Summary
Stochasticity is a hallmark of cellular processes and different classes of genes show large differences in their cell-to-cell variability (noise). To decipher the sources and consequences of this noise, we systematically measured pairwise correlations between large numbers of genes, including those with high variability. We find that there is substantial pathway variability shared across similarly regulated genes. This induces quantitative correlations in the expression of functionally related genes such as those involved in the Msn2/4 stress response pathway, amino-acid biosynthesis, and mitochondrial maintenance. Bioinformatic analyses and genetic perturbations suggest that fluctuations in PKA and Tor signaling contribute to pathway-specific variability. Our results argue that a limited number of well-delineated “noise regulons” operate across a yeast cell, and that such coordinated fluctuations enable a stochastic but coherent induction of functionally related genes. Finally, we show that pathway noise is a quantitative tool for exploring pathway features and regulatory relationships in un-stimulated systems.
Introduction
Isogenic populations of cells grown in the same environment show diversity in size, shape, cell cycle position, and gene expression. Evidence of such variability, or “molecular noise”, was reported in early studies of bacterial persistence during antibiotic treatment, λ phage burst size, and bacterial chemotactic behavior (Bigger, 1944; Delbruck, 1945; Spudich and Koshland, 1976). In addition to these early examples, cell-to-cell variability is increasingly being identified as a ubiquitous property of cellular networks, contributing meaningfully to the operation of many systems such as HIV latency and the response to chemotherapy (Burnett et al., 2009; Spencer et al., 2009).
Single cell studies documented two clearly distinct sources of cell-to-cell non genetic variability. First, the fundamentally stochastic nature of transcription and translation are a source of variation at the level of a single gene or transcript. Second, global differences in cellular physical properties such as size, energy state, or concentrations of key transcriptional, translational or metabolic components generate cell-to-cell variability (Thattai and van Oudenaarden, 2001; Blake et al., 2003; Raser and O’Shea, 2004; Pedraza and van Oudenaarden, 2005, das Neves et al., 2010).
Much less studied as a source of noise are fluctuations that are generated within a given pathway and propagate to its members, but do not percolate globally within the cell. Studies in the mating and galactose pathways in yeast (Colman-Lerner et al., 2005; Volfson et al., 2006), in addition to competence and sugar sensing in bacteria (Suel et al., 2006; Dunlop et al., 2008) have suggested that such pathway-specific fluctuations can have crucial phenotypic consequences.
Assays for decomposing noise have been instrumental in determining fluctuations that are specific to a single gene (intrinsic, uncorrelated) and those experienced by multiple genes (extrinsic, correlated). In these assays, intrinsic and extrinsic fluctuations are quantified by measuring the covariance between identical promoters in the same cell (Thattai and van Oudenaarden, 2001; Swain et al., 2002; Elowitz et al., 2002; Raser and O’Shea, 2004). Uncorrelated variation in expression of these two promoters reflects intrinsic noise resulting from stochastic fluctuations in the process of gene expression itself. In contrast, extrinsic noise is defined as the correlated variation in the expression of the two genes resulting from either global or pathway-specific fluctuations. While studies in yeast and bacteria demonstrated that intrinsic noise was dominant for weakly expressed genes and documented the determinants of such noise (Ozubdzk et al., 2002; Blake et al., 2003; Raser and O’Shea, 2004; Golding et al., 2005; Raj et al., 2005; Newman et al., 2005; Becskei et al., 2005; Taniguchi et al., 2010), the contribution of extrinsic noise to gene expression heterogeneity varies greatly across protein types and has not been systematically studied. Furthermore, the impact of pathway-specific versus global fluctuations on extrinsic noise remains unclear.
In this paper, we probe the contribution of global versus pathway-specific sources to the expression noise of a broad array of S. cerevisiae genes. We show that while all genes experience a modest amount of noise due to generic cellular factors, a substantial subset of genes experience high extrinsic noise produced by pathway-specific fluctuations. This noise is shared across similarly regulated genes, and induces quantitative correlations which we use to identify co-regulated groups of genes. We further show that this correlation measure, which is derived from steady-state measurements in un-stimulated cells, is a quantitative metric of the sensitivity of a given gene to its upstream pathway, and is therefore predictive of the response of a gene to environmental perturbations. Using this approach, we identify distinct noise ‘regulons’ involved in the general stress response, mitochondrial regulation, and amino acid biosynthesis. Genetic perturbations to the PKA and Tor pathway quantitatively alter noise-induced correlations, implicating these pathways as important causative noise sources in budding yeast.
Results
A strategy for measuring covariance in protein expression using a single fluorescent protein
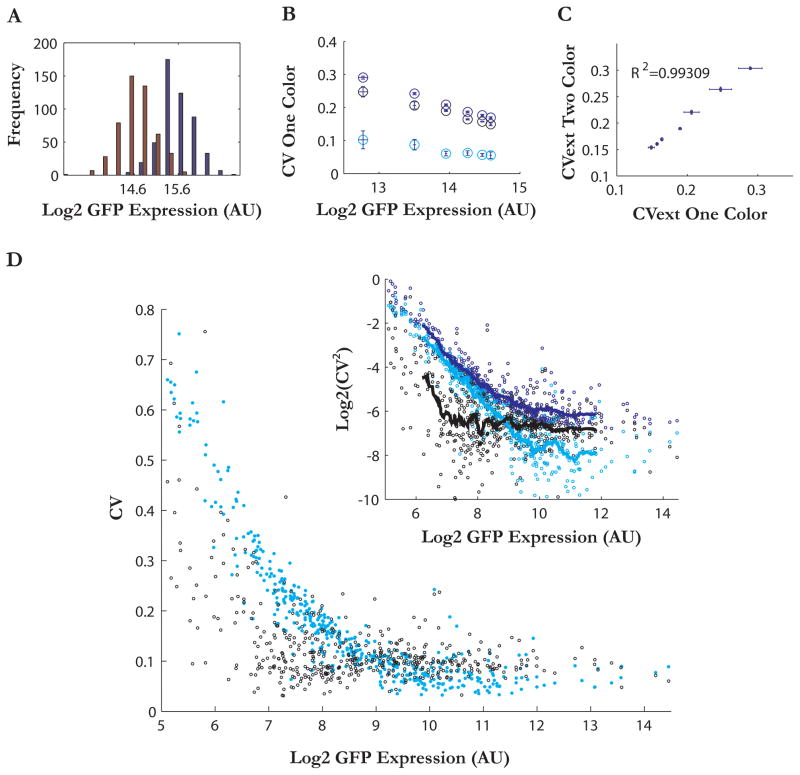
To define the role of pathway specific noise in gene expression, we sought to identify groups of co-regulated genes which exhibit significant extrinsic noise. To determine the relative contributions of intrinsic and extrinsic noise in the expression of a diverse set of genes, we adapted a noise decomposition strategy utilizing isogenic diploid cells expressing either one or two copies of the same fluorescently-tagged protein (FP). This single color approach avoided problems with equivalence between different color FPs and allowed us to take advantage of existing libraries of S. cerevisiae strains in which each protein is expressed as a carboxy-terminal GFP fusion from its endogenous promoter and natural chromosomal position (Howson et al., 2005). To validate this approach which has previously been used in low throughput (Volfson et al., 2006), we compared one and two color noise decompositions of a titratable galactose expression system driven by the small molecule estradiol (see supplemental methods). The strain expressing two copies of pGAL1-YFP exhibited a unimodal distribution whose mean was twice as fluorescent as the strain expressing only one copy (Figure 1A). Consistent with previous reports, extrinsic noise of pGAL1 was significantly higher than its intrinsic noise (Volfson et al., 2006; Figure 1B). The need for variance measurement in two populations of cells (rather than a single measurement in the two color assay) resulted in more cumulative experimental error in the one FP strategy (Figure 1C). However, the extrinsic noise values estimated by the two techniques were linearly correlated (R2 = 0.99, Figure 1C).
Figure 1. Extrinsic versus intrinsic noise decomposition across the proteome.
(a) Histograms of the single cell fluorescence of populations of cells expressing either one (red) or two (blue) copies of pGal1-YFP. The mean of the two distributions are separated by one log2 unit.
(b) Total, intrinsic, and extrinsic noise plotted against log mean expression for seven levels of induction of pGal1-YFP. Noise was quantified as the Coefficient of Variation (CV=σ/μ) of the YFP distribution.
(c) Comparison of extrinsic noise values calculated using a two color (pGal1-YFP, pGal1-mCherry) or one color approach.
(d) Intrinsic (cyan) and extrinsic(black) noise plotted against log2 mean expression for 465 genes. Inset: log2(CV2) plotted against log2(mean), running means (smoothing window of 30) for intrinsic(cyan), extrinsic(black), total (dark blue) noise.
Intrinsic noise scales with protein expression level, whereas extrinsic noise shows a more complex pattern
We constructed isogenic diploid strains each harboring one or two copies of 456 highly expressed GFP-tagged proteins. Using this single-color approach and flow cytometry, we computed intrinsic and extrinsic noise decompositions in the expression of these proteins (Figure 1D, S1, Table S1). After correcting for cell size variability (Supplemental information), our data confirmed that at low expression levels, noise is dominated by intrinsic fluctuations in agreement with previous work (Raser and O’Shea, 2004; Newman et al., 2005; Taniguchi et al., 2010). Indeed, the coefficient of variation for intrinsic noise (CVint) declines according to an inverse square-root relationship as mean expression increases, as would be expected from fluctuations resulting from uncorrelated stochastic processes such as production and destruction of mRNA and protein, or random partitioning of cellular components during cell division (Paulsson, 2004; Huh and Paulsson, 2011). The overall trend of intrinsic noise was well captured (R2=0.88) by a two parameter model CVint= α +β/(μ)0.5, where α represents the plateau of intrinsic noise observed in the data and β the influence of the mean (μ). In contrast, extrinsic noise fits more poorly to the same model functional form (R2=0.35). Most proteins exhibited extrinsic noise values around a well-delineated CVext=0.09 “floor” (Figure 1D, inset). However, around 20% of the proteins in the dataset (N=86) displayed elevated extrinsic noise. Therefore, noise in S. cerevisae can be separated into local stochastic components that dominate at low expression, a moderate level of global variation common to all genes due to variations in the overall transcriptional, translational, and metabolic capacity of the cell, and substantial extrinsic noise of unknown origin affecting only a subset of genes.
Promoters of high extrinsic noise proteins are enriched for specific transcription factor binding sites
Examination of the proteins that exhibited the top 10% highest extrinsic noise revealed that their respective gene promoters were enriched for several transcription factor binding motifs. These included binding sites for the stress responsive transcription factors Msn2/4, in addition to amino acid synthesis linked transcription factors such as Gzt4 and Met31/Met32 (p-value<0.05). Further analysis of high noise genes revealed ‘AGGGG’, the consensus Msn2/4 binding site (Schmit et al., 1996), as the most enriched 5-mer sequence. The presence of specific transcription factor binding sites could reflect the presence of pathway-specific noise that induces correlated fluctuations across a set of target genes. Alternatively, it could reflect an enrichment of these pathways with genes that are particularly vulnerable to shared global fluctuations. If the enrichment was due to global fluctuations, then genes exhibiting high extrinsic noise would be expected to be strongly correlated irrespective of their pathway membership. By contrast, if noise is pathway specific, then correlations should appear only between genes that are members of the same pathway.
Genes regulated by Msn2/4 show correlated steady-state expression
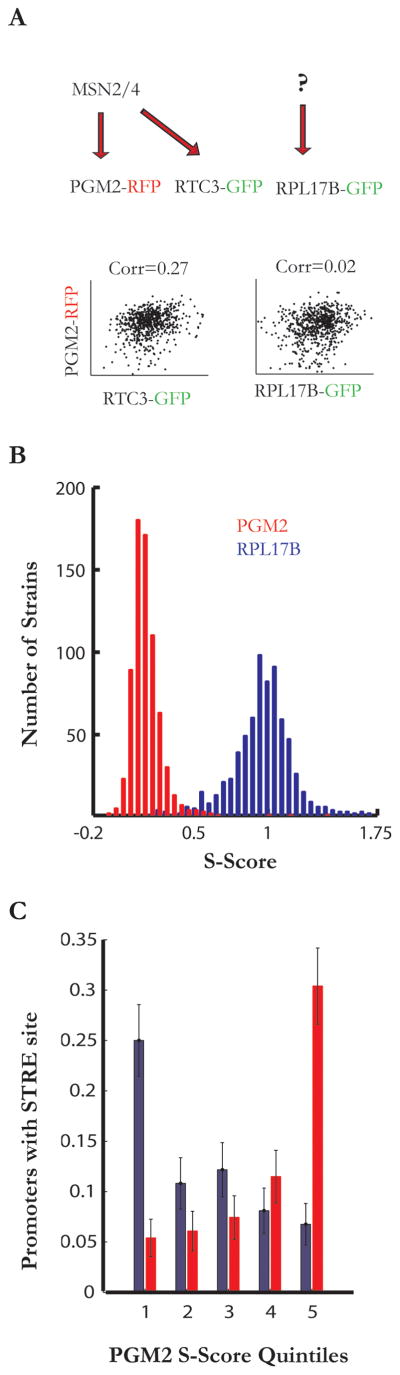
To discriminate between these two scenarios, and test whether noise-induced correlations are predictive of pathway membership, we first focused on proteins whose gene promoters are regulated by the Msn2/4 general stress response since several of its canonical members (Pgm2, Hsp12, Tfs1) were present in the high noise category. We selected the extrinsically noisy protein Pgm2-RFP as a specific reporter of the pathway, and verified that its expression was dependent on Msn2/4 activity (data not shown). Since the single FP approach to estimating covariance becomes increasingly error prone as the proteins diverge in expression levels, we turned to a two-color strategy to measure the covariance of the most abundant proteins in the GFP library (743 genes) with either Pgm2 or the “quiet” ribosomal protein Rpl17B (Table S1, Figure 2A, B). To compare covariance without bias, we defined the S-score as the covariance of two proteins, normalized by the query proteins’s (Pgm2 or Rpl17B) extrinsic noise (see supplemental methods). The distribution of Pgm2 S-scores was reproducible (R2=0.87) and right skewed, with the majority of proteins weakly correlated with Pgm2 and only a subset showing high S-scores (Figure 2B). Furthermore, for proteins in this set, the value of their Pgm2 S-Score was highly predictive of the presence of Msn2/4 binding sites in their gene promoters (17 of the top 20 genes have consensus ‘AGGGG’ sites in their promoters, Figure 2C, S2B). In contrast Rpl17B S-scores showed a distribution that was symmetrical and centered near one, reflecting the sensitivity of most cellular components to this ribosomal gene. Furthermore, promoters of genes with high Rp17B S-scores showed no enrichment for Msn2/4 binding sites (Figure 2C).
Figure 2. Stress genes inherit fluctuations from the transcription factors Msn2/4.
(a) Schematic illustration of correlation analysis of GFP-tagged and RFP-tagged proteins expressed within a single cell. Proteins encoded by genes regulated by the same upstream factor (the transcription factor Msn2/4) show strong correlation while proteins regulated by unrelated processes show weak correlation.
(b) Histograms of the Pgm2 (red) and Rpl17B (blue) S-scores for 750 of the most abundant protein in the S. cerevisiae GFP collection.
(c) Percent genes with one or more STRE elements in their promoters (700 bp before the start codon) versus quintiles of Pgm2 or Rpl17B S-score, error bars show standard error based on a binomial model.
Correlations induced by steady-state fluctuations are predictive of dynamic response to stimulus
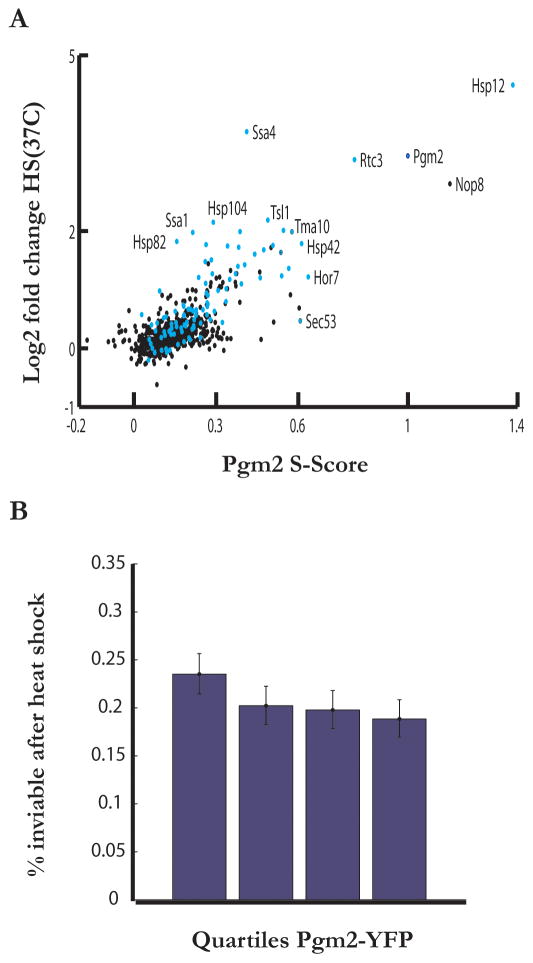
The S-score metric reports on local sensitivity in the expression of a gene to the activity of an upstream regulator using a simple linear model (Supplementary material). The applicability of such a model should be extendable to larger input perturbations when genes respond linearly to changes in activity of the transcription factors. This is plausibly the case for stress responsive genes, which have been observed to exhibit graded induction profiles (Giorgetti et al., 2011; also our own observations with HSP12 and PGM2, unpublished). To test whether the low amplitude variability resulting from noise at steady-state, as captured by an S-score, is quantitatively predictive of the response of a gene to changes in the environment, we compared the Pgm2 S-score of a protein with the protein’s expression in response to a mild heat shock—37°C for 30min. In general, we find a strong positive correlation (Pearson correlation 0.74, p-value= 3.69e-130) between these two quantities (Figure 3A). Interestingly, the subset of genes such as HSP82, SSA4, and SSA1 whose expression (quantified by protein abundance) responded strongly to heat shock while exhibiting low S-scores were targets of the heat shock transcription factor Hsf1 in addition to Msn2/4. These results argue that extrinsic noise at steady-state is shared across the members of the Msn2/4 stress response pathway. The presence of this pathway-specific noise defines a well-delineated Msn2/4 unit, easily separable from heat shock genes, with the S-score representing a quantitative measure of a component’s pathway membership.
Figure 3. Fluctuations in Msn2/4 target genes are predictive of response to stress.
(a) Plot of the fold induction of a gene following heat shock against its Pgm2 S-value for assayed genes. Genes with two or more Msn2 binding sites (‘AGGGG’) in the 700bp before their start codon are colored blue.
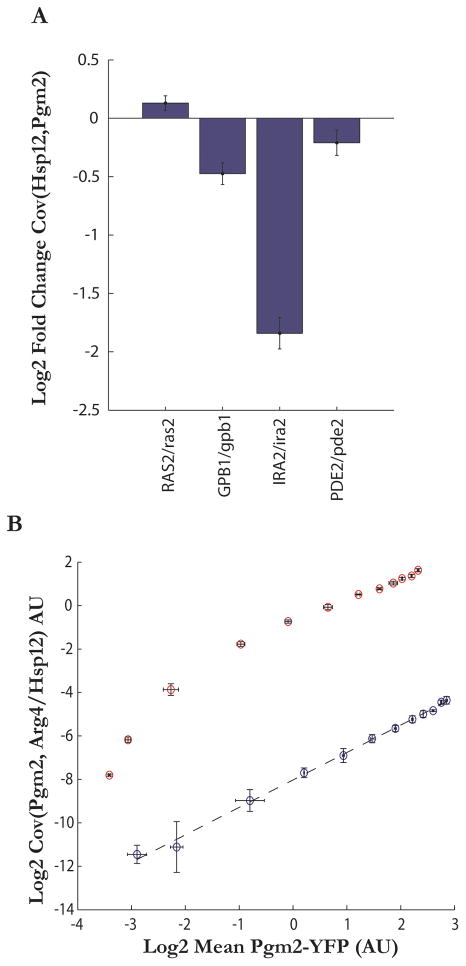
(b) Survival of cells as a function of their basal Pgm2 levels. Pgm2-YFP expression was determined for individual cells in mid-exponential phase (OD=0.5). Cells were then heat shocked (50C, 20 min), and stained with propidium iodide to detect dead cells. The probability of cell death was lower (18.84% (95% CI = 16.96–20.84)) in the top 25% of Pgm2 expressing cells compared to the bottom quarter (23.51% (95% CI = 21.45–25.66%)). Statistics were computed using a binomial test (N=1608 in each quartile, error bars show 95% CI).
Noise in MSN2/4 general stress pathway is predictive of survival in stress conditions
Cell-to-cell variability has been implicated in population survival of yeast (Smith et al., 2007; Blake et al., 2006) and cancer cells (Singh DK et al., 2010) exposed to acute environmental stressors. In terms of the general stress response, genetic (Sadeh et al., 2011) and environmental (Yamamoto et al., 2008) perturbations that activate Msn2 were shown to result in increased survival in severe stress conditions. As a result, we hypothesized that higher stochastic expression of Msn2/4 target genes allows for enhanced resistance to stress. This model requires, first, that Msn2 activation is sufficient to provide resistance to stress. Indeed, we find that overexpression of Msn2 provides resistance to heat shock, and that most importantly, this resistance is graded and proportional to the expression level of the Msn2 target genes such as HSP12 (Figure S3A). To pinpoint the physiological circumstances under which natural variation in Msn2 is protective, we imaged exponentially growing cells expressing Pgm2-YFP and determined basal Pgm2 levels in individual cells. We then re-examined the same cells after severe heat stress (50°C for 20min on a hot plate) and determined their viability using the dead cell marker Propidium Iodide (PI). For a very low density exponentially growing population (OD~0.05), we did not observe a statistically significant correlation between basal Pgm2-YFP levels and enhanced survival (Figure S3B). However, interestingly, cells grown to late exponential (OD=0.5) exhibited a quantitative correlation between survival and basal Pgm2 expression level. Specifically, if the Pgm2-YFP distribution is divided into quartiles, and cells within each of these quartiles scored for viability with PI staining, then 23.51% (95% CI = 21.45–25.66%) of cells in the bottom quarter of Pgm2 were inviable (Figure 3B). In contrast, only 18.84% (95% CI = 16.96–20.84) of cells in the top quarter of the Pgm2 distribution were inviable (N=1608), amounting to a 20% increase in survival (Figure 3B). These data establish an intricate connection between phenotypic diversity and stress resistance.
Multiple noise regulons containing a coherent set of genes exist in S. cerevisiae
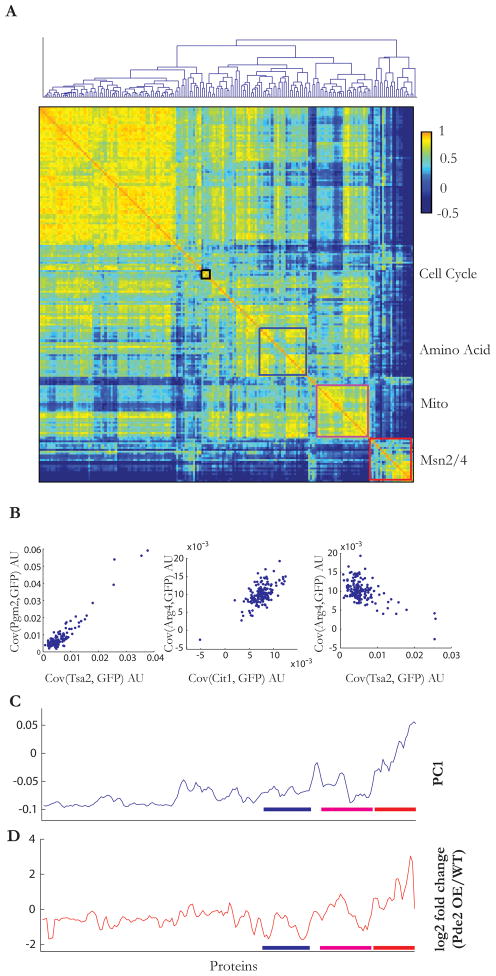
What other noise regulons, in addition to targets of Msn2/4, might exist in a yeast cell? To address this question more globally, we picked 44 proteins (in addition to Pgm2) whose expression exhibited high extrinsic noise to tag with mCherry (see Supplemental Material). We then measured their covariance with a set of 182 GFP-tagged proteins spanning a range of pathways, noise, and expression levels (Table S3). To group proteins according to their noise patterns, we computed the correlations across the dataset to produce a 182 square matrix which was then hierarchically clustered, revealing four major groups (Figure 4A). GO term analysis indentified three of these blocks as corresponding to stress response, mitochondrial, and amino acid biosynthesis functional categories (Figure S4). The fourth block, which dominates the upper left quadrant, was a more heterogeneous mix which may represent generic cellular noise. Other smaller groups could also be identified, including a group containing three out of the four histone proteins present in the dataset (Htz1, Hta2, Htb2) in addition to Tcb3 and YMR295C, two cell cycle regulated proteins (Spellman et al., 1998).
Figure 4. Noise measurements divide the genome into distinct regulons and inform an understanding of the dimensionality of a cell.
(a) A map of noise correlations among 182 proteins showing distinct blocks that share patterns of covariance. Prominent among these are Amino Acid biosynthesis (magenta outline), Mitochondrial (blue outline), and Stress responsive (red outline) clusters. A smaller group of cell cycle regulated genes are also apparent (black).
(b) Scatter plots comparing the covariances of Pgm2, Tsa2, Arg4, and Cit1 with the 182 proteins show similar covariance patterns for two members of the same stress responsive cluster (Pgm2, Tsa2), anti-correlated patterns for two members (Arg4 and Tsa2) of distinct cluster, and the presence of ‘off-diagonal’ interactions between different clusters (Cit1 and Arg4).
(c) A principal component analysis of the covariance dataset shows that five principle components (PCs) can describe ~80% of the observed variance. Contribution of the noise in different genes to the first PC, PC1, is plotted in the order in which they appear in (a). Values are smoothed using a sliding window of size 3.
(d) Expression of 184 GFP-tagged proteins in a Pde2 over-expression background. The results are reported as a log2 of the ratio of GFP in the overexpression strain normalized to that of the wild-type. Overexpression of Pde2 was achieved using an estradiol inducible GAL1 promoter. Proteins are in the order in which they appear in (a). Induction values were smoothed with a sliding window of size 3.
If the putative amino acid, mitochondrial, and stress responsive clusters are co-regulated as the noise analysis predicts, then the promoters of the genes encoding these proteins should be enriched for binding sites of the appropriate transcription factors. Drawing on published ChIP data (Harbison et al., 2004), we determined that the amino acid biosynthesis group was enriched for the amino acid regulatory transcription factors Gcn4, Met4, and Arg80/81, that the mitochondrial group was enriched for members of the Hap2/3/4/5 complex which is known to regulate heme synthesis and oxidative metabolism, and that the stress responsive group was significantly enriched for Msn4 binding (Figure S4).
The presence of these well-delineated clusters indicates that members in the same cluster show related patterns of covariances with the 182 proteins in the dataset. For example, the covariances of Pgm2 and Tsa2 (encoded by two genes in the ‘stress’ group downstream of Msn2/4) with the 182 test proteins were strongly linearly related (Pearson Correlation = 0.94, Figure 4B). A positive linear relationship also existed between the covariances of the mitochondrial protein Cit1 with the 182 test proteins and those of the amino acid biosynthesis protein Arg4 with the same proteins (Pearson Correlation = 0.65). Furthermore, the covariances of Arg4 with the 182 test proteins were negatively correlated with those of Tsa2 (Pearson Correlation = −0.58). These robust ‘off-diagonal’ interactions indicate that this fluctuation-based analysis is capable of detecting pathways that are correlated by specific upstream signaling, in addition to a shared transcription factor. It also suggests that at least part of the measured correlative fluctuations is generated upstream of the particular transcription factors which regulate these groups of genes (see Supplementary Materials for an extended analysis).
These results suggest that in addition to generic extrinsic fluctuations resulting from variability in general cellular factors (noise floor), genes across a yeast cell are subject to a small number of structured and pathway-specific extrinsic noise sources. Such noise sources induce correlated fluctuations in the expression of pathway genes, and hence can be used to assign genes to pathways, and as a measure of pathway activity. To explore this idea further, we took advantage of the data representation in terms of a covariance matrix which renders it interpretable in terms of standard dimensionality reduction techniques such as Principal Component Analysis (PCA). By subjecting the covariance matrix to PCA, we can compute the number of dimensions necessary to describe the noise patterns under unperturbed conditions. PCA analysis revealed that ~80% of the variance in the data could be explained by the first five principal components (Figure S4).
The principal components determined by PCA appeared readily interpretable in terms of cellular pathways. For example, the contribution of the first principal component to each gene, plotted in the same order as the clustered data, showed a strong peak spanning the Msn2/4 stress responsive cluster and a broad negative region corresponding to the remaining genes (Figure 4C). Comparison of the first principal component to published mRNA expression data over a range of environmental conditions (Gasch et al., 2000) showed a correlation (Pearson correlation > 0.4) to a range of stresses including heat shock and growth on non-glucose carbon sources.
Growth on poor carbon sources, as well as heat shock, is known to inactivate the PKA pathway resulting in Msn2/4 activation (Gorner et a., 1998; Gorner et a., 2002). To directly test if the pattern of gene expression predicted by PCA analysis is consistent with PKA inactivation, we overexpressed Pde2, a negative regulator of PKA activity and measured the change in the expression of the 182 query genes. The log2 change in gene expression after 8hrs of Pde2 overexpression from an estradiol regulated galactose promoter correlated with the first principal component (Pearson correlation 0.62, p-value 8.60e-21), showing a pattern of stress gene expression that again mirrored the steady-state Pgm2 S-scores (Figure 4D).
Genetic analysis traces noise in regulons to PKA and TOR pathways
We next asked if fluctuations in PKA activity causally affect the fluctuations in the Msn2/4 pathway. since deletions or overexpression of PKA-related genes have dramatic phenotypic effects on cellular physiology, making it difficult to tease out specific effects on noise, we turned to heterozygous deletions of PDE2, RAS2, GPB1, and IRA2—core regulators of PKA. The mean normalized covariance of Pgm2 and Hsp12 increased in RAS2/ras2 and decreased in PDE2/pde2, GPB1/gpb1, and most significantly in IRA2/ira2 (Figure 5A, p-values<0.05 in all cases). These results support the notion that alterations in PKA activity affect the noise properties of the Msn2/4 cluster specifically, and may be a major driving force for the correlations observed between its members. These results are further corroborated by a simple linear model of gene expression which predicts that the covariance of two proteins (e.g. Pgm2 and Arg4) which don’t share a transcription factor should change linearly as a function of mean expression when one of the transcription factors (Msn2) is titrated. On the contrary, under this model, the same titration of Msn2 should cause the covariance between two downstream targets of Msn2, Hsp12 and Pgm2, to increase non-linearly (Figure 5B). This non-linear effect is indeed observed when the upstream regulator PKA is disrupted (Figure S5, and Supplemental methods). These results argue that covariance measures within a pathway have an intricate, potentially information rich, relation with mean expression.
Figure 5. Genetic perturbations tie the Msn2/4 noise regulon to PKA signaling and demonstrate that mean expression of a pathway and covariance within a pathway have a complex relationship.
(a) Noise-induced covariance between Hsp12-RFP and Pgm2-YFP is altered in heterozygous deletes of PKA pathway members, showing significant decreases in PDE2/pde2, IRA2/ira2, and GBP1/gbp1 strains and an increase in the RAS2/ras2 strain. Error bars represent standard error of means (n=6)
(b) Covariance of Pgm2 and Arg4 as a function of mean Pgm2 expression as the levels of Msn2 are varied. Overexpression of constitutively active Msn2 to various levels results in a log-linear increase in covariance between Arg4 and Pgm2 as a function of Pgm2, and non-loglinear increase in covariance between Pgm2 and Hsp12, two proteins whose genes promoters are targets of Msn2. Error bars represent standard error of means (n=3).
Extensive knowledge of Msn2/4 regulation was used to guide the identification of PKA as a plausible noise regulator in the system. To investigate noise sources in other regulons, we used a less biased screening approach based on the hypothesis that, much like our findings for the Msn2/4 pathway, disruption of key regulators would result in quantitative changes in covariances between pathway genes.
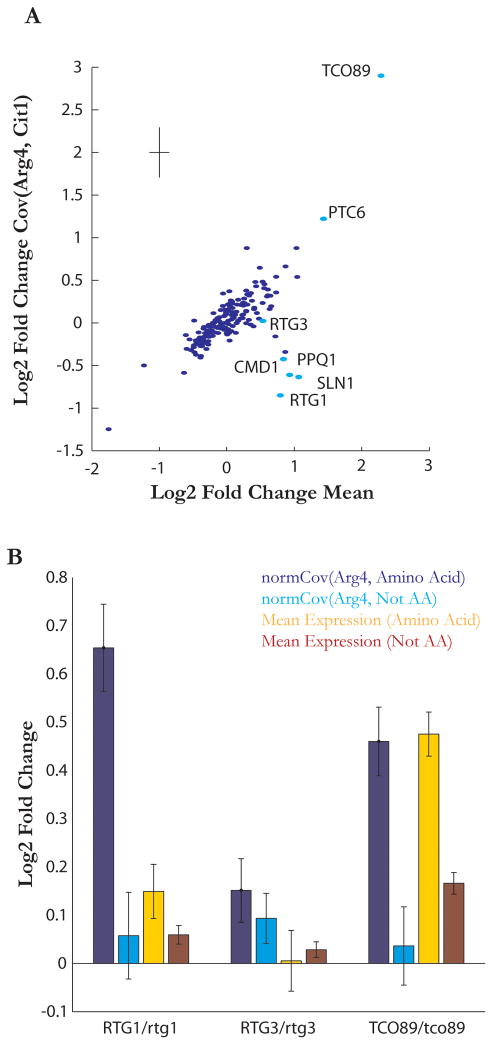
Although the amino acid biosynthesis and mitochondrial genes formed well-delineated and distinct clusters in our dataset, off- diagonal interactions are observed between the two groups (Figure 4A). Therefore, we treated these clusters as one large group for this analysis. We crossed a strain expressing Arg4-GFP (an amino acid biosynthesis gene) and Cit1-mCherry (a mitochondrial TCA cycle gene) to a collection of 188 diploid strains deleted for one copy of a range of signaling related factors, including kinases and transcription factors (Table S4). We then measured the expression of Arg4 and Cit1 and the covariance between them in each such heterozygous strain (Figure 6A). Here again, heterozygous deletions showed negligible growth phenotypes and limited impact on average gene expression, therefore presenting us with the opportunity to probe noise without strong confounding effects (Springer et al., 2010). In the mean-covariance space, most heterozygote strains fall on a straight line where increases or decreases in expression result in proportional changes in covariance, a behavior that is expected of multivariate Poisson random variables (Holgate, 1964). There are, however, a few outliers. For example, TCO89/tco89 heterozygous deletion substantially increases the mean and the Arg4/Cit1 covariance. Tco89 is a Tor component, and Tor is known to regulate nitrogen metabolism and mitochondria (Reinke et al, 2004). More interestingly, heterozygous deletions of a cluster of genes, such as RTG1, SLN1, CMD1, and PPQ1, increased Cit-Arg4 geometric mean but reduced their covariance, violating the expected relationship between mean and covariance and showing a ‘noise phenotype’. This is plausibly the case because these genes play a substantive role in modulating pathway fluctuations.
Figure 6. Covariance measurements of heterozygous strains link noise in amino acid biosynthesis to Tor signaling.
(a) Covariance of the amino acid biosynthesis protein Arg4-mCherry and the mitrochondrial protein Cit1-GFP plotted against the geometric mean of Arg4-mcherry and Cit1-GFP for 188 heterozygous deletion mutants. Values are displayed as log2 fold change over WT. Strains referred to in the text are highlighted in cyan. Error bars in upper left show standard deviation of replicate measurements.
(b) Change of Arg4 mean and covariance between Arg4 and members in the amino acid biosynthesis group in RTG1/rtg1, RTG3/rtg3, and TCO89/tco89 heterozygous strains. Values are displayed as log2 fold change over WT. RTG1/rtg1 and TCO89/tco89 heterozygous deletions cause significant increases in covariance, while RTG3/rtg3 has no significant effect on the covariance. TCO89/tco89 heterozygous deletion, but not RTG1/rtg1 heterozygous deletion, increases mean gene expression of amino acid biosynthesis genes. Error bars represent standard error of means.
To further dissect the role of the TOR pathway in the noise phenotype of the amino acid biosynthesis regulon, we measured the covariance between the biosynthetic protein Arg4-mCherry and the set of 182 GFP query strains in heterozygous deletions of RTG1, RTG3, and TCO89. Heterozygous deletions of RTG1 and TCO89 resulted in significant and specific increase of Arg4 covariance with members of the amino acid cluster. In contrast, heterozygous deletion of RTG3, a RTG1 binding partner, had no significant effect on amino acid S-scores consistent with observations in the initial screen (Figure 6B). Interestingly, the increase in covariance observed among the amino acid biosynthesis proteins in the RTG1/rtg1 background stands in contrast to the decrease observed in the covariance between Arg4 and Cit1 in the same strain. This suggests that in the RTG1/rtg1 strain, there is a de-correlation between the mitochondrial and amino acid regulons, and an enhanced coherence within the members of the amino acid regulon.
Concomitant with increased Arg4 covariance in TCO89/tco89, members of the amino acid regulon showed increased mean expression. Interestingly, however, these genes did not show any appreciable mean increase in the RTG1/rtg1 background, demonstrating that fluctuations-induced correlations contain information complementary to mean expression. In this case, RTG1 is involved in a negative feedback loop with TOR through nitrogen metabolic processes (Liu and Butow, 1999). Disruption of this loop is shown here to increase noise, in accordance with a role of negative feedback in attenuating fluctuations disproportionate with its effect on mean expression. Overall, these results argue that much like Msn2/4 responsive genes, amino acid biosynthesis genes are correlated at the single cell level by upstream regulators. In this case, our data implicate the Tor pathway as a major noise source.
Discussion
Stochasticity and noise are universal features of cellular systems and a key source of non-genetic diversity in populations of cells and organisms. Here, we have defined a strategy for dissecting the contributions of different processes to noise in S. cerevisiae. We find that, in accordance with previous reports, intrinsic noise is strongly predicted by expression level. Variability in global transcriptional and translational capacity between cells also impact gene expression. These sources of noise affect the expression of all genes and manifest as an extrinsic noise floor. In addition to such global sources of extrinsic variability, genes are also subject to specific noise that is generated within the pathway in which they operate. When noise is pathway exclusive, it should induce covariances among members of the pathway, defining structured “noise regulons”. We have shown that this is indeed the case across a yeast cell, and that genes that are members of a noise regulon are also functionally related. Noise regulons further map onto well-defined cellular pathways such as the general stress response, amino acid biosynthesis and mitochondrial regulation.
What is the underlying source of these noise regulons? The existence of large groups of correlated genes in populations of isogenic cells is unlikely to result from external inhomogeneity in the growth environment of the cells as similar noise values have been independently measured under different conditions including in a chemostat-controlled environment and by flow cytometry or microscopy (Raser and O‘Shea, 2004; Newman et al., 2005). The specificity of the gene groups and the nutrient rich growth conditions for our experiments also argue against the possibility that correlated noise is the result of distinct internal states due to variations in metabolism or energy state among cells. As a result, we believe that such regulons, consisting of fluctuation-correlated genes, reflect biologically relevant modular structures that might exist to maintain useful but controlled diversity across a population. Such a ‘bet-hedging’ strategy might be instrumental in allowing a population of cells to successfully navigate unanticipated changes in its environment (Thattai and van Oudenaarder, 2004). A scheme based on a coherently fluctuating program, rather than on a set of discordantly fluctuating individual genes, would constitute a robust implementation of a successful bet-hedging program. The noise regulons we have identified, which are coherently coordinated within one cell but variable across a population, might represent such a scenario. In particular, it is interesting to note the presence of the MSN2/4 pathway which promotes survival in the face of acute stress as one such noise regulon. It is also interesting to note the absence of programs (for example an HSF1 regulon) that mediate long term adaption and recovery from stress (Yamamoto et al., 2008).
Regardless of the source or functional repercussions of pathway noise, we have shown that noise measurements can be used as a critical non perturbative tool for tracing co-regulation. In particular, our data demonstrate a correlation between fluctuations at steady-state and dynamic induction of genes in response to environmental stimuli for stress responsive genes. This relationship between noise at steady state and dynamic response has previously been observed in the context of bacterial chemotaxis (Park et al., 2010). Our results argue that the quantitative relationship between local fluctuations and pathway induction is likely to hold in much more general contexts. This is further supported by analyses illustrating that noisy genes in S. cerevisiae also exhibit high expression variability across conditions (Lehner, 2010). Overall, this positions molecular noise as a quantitative phenotype for probing pathway regulation (Dunlop et al., 2008). The ability of noise to probe cellular organization is appealing as it does not require external perturbations. Thus, no knowledge of when, how, or what induces the pathway of interest is necessary. Furthermore, no severe genetic or physiological perturbations are required, avoiding pleiotropic effects. Importantly, noise measurements allow for the identification and dissection of distinct pathways that have overlapping responses to the same set of inputs. For instance, stress-mediated induction of HSF1 and MSN2/4, which are components of two distinct pathways (heat shock and general stress response respectively), induce overlapping genes making them challenging to disentangle based on mean expression levels alone. Nonetheless, noise-based measurements in terms of the S-scores distinguished between the components of the two systems.
While steady-state noise measurements contain a wealth of information about cellular organization, there is no assumption that cellular wiring should be constant across conditions or time. Therefore, perturbations could be easily incorporated into this general analysis framework. For example, applying a similar covariance analysis to a network as it evolves in response to an input might allow a mapping of network connections as they dynamically occur. Genetic perturbations such as heterozygous deletions are particularly well-suited for noise analysis. First, heterozygous deletions are simple to apply universally and amenable for high-throughput screening strategies. Furthermore, previous studies in the yeast S. cerevisiae (Springer et al., 2010), also corroborated by our data, indicate that the mean output of pathways is robust to copy number of pathway constituents. Therefore, changes in noise patterns in these deletions may carry information that is not present in the mean expression and suggests that quantitative noise measurements may be a fruitful strategy for studying complex regulatory interactions.
Experimental Procedures
Yeast Strains/protocols
All yeast strains used for these experiments are derived from W303, BY4741, or BY4742. Isogenic diploid strains containing one or two copies of a given protein were constructed by mating the GFP collection strains to a Synthetic Genetic Analysis (SGA) strain, sporulating and mating the resulting haploids back into the GFP collection (see supplemental methods). Individual reporter strains were constructed by homologous insertion of an RFP protein (mCherry or mKate2) at the C-Terminal end of the open reading frame with a URA3 marker immediately 3′. Reporter strains marked with mCherry (or mKate2) were crossed to strains from the GFP library (Open Biosystems), with a selection step for diploids in SD-Ura/-His. For deletion analysis, strains were constructed with one gene tagged with mCherry (URA3), and one with GFP (HIS3). These SGA strains were mated into subsets of the deletion collection and heterozygous diploids selected in SD-Ura+G418 (500ug/ml). Overexpression of PDE2 was accomplished by placing PDE2 under the control of a GAL1 promoter in an SGA strain expressing an estradiol inducible construct, this strain was mated to GFP strains and diploids selected as described above. Expression of PDE2 was induced by the addition of 100nm estradiol (Sigma) for 8hrs before measurement.
Growth and fluorescence measurements by flow cytometry
For noise and correlation measurements (Figures 1 and 2) cells were grown to saturation in 96-shallow well plates (Costar) and then diluted into fresh media, grown at 30C on orbital shakers (Elim) for 12hrs to an OD of ~0.2, and subsequently diluted and grown for 8hrs to an OD of ~0.05 before measurement. Larger scale correlation measurements (Figure 3) were preformed in 384-well plates (Thermo).
All cytometry measurements were made on a Becton Dickinson LSRII flow cytometer, along with an autosampler device (HTS) to collect data over a sampling time of 6–12 seconds, typically corresponding to 5000–10000 cells. GFP and YFP were excited at 488nm, and fluorescence was collected through a HQ530/30 bandpass filters (Chroma), mCherry and mKate2 were excited at 561 nm and fluorescence collected through 610/20 bandpass filter (Chroma).
Microscopy and image analysis
Cells expressing Pgm2-YFP were plated in SD complete onto ConcanavalinA coated 96 well glass bottom plates, allowed to settle and then washed twice with fresh media. Samples were imaged on a Nikon TE-2000 inverted scope with arc-lamp illumination using RFP(610/20nm) and YFP (530/30nm) chroma filters. To heat shock cells, the 96 well plate was placed on a Elim plate shaker set at 50C for 20min. The media was then removed and replaced with 80ul of TE+1ug/ml propidium iodide. Cells were then incubated for 10minutes at room temperature, and imaged. Non-viable cells were determined by bright RFP staining. Images before and after heat shock were aligned using the ImageJ StackReg utility, and cells segmented and fluorescence computed using custom Matlab code. Relative survival probabilities and the associated errors were computed using a binomial model of survival.
Flow cytometry Data analysis
All data was analyzed with custom Matlab software. Raw cytometry data were filtered to remove errors due to uneven sampling and remove outliers using an MCD method (Rousseau and Van Driessen, 1999). Variability in cell size was corrected using a linear transformation from the side scatter parameter (see supplemental materials for detail). Extrinsic noise was calculated from the one and two-FP strain measurements using the following formula:
Here, a1 and a2 are indistinguishable alleles of the same gene. Var(a1) = Var(a2) is measured in the one-FP strains. Var(a1 + a2) is measured in the two-FP strains. Extrinsic noise is then given by the normalized covariance [Swain et al., 2002]
The “S-score” of gene b with respect to gene a1 is defined as . Here, G is given by , where a1 and a2 are two alleles of the same gene tagged with different FPs.
TF binding site and GO term Analysis
To map S-score measures from flow cytometry data to cis regulatory motifs, we calculated transcription factor binding sites in the 700 base pairs immediately prior to the start condon using the database of motifs from the Swiss regulon (Pachkov et al., 2007). ChIP data were obtained from (http://web.wi.mit.edu/young/regulatory_code/) based on the work of Harbison et al. (Harbison et al, 2004). As the ChIP data has very weak representation of Msn2 or Msn4 showing no enrichment at many canonical targets such as PGM2, HSP12, TLS1, TFS1, or GSY1, we used transcription regulatory motifs to analyze data sets where the stress response dominated (Figs. 1 and 2), and ChIP data for the more general analysis (Fig. 3). The most recent GO term database was downloaded from SGD. For both data types p-values for enrichment were calculated with a hypergeometic test (N=465 for ext. noise, 182 for covariance analysis). All analysis above was carried out using custom Matlab code.
Clustering and Principal Component Analysis (PCA)
Hierarchical clustering with a correlation distance metric was performed in Matlab on a 45 by 182 matrix composed of the mean normalized covariances between query and array genes. The row-wise correlations were then computed to produce a 182 by 182 correlation matrix. PCA of this matrix was calculated using Matlab functions.
Supplementary Material
Highlights.
Pathway noise impacts S. cerevisiae stress and amino acid biosynthesis genes
Single cell covariance measurements reveal functionally coherent noise regulons
Genetic perturbations point to PKA and TOR as significant sources of fluctuations
Correlated noise is a quantitative tool to probe regulatory relationships
Acknowledgments
We would like to thank David Pincus, Onn Brandmann, Charles Biddle-Snead, Michael Chevalier, David Breslow, Simon Vidal, and the Weissman and El-Samad labs for scientific discussion and comments on the manuscript. Imaging was performed in the UCSF Nikon Imaging Center. This work was funded by the NIGMS system biology center (P50 GM081879) and the David and Lucille Packard Foundation (H.E.S.), the Howard Hughes Medical Institute (J.S.W.), and an NSERC post-graduate scholarship (J.S.O). J.S.O, H.E.S, and J.S.W designed the experiments and wrote the manuscript. J.S.O preformed the experiments and analyzed the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becskei A, Kaufmann BB, Oudenaarden A. Contributions of low molecular number and chromosomal positioning to stochastic gene expression. Nature Genetics. 2005;37:937–944. doi: 10.1038/ng1616. [DOI] [PubMed] [Google Scholar]
- Bigger W. Treatment Of Staphylococcal Infections With Penicillin by Intermittent Sterilisation. The Lancet. 1944;244(6320):497–500. [Google Scholar]
- Blake WJ, Kaern M, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- Blake WJ, Balazsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, Cantor CR, Walt DR, Collins JJ. Phenotypic consequences of promoter-mediated transcriptional noise. Molecular Cell. 2006;24:853–865. doi: 10.1016/j.molcel.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Miller-Jensen K, Shah PS, Arkin AP, Schaffer DV. Control of Stochastic Gene Expression by Host Factors at the HIV Promoter. PLoS Pathog. 2009;5(1):e1000260. doi: 10.1371/journal.ppat.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman-Lerner A, Gordon A, Serra E, Chin T, Resnekov O, Endy D, Pesce CG, Brent R. Regulated cell-to-cell variation in a cell-fate decision system. Nature. 2005;427:699–706. doi: 10.1038/nature03998. [DOI] [PubMed] [Google Scholar]
- Delbruck M. The Burst Size Distribution in the Growth of Bacterial Viruses (Bacteriophages) J Bacteriol. 1945;50(2):131–5. [PMC free article] [PubMed] [Google Scholar]
- Dunlop MJ, Cox RS, 3rd, Levine JH, Murray RM, Elowitz MB. Regulatory activity revealed by dynamic correlations in gene expression noise. Nat Genet. 2008;40(12):1493–8. doi: 10.1038/ng.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297(5584):1183–6. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic Expression Programs in the Response of Yeast Cells to Environmental Changes. Molecular Biology of the Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti L, Siggers T, Tiana G, Caprara G, Notarbartolo S, Corona T, Pasparakis M, Milani P, Bulyk ML, Natoli G. Noncooperative Interactions between Transcription Factors and Clustered DNA Binding Sites Enable Graded Transcriptional Responses to Environmental Inputs. Molecular cell. 2011;37(3):418–428. doi: 10.1016/j.molcel.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-Time Kinetic of Gene Activity in Individual Bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schüller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schuller C. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO. 2002;21:135–144. doi: 10.1093/emboj/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne JB, Reynolds DB, Yoo J, Jennings EG, Zeitlinger J, Pokholok DK, Kellis M, Rolfe PA, Takusagawa KS, Lander ES, Gifford DK, Fraenkel E, Young RA. transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431:99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate P. Estimation for the bivariate Poisson distribution. Biometrika. 1964;51:241–245. [Google Scholar]
- Howson R, Huh WK, Ghaemmaghami S, Falvo JV, Bower K, Belle A, Dephoure N, Wykoff DD, Weissman JS, O’Shea EK. Construction, verification and experimental use of two epitope-tagged collections of budding yeast strains. Comp Funct Genomics. 2005;6(1–2):2–16. doi: 10.1002/cfg.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Paulsson J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nature Genetics. 2011;43:95–100. doi: 10.1038/ng.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B. Conflict between Noise and Plasticity in Yeast. PLoS Genet. 2010;6(11):e1001185. doi: 10.1371/journal.pgen.1001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Butow RA. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol. 1999;10:6720–8. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- das Neves RP, Jones NS, Andreu L, Gupta R, Enver T, Iborra FJ. Connecting Variability in Global Transcription Rate to Mitochondrial Variability. PLoS Biol. 2010;8(12):e1000560. doi: 10.1371/journal.pbio.1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveal the architecture of biological noise. Nature. 2005;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- Ozbudzk EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nature Genetics. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- Pachkov M, Erb I, Molina N, van Nimwegen E. SwissRegulon: a database of genome-wide annotations of regulatory sites. Nucleic Acids Research. 2007;35(Database issue):D127–D131. doi: 10.1093/nar/gkl857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Pontius W, Guet CC, Marko JF, Emonet T, Cluzel P. Interdependence of behavioural variability and response to small stimuli in bacteria. Nature. 2010;468(7325):819–23. doi: 10.1038/nature09551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- Pedraza JM, van Oudenaarden A. Noise Propagation in Gene Networks. Science. 2005;307:1965–1969. doi: 10.1126/science.1109090. [DOI] [PubMed] [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biology. 2006;4(10):e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O’Shea EK. Control of Stochasticity in Eukaryotic Gene Expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A, Anderson S, McCaffery JM, Yates J 3rd, Aronova S, Chu S, Fairclough S, Iverson C, Wedaman KP, Powers T. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J Biol Chem. 2004;279(15):14752–62. doi: 10.1074/jbc.M313062200. [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ, Van Driessen K. A Fast Algorithm for the Minimum Covariance Determinant Estimator. Technometrics. 1999;41:212–223. [Google Scholar]
- Schmit AP, McEntee K. Msn2p, a zinc finger DNA-Binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. PNAS. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Ku CJ, Wichaidit C, Steininger RJ, 3rd, Wu LF, Altschuler SJ. Patterns of basal signaling can distinguish cellular populations with different drug sensitivities. Molecular Systems Biology. 2010;6:369. doi: 10.1038/msb.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MCA, Sumner ER, Avery SV. Glutathione and Gts1p drive beneficial variability in the cadmium resistances of individual yeast cells. Molecular Microbiology. 2007;66:699–712. doi: 10.1111/j.1365-2958.2007.05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive Identification of Cell Cycle-regulated Genes of the Yeast Saccharomyces cerevisiae by Microarray Hybridization. Molecular Biology of the Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459(7245):428–32. doi: 10.1038/nature08012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M, Weissman JS, Kirschner MW. A general lack of compensation for gene dosage in yeast. Mol Syst Biol. 2010;6:368. doi: 10.1038/msb.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JL, Koshland DE., Jr Non-genetic individuality: chance in the single cell. Nature. 1976;262(5568):467–71. doi: 10.1038/262467a0. [DOI] [PubMed] [Google Scholar]
- Suel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- Swain PS, Elowitz MB, Siggia ED. Intrinsic and Extrinsic contributions to stochasticity in gene expression. PNAS. 2002;99:12795–12800. doi: 10.1073/pnas.162041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. Coli Proteome and Transcriptome with Single-Molecule Sensitivity in Single Cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thattai M, van Oudenaarden A. Intrinsic Noise in Gene Regulatory Networks. PNAS. 2001;98:8615–8619. doi: 10.1073/pnas.151588598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thattai M, van Oudenaarden A. Stochastic Gene Expression in Fluctuating Environments. Genetics. 2004;167:523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volfson D, Marciniak J, Blake WJ, Ostroff N, Tsimring LS, Hasty J. Origins of extrinsic variability in eukaryotic gene expression. Nature. 2006;439:861–864. doi: 10.1038/nature04281. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Maeda Y, Ikeda A, Sakurai H. Regulation of Thermotolerance by Stress-Induced Transcription Factors in Saccharomyces cerevisiae. Eukaryotic Cell. 2008;7:783–790. doi: 10.1128/EC.00029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.