 1.3b | |
| 2a | N,N-dimethylacetamide | 100 | 12 | 68 | 1.0 : 1.0 : 1.8b | |
| 2b | N,N-dimethylacetamide | 7100 | 16 | 64 | 1.0 : 1.5 : 2.9b | |
| 3 | Boc-Asn(Me2)-DAla-DAla-OH (10) | 100 | 2 | 97 | 1.0 : 6.8 : 2.6b | |
| 4 | Boc-DAsn(Me2)-DAla-DAla-OH (11) | 100 | 2 | 90 | 1.0 : 4.9 : 1.8b | |
| 5 | Boc-Gln(Me2)-DAla-DAla-OH (12) | 100 | 2 | 99 | 1.0 : 4.0 : 9.3b | |
| 6 | Boc-DGln(Me2)-DAla-DAla-OH (13) | 100 | 2 | 99 | 1.0 : 3.0 : 3.4b | |
| 7 | Boc-Leu-DAla-DAla-OH (14) | 100 | 12 | 60 | 1.0 : 5.7 :2.5b | |
| 8a | Boc-Asn(Me2)-DAla-DAla-OH (10) | 100 | 1.5 | 98 | 1.0 : 14.6 : 2.8c | |
| 8b | 200 | 1.5 | 99 | 1.0 : 19.0 : 6.0c | ||
| 8c | 50 | 2 | 97 | 1.0 : 3.4 : 1.1c | ||
| 8d | 25 | 1.5 | 83 | 1.0 : 1.9 : 0.7c | ||
| 9 | Boc-Leu-DAsn(Me2)-DAla-OH (15) | 200 | 12 | 96 | 1.0 : 6.5 : 2.7c | |
| 10 | Boc-Leu-DAla-DAsn(Me2)-OH (16) | 200 | 12 | 85 | 1.0 : 3.5 : 1.0c | |
| 11 | Boc-Asn(Me2)-DAla-DAla-OH (10) | 100 | 1.5 | 16 | 1.0 : 10.5 : 0.9d | |
| 12 | Boc-Leu-DAla-DAsn(Me2)-OH (16) | 100 | 1.5 | 12 | 1.0 : 3.5 : 1.6d | |
Ratios were measured by HPLC at 280 nm wavelength.
2.0 equiv of NBP, 250 μL of water, 50 μL of MeOH, 8 μmol of 4.
2.0 equiv of NBP, 1000 μL of water, 200 μL of MeOH, 8 μmol of 4.
50 mol% NBP, 0.033 mmol of 4.
The most striking observation throughout the study was the profound rate acceleration in the presence of peptides that contain the N,N-dimethyl amide functionality at a particular position. As noted in Figure 3, the uncatalyzed reaction delivers essentially equal quantities of 5 and 6 (Table 1, entry 1). The exploration of N,N-dimethylacetamide itself as a promoter (in various concentrations, entries 2a and 2b) did not provide noticeable rate acceleration, nor significant perturbation of the ratio of 5:6, although slightly more 8 is observed. However, as shown in entries 3–6, when peptides containing the DAla-DAla sequence, along with the N,N-dimethylamidoside chain are examined, essentially all of the starting material (4) is consumed within 2 h. These results stand in stark contrast to the rate of consumption of 4 in our control experiments. Moreover, the product distribution responds to structural changes in the peptide. For example, employing peptide 10, with a Asn(Me2)-DAla-DAla structure, we observe 7d-Br derivative 6 as the major product with a 6.8:1 preference over mono-bromide 5, with the dibromide 8 also formed in considerable quantity (entry 3). Altering the stereochemistry to the DAsn(Me2) residue (peptide 11, entry 4) also delivers 6 as the major product, but with a lower 6:5 ratio (4.9:1). We are cautious about over interpreting these differences in selectivity due to the tendency of polyfunctionalization reactions to exhibit different product distributions as a function of conversion (e.g., as monofunctionalized products (e.g., 5 and 6) are depleted and polyfunctionalized products (e.g., 8) are formed).13 In any event, we also observe that exchange of the Asn residue to Gln(Me2) (catalyst 12, entry 5) also contributes to efficient consumption of 4, with the dibromide 8 emerging as the dominant product of the reaction. Yet, this effect is attenuated with the epimeric Gln(Me2) structure 13 (entry 6). Notably, if the N,N-dimethylamide moiety is excised from the peptide structure (peptide 14, entry 7), much lower conversion is observed. Even so, in this case, a preference for 6 is observed.
The effect of peptide concentration and stoichiometry appear to substantially influence both rate and product distribution. Lowering the concentration leads to significant improvement in the ratio of 6:5 (14.6:1; entries 3 and 8a) without appreciable loss of rate. Furthermore, increase in peptide loading to 200 mol% leads to modest improvement in the ratio of 6:5 (19:1; entry 8a vs. 8b). On the other hand, lowering peptide loading to 50 mol% results in a lower 6:5 ratio (3.4:1; entries 8a vs. 8c). We noted that with 50 mol% peptide loading, full consumption of vancomycin was still observed within 2 h. Yet, reduction of the peptide loading to 25 mol% leads to a further erosion of the selectivity in comparison to the results observed with higher amounts of the peptide (entry 8d, 1.9:1.0). These results imply that while the peptide-based promoters exhibit a principle hallmark of catalysis – rate acceleration – turnover rates do not appear to be high in the present case. This latter phenomenon may be due to the high affinity of 4 for the DAla-DAla motif.14
Furthermore, the effect of relocating the Asn(Me2) residue within the tripeptide also appears to influence both the rate and product distribution (c.f., entries 8b, 9 and 10). When the residue is disposed closer to the C-terminal position, the result appears to be a less effective promoter as rates diminish, and selectivity for 6 decreases. These facts may reveal the importance of situating the putative directing functional group at the right locus for effective reactions. Finally, when a limiting quantity (0.5 equiv) of NBP is used, the selectivity trends are preserved and the quantity of di-bromide 8 is reduced (entries 11 and 12). The observations in these two experiments suggest that the preference for monobromide 6 is not simply a function of over-conversion, and depletion of monobromide 5.
With encouraging data regarding the use of peptide 10,14 we chose to explore its use for preparative reactions of particular brominated vancomycins. Under optimized reaction conditions, treatment of 100 mg of 4 provided 43.1 mg (41% yield) of analytically pure 7d-Br derivative 6 in a single experiment. By way of comparison, 100 mg of 4 under uncatalyzed reaction conditions delivers only 11.6 mg (11% yield) of 7d-Br derivative 6 (under the conditions shown in Figure 2), with a quite tedious purification required. Figure 5 compares the HPLC traces for the peptide-mediated reaction of 4 with NBP (bottom trace) with that of the corresponding uncatalyzed reaction (top trace) under identical conditions for direct comparison.
Figure 5.
HPLC traces of uncatalyzed (top) and peptide-catalyzed (bottom) bromination of 4 obtained on reverse phase HPLC.
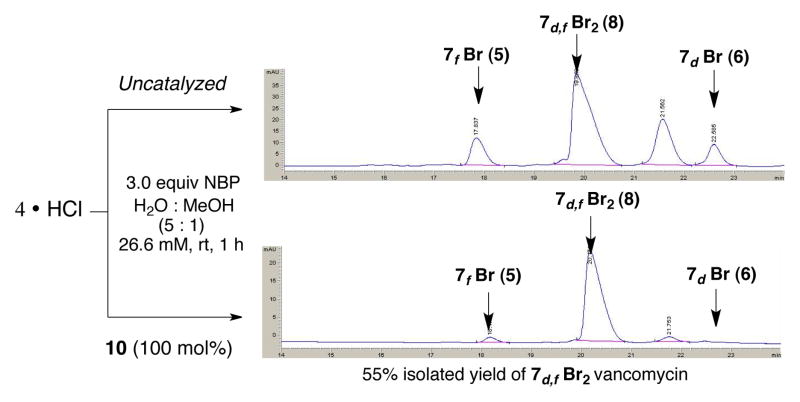
Next, we addressed efficient synthesis of dibrominated vancomycin (8). This particular derivative is generally the major product in the uncatalyzed bromination of vancomycin with excess of brominating reagent. However, as noted, these reactions are generally unselective, which makes product isolation difficult. On the other hand, as shown in Figure 6, treatment of 4 with NBP (3 equiv) in the presence of 100 mol% of peptide 10 provides the desired product with excellent efficiency (Figure 6, bottom). The control reaction provides 8 as a major product, but as a constituent along with significant quantities of the other brominated vancomycins (Figure 6, top). The treatment of 100 mg of 4, under peptide-promoted reaction conditions, provided 60.2 mg (55% yield) of analytically pure 7d,f–Br2 derivative 8 in a single experiment.
Figure 6.
HPLC traces of uncatalyzed (top) and peptide-catalyzed (bottom) dibromination of 4 obtained on reverse phase HPLC.
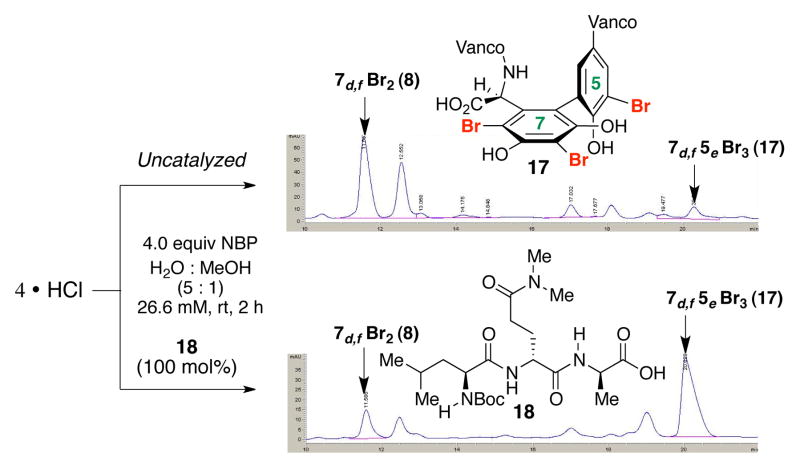
Our explorations of the peptide-based mediators for the bromination of 4 also delivered some additional outcomes, and new design opportunities. For example, during a study of peptide-dependent capacity to deliver highly brominated derivatives, we observed a hint of a new compound (17; Figure 7, top), which proved to be a tri-brominated analog. Intriguingly, treatment of 4 with NBP, in the presence of 100 mol% 10 under otherwise identical conditions enabled observation of compound 8 and new compound 17,10a although with ratios no better than approximately 1:1. Characterization of 1710a allowed assignment of 17 as the illustrated tri-brominated vancomycin, wherein the phenolic moiety of residue 5 had also undergone bromination. This structural assignment stimulated exploration of catalyst 18, wherein the central Gln(Me2) residue was postulated to place dimethylamido moiety in proximity to this previously recalcitrant site of bromination. When 4 was exposed to NBP under the reaction conditions in the presence of catalyst 18, tribromide 17 was produced in significant quantity, preferentially over the dibromide (17:8, 3–4:1; Figure 7, bottom), in a substantially cleaner reaction. This observation demonstrates power of a peptide catalyst to provide a new brominated vancomycin, which is otherwise difficult to acquire. In a preparative mode, 49.0 mg of 4 led to isolation of 20.2 mg (35% yield) of 17.
Figure 7.
HPLC traces of uncatalyzed (top) and peptide-catalyzed (bottom) tribromination of 4 obtained on reverse phase HPLC.
We then turned our attention to the question of reversal of the selectivity exhibited by peptide 10, which preferentially delivers monobromide 6. A preliminary examination of DAla-DAla-based peptides did not unveil a 5-selective catalyst. However, in the course of these studies, we made the surprising observation that the reaction medium had a substantial effect on the site-selectivity of the initial bromination. When 4 is exposed to 2 equiv of NBP in either H2O:MeOH (5:1) or MeOH alone, sluggish reactions occur, and the ratio of 5:6 is near unity (Table 2, entries 1 and 2). Yet, when a larger quantity (4 equiv of NBP) is employed, in the absence of a catalyst, a surprising preference for 5 is observed, with a substantial increase in the observed quantity of dibromide 8 (entry 3). Peptide 10 maintains its capacity to favor 6 in MeOH solvent, although the selectivity is strongly attenuated (entry 4).
Table 2.
Optimization of reaction conditions aiming analog 5.a
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Additive | Solvent | NBP (equiv) | t(h) | % conv. | 5 6 8 |
| 7f Br : 7d Br : 7d,f Br2 | ||||||
| 1 | No catalyst | H2O:MeOH (5:1) | 2 | 12 | 71 | 1.0 : 1.0 : 1.3b |
| 2 | No catalyst | MeOH | 2 | 2 | 17 | 1.0 : 1.0 : 3.0b |
| 3 | No catalyst | MeOH | 4 | 1 | 92 | 14.3 : 1.0 : 53.8c |
| 4 | 10 | MeOH | 4 | 1 | 31 | 0.5 : 1.0 : 1.3b |
| 5 | Guanidine•HCl | MeOH | 4 | 1 | 85 | 12.7 : 1.0 : 10.8c |
| 6 | Guanidine•HCl | MeOH | 4 | 1 | 92 | 11.8 : 1.0 : 11.4d |
Ratios were measured by HPLC at 280 nm wavelength.
8 μmol of 4, 6.6 mM.
16 μmol of 4, 3.3 mM, 18 equiv of guanidine•HCl.
simmilar to (c) except with 6 equiv of guanidine•HCl.
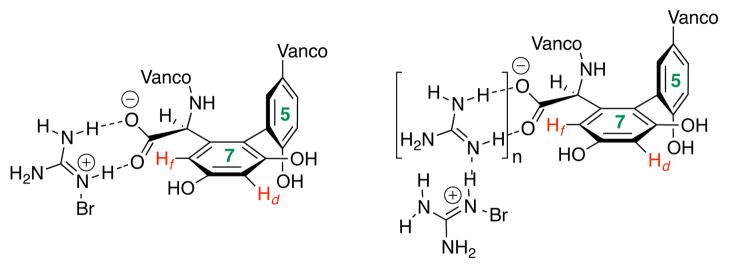
We then chose to examine guanidine as an additive, due to its propensity to accelerate bromination reactions.15 Additionally, guanidine possesses the capacity to bind carboxylate under a variety of conditions.16 Thus, we hypothesized that guanidine might effectively target the acid of 4, while simultaneously delivering bromide to the proximal 7f-site of 4 (Figure 8). The assessment of this hypothesis provides a striking result. As illustrated in entries 5 and 6, guanidine not only provides 5 with good selectivity over 6 but it also provides significant improvement in ratio of monobromide (5) to dibromide (8), which makes isolation of 5 in good quantity viable (entry 3 versus entries 5 and 6). On preparative scale, reaction of 100 mg of 4 with 4 equiv of NBP, in the presence of guanidine, provided 23.3 mg (21% yield) of analytically pure 5, which is otherwise very difficult to isolate cleanly in significant quantity (Figure 2). These results are consistent with the hypothesis that guanidine may associate with the acid of 4, and simultaneously deliver the bromide ion.17,18 This effect may lead to kinetically favored formation of 5, and also the formation of 8 via the depletion of 6. Additional studies are needed to definitively establish the putative mechanism shown in Figure 8.
Figure 8.
Speculative concept for bromide delivery by guanidine.
In conclusion, we have developed simple and efficient site-selective brominations of vancomycin. The approach, in a single step, provides peptide-based promoter- and additive-dependent product ratios, and access to compounds that are otherwise not easily accessible. The concepts we evaluated were based on the storied DAla-DAla binding motif exploited by natural systems, as well as chemically grounded ideas about carboxylate targeting. The introduction of Br into complex, bioactive structures has the potential to modulate biological activity.10b Moreover, from a more chemical standpoint, the Ar-Br moiety may also serve as a platform for further functionalization of glycopeptide antibiotics.9 Studies of this type are current topics of interest in our lab.
Supplementary Material
Acknowledgments
We thank Dr. Jeffery L. Gustafson for initial experiments. We are grateful to the National Institutes of General Medical Sciences of the NIH (GM-068649) for support. We thank Eli Lilly for initial donations of vancomycin.
Footnotes
Supporting Information. Experimental procedures for all experiments, characterization data and biological activity data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Piel J, editor. Top Curr Chem. Vol. 297. Springer GmbH; 2010. Natural Products Via Enzymatic Reactions. [PubMed] [Google Scholar]; b) Steffan N, Grundmann A, Yin WB, Kremer A, Li SM. Curr Med Chem. 2009;16:218. doi: 10.2174/092986709787002772. [DOI] [PubMed] [Google Scholar]; c) Schultz C, Groeger H, Dinkel C, Drauz K, Waldmann H. Vol. 2. Wiley-VCH Verlag GmbH; 2002. p. 872. [Google Scholar]; d) Gatto GJ, Boyne MT, Kelleher NL, Walsh CT. J Am Chem Soc. 2006;128:3838. doi: 10.1021/ja0587603. [DOI] [PubMed] [Google Scholar]
- 2.a) Lewis CA, Miller SJ. Angew Chem, Int Ed. 2006;45:5616. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]; b) Jordan PA, Kayser-Bricker KJ, Miller SJ. Proc Natl Acad Sci. 2010;107:20620. doi: 10.1073/pnas.1001111107. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lewis CA, Merkel J, Miller SJ. Bioorg Med Chem Lett. 2008;18:6007. doi: 10.1016/j.bmcl.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lewis CA, Longcore KE, Miller SJ, Wender PA. J Nat Prod. 2009;72:1864. doi: 10.1021/np9004932. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Jordan PA, Miller SJ. Angew Chem, Int Ed. 2012;51:2907. doi: 10.1002/anie.201109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MS, White MC. Science. 2007;318:783. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]; b) Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc Chem Res. Vol. 45. ASAP; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chamni S, He QL, Dang Y, Bhat S, Liu JO, Romo D. ACS Chem Biol. 2011;6:1175. doi: 10.1021/cb2002686. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Snyder SA, Gollner A, Chiriac MI. Nature. 2011;474:461. doi: 10.1038/nature10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafson JL, Lim D, Miller SJ. Science. 2010;328:1251. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane CM, Pierce JG, Leung SSF, Tirado-Rives J, Jorgensen WL, Boger DL. J Med Chem. 2010;53:7229. doi: 10.1021/jm100946e.Weist S, Kittel C, Bischoff D, Bister B, Pfeifer V, Nicholson GJ, Wohlleben W, Sussmuth RD. J Am Chem Soc. 2004;126:5942. doi: 10.1021/ja0499389.Xie J, Pierce JG, James RC, Okano A, Boger DL. J Am Chem Soc. 2011;133:13946. doi: 10.1021/ja207142h.For recent review see: Ashford P-A, Bew SP. Chem Soc Rev. 2012;41:957. doi: 10.1039/c1cs15125h.
- 6.See, for example: Xie J, Pierce JG, James RC, Okano A, Boger DL. J Am Chem Soc. 2011;133:13946. doi: 10.1021/ja207142h.Nicolaou KC, Boddy CNC, Brase S, Winnssinger N. Angew Chem Int Ed. 1999;38:2096. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f.Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew Chem Int Ed. 1998;37:2700. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P.
- 7.Representative examples: Losey HC, Peczuh MW, Chen Z, Eggert US, Dong SD, Pelczer I, Kahne D, Walsh CT. Biochemistry. 2001;40:4745. doi: 10.1021/bi010050w.Losey HC, Jiang J, Biggins JB, Oberthur M, Ye XY, Dong SD, Kahne D, Thorson JS, Walsh CT. Chem Biol. 2002;9:1305–1314. doi: 10.1016/s1074-5521(02)00270-3.
- 8.Nicolaou KC, Hughes R, Cho SY, Winssinger N, Smethurst C, Labischinski H, Endermann R. Angew Chem, Int Ed. 2000;39:3823. doi: 10.1002/1521-3773(20001103)39:21<3823::AID-ANIE3823>3.0.CO;2-3.b) Also see reference 5d.
- 9.Nakama Y, Yoshida O, Yoda M, Araki K, Sawada Y, Nakamura J, Xu S, Miura K, Maki H, Arimoto H. J Med Chem. 2010;53:2528. doi: 10.1021/jm9017543. [DOI] [PubMed] [Google Scholar]
- 10.a) See supporting information for more detail. b) MIC’s for all new derivatives were measured and are no better than 4, see SI for data.
- 11.Nieto M, Perkins HR. Biochem J. 1971;123:789. doi: 10.1042/bj1230789.For review see: Perkins HR. Pharmacol Ther. 1982;16:181. doi: 10.1016/0163-7258(82)90053-5.McComas CC, Crowley BM, Boger DL. J Am Chem Soc. 2003;125:9314. doi: 10.1021/ja035901x.For crystal structure data see: Nitanai Y, Kikuchi T, Kakoi K, Hanamaki S, Fujisawa I, Aoki K. J Mol Biol. 2009;385:1422. doi: 10.1016/j.jmb.2008.10.026.
- 12.Mitchell RH, Lai YH, Williams RV. J Org Chem. 1979;44:4733. [Google Scholar]; b) Denmark SE, Burk MT. Proc Nat Acad Sci. 2010;107:20655. doi: 10.1073/pnas.1005296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber SL, Schreiber TS, Smith DB. J Am Chem Soc. 1987;109:1525. [Google Scholar]
- 14.We also note that several other brominating agents were examined, with comparable findings. See SI for details.
- 15.a) Fu X, Tan CH. Chem Commun. 2011;47:8210. doi: 10.1039/c0cc03691a. [DOI] [PubMed] [Google Scholar]; b) Ahmad SM, Braddock DC, Cansell G, Hermitage SA. Tetrahedron Lett. 2007;48:915. [Google Scholar]
- 16.Blondeau P, Segura M, Perez-Fernandez R, de Mendoza J. Chem Soc Rev. 2007:36. doi: 10.1039/b603089k. [DOI] [PubMed] [Google Scholar]
- 17.We prepared the corresponding methyl ester of 4, and we find that the analogous reactions with NBP, in the presence, or absence, or guanidine, deliver similar results, and neither reaction produces vancomycin-derived mono-bromides cleanly. Please see the Supporting Information.
- 18.Other additives, such as simple urea-based compounds, also exhibit this effect. Please see the Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.