Abstract
Schistosoma mansoni is responsible for schistosomiasis, a parasitic disease that affects 200 million people worldwide. Molecular mechanisms of host-parasite interaction are complex and involve a crosstalk between host signals and parasite receptors. TGF-β signaling pathway has been shown to play an important role in S. mansoni development and embryogenesis. In particular human (h) TGF-β has been shown to bind to a S. mansoni receptor, transduce a signal that regulates the expression of a schistosome target gene. Here we describe 381 parasite genes whose expression levels are affected by in vitro treatment with hTGF-β. Among these differentially expressed genes we highlight genes related to morphology, development and cell cycle that could be players of cytokine effects on the parasite. We confirm by qPCR the expression changes detected with microarrays for 5 out of 7 selected genes. We also highlight a set of non-coding RNAs transcribed from the same loci of protein-coding genes that are differentially expressed upon hTGF-β treatment. These datasets offer potential targets to be explored in order to understand the molecular mechanisms behind the possible role of hTGF-β effects on parasite biology.
Keywords: Schistosoma mansoni, TGF-β signaling, host-parasite cross talk, microarray analysis, ncRNAs, gene network interactions
1. Introduction
Schistosomes are complex trematode parasites that have six developmental stages and two hosts: a snail as intermediate host belonging to genus Biomphalaria and a vertebrate as definitive host. They cause schistosomiasis, a serious parasitic disease worldwide, with an estimated 200 million people afflicted in 76 countries and territories located in tropical and subtropical areas [1].
The parasites have a highly adapted relationship with their hosts that appears to involve the schistosome exploitation of host endrocrine and immune signals, in addition to nutrient uptake, for their development and differentiation [2–6]. Recent description in the parasite of homolog genes to vertebrate receptors suggests that schistosomes are potentially responsive to host molecules [7–13], and as a consequence the role of host factors in schistosome development has been explored [4,14–17].
Transforming Growth Factor Beta (TGF-β) is a cytokine that regulates many processes central to life of metazoans such as growth and differentiation, developmental patterning, tissue repair and cell death [18]. The basic signaling pathway of TGF-β comprises the TGF-β receptors type I and type II that are structurally similar transmembrane serine / threonine kinases; SMADs proteins: R-Smads (e.g., Smad 1/Smad 2) in vertebrates) that interact with the receptors, are phosphorylated by receptors and then will associates with other SMADs, co-SMADs (Smad4) to form a transcription regulatory complex that translocates to the nucleus. Through interaction of co-regulators such as CBP (CREB binding protein) or p300, the complex activates/represses transcription of target genes [18,19].
TGF-β signaling elements have been described in Schistosoma mansoni; two types of TGF-β receptors I (SmTβR1) and II (SmTβR1) [9]; four SMADs: SmSMAD1 [20], SmSMAD1b [21], SmSMAD2 [22], SmSMAD4 [23]; one homolog gene to Inhinbin/Activin SmInAct [24]; a homolog to BMP (Bone Morphogenic Protein), SmBMP [25] and Smp300 /CBP was also identified [26]. It has been shown that these elements can act together in the signaling process in S. mansoni and play an important role in the development of vitelline cells in female worms and in embryogenesis of eggs by male stimuli [24,27–29].
In spite of all the evidence of a TGF-β effect in parasite biology, the molecular basis to understand which genes are affected at the transcriptional level have not until this study been explored. In this work we present the effect of human TGF-β on the gene expression profile of adult worms, resulting in increased knowledge about host-parasite molecular cross talk.
2. Materials and Methods
2.1. Parasite treatment and RNA extraction
Schistosoma mansoni (NMRI) maintained in a Puerto Rican Biomphalaria glabrata and Golden Syrian hamsters was used for these studies. Parasites were recovered by portal perfusion of infected hamsters 45 days after infection [30].
Freshly perfused adult worms were washed in M169 medium plus antibiotics 100 U/ml penicillin, 100 mg/ml streptomycin, 1 mg/ml amphotericin B (antibiotic antimycotic solution) and cultured 22–24 h in M169 medium supplemented with 10% Fetal Bovine Serum and antibiotics. 1 nM of recombinant human TGF-β (R&D Systems, Inc.) was added and the worms cultured for 22–24 h. We performed three biological replicas of treated worms (annotated as TGF_1, TGF_4 and TGF_5) and two biological replicas of control non-treated parasites (Ctrl_2 and Ctrl_3).
RNA from each sample was extracted using Trizol reagent (Invitrogen), and then treated with DNAse using RNAeasy kit (QIAGEN) according to the manufacturer´s instructions. RNA integrity was evaluated using Bioanalyzer microfluidic electrophoresis (Agilent Technologies).
2.2. Microarray experiments
We used 300 ng of each sample to perform linear amplification using Low input RNA amplification kit (Agilent Technologies); for each TGF treated sample (TGF_1, TGF_4 or TGF_5) we performed 4 technical replicas. The control samples (Ctrl_2 and Ctrl_3) were pooled together (using the same amount of RNA) and were used for each amplification reaction. In a similar manner, we performed 12 technical replicas of the control pool (to combine to each one of the four technical replicas from three biological samples). Treated samples were labeled with Cy3 or Cy5 dye, control samples were labeled with the opposite dye and 825 ng of each labeled sample was combined for hybridization on a microarray slide, in a dye-swap approach.
Hybridization was carried out overnight on a custom 4 × 44k oligonucleotide array designed by our research group [31] and produced by Agilent Technology following the manufacturer´s instructions. Detailed description of 4 × 44k platform is found in [31] and the platform probe annotation is available on Gene Expression Omnibus (GEO) under the accession number GPL8606.
Slides were washed and scanned according to Agilent instructions using GenePix 4000B scanner (Molecular Devices). Raw data were extracted using Feature Extraction software (Agilent Technologies). Raw data is available in GEO under the accession number GSE27050.
We kept in the analysis genes that have a significantly detectable signal (using the IsPosAndSig column from Feature Extraction data output) in at least 75% of all replicas in at least one biological condition (Treated or Control). The intensities were normalized by LOWESS algorithm [32] and the log2 ratio between treated and control was calculated. Under our experimental conditions, biological and technical variability were in the same range as evaluated by the intensity signal Pearson correlation coefficients: for the three biological replicas of Treated samples the average correlation = 0.901 (range = 0.837 to 0.939), whereas for the technical replicas of these Treated samples the average correlation = 0.951 (range 0.904 to 0.975). For the technical replicas of Control samples the average correlation = 0.983 (range 0.971 to 0.996).
To find the differentially expressed genes between treated and control we used SAM (Significance Analysis of Microarray) approach [33], and genes with a q-value < 0.05 and were selected as differentially expressed. Next, we used the Leave-one-out [34] statistical approach to find genes that are consistently differentially expressed between treated and control; we selected as differentially expressed those genes that have a q-value lower than 0.05 in all possible comparisons between two biological replicas (TGF_1 and TGF_4; TGF_1 and TGF_5; TGF_4 and TGF_5). Finally, we applied a cutoff filter to keep as differentially expressed only those genes with an average |log2ratio (treated/control)| ≥ 1.0 (i.e., minimum two fold increase or decrease in gene expression in TGF-β treated in relation to control worms).
Analysis of Gene Ontology (GO) was performed using Ontologizer [35], and the p-value was adjusted using Benjamin-Hochberg method [36]. Functional analysis was performed using Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/). For this purpose we annotated S. mansoni genes encoding putative homologs to human proteins; the putative homolog should have similarity with a BlastX e-value lower than 1× 10−10 and coverage of at least 60% of the human homolog. The RefSeq number of each human homolog was associated to each S. mansoni gene and the expression data was uploaded to Ingenuity Pathway Analysis System version 7.6. We included all gene/protein relationships described as experimentally observed and/or predicted with high confidence.
2.3. RT- Real Time PCR experiments
In order to confirm the expression pattern of some candidates, we performed RT-Real Time PCR experiments. Five hundred ng of RNA from each sample was used for reverse transcription using QuantiTect reverse transcription kit (QIAGEN) following the manufacturer´s instructions. In parallel, 500 ng of RNA from each sample was also incubated in reaction medium without reverse transcriptase enzyme as a negative control.
From the previous step, 0.15 µl of the reverse transcription reaction was used to perform Real Time PCR using Power SYBR Green Master Mix (Applied Biosystems) in the 7500 Real Time PCR System (Applied Biosystems) using the delta-CT method default parameters. Primer express software (Applied Biosystems) was used to design primers for selected genes, and the list of primers used is available in supplementary table 1. Data was analyzed according to the comparative CT method [37], Student t-test was used to calculate the significance. S. mansoni alpha-tubulin (sat-1, gi|161071, M80214.1) was used as the reference gene; we performed an absolute qPCR determination (with primers described in Supplementary Table 1) to evaluate sat-1 expression and the result is shown in Supplementary Figure 1. There was no detectable Ct changes between hTGF-β treated worms and control worms.
3. Results
3.1. Identification of differentially expressed genes and functional analysis of protein coding genes
Human TGF-β (hTGF-β) at a concentration of 1nM has been shown to affect S. mansoni adult worms, when parasites are incubated overnight in culture [9]. We used similar treatment conditions and total RNA was extracted from parasites. mRNA was linearly amplified and labeled as described under Material and Methods, and used for microarray hybridization experiments with an oligoarray platform containing 44-thousand gene probes [31].
We identified 8649 expressed genes, for which the intensity signals were significantly above the background in at least 75% of the replicas of at least one biological condition, i.e. either in the control or in the TGF-β treated worms (Table 1).
Table 1.
General information about TGF-beta microarray experiments
| S. mansoni gene category | Number of expressed genesa |
Number of differentially expressed genesb |
Number of genes down- regulated by TGF-betab |
Number of genes up- regulated by TGF-betab |
|---|---|---|---|---|
| Predicted in the genome | 6160 | 240 | 200 | 40 |
| Not predicted in the genome however predicted in S. japonicum | 573 | 32 | 27 | 5 |
| Not predicted in the genome with GenBank homolog | 1241 | 64 | 48 | 16 |
| Not predicted in the genome without gene homolog (No match) | 675 | 45 | 41 | 4 |
| TOTAL | 8649 | 381 | 316 | 65 |
Expressed in at least one condition, i.e. in control or in TGF-β treated worms.
q-value < 5% in all three Leave-one-out comparisons and |log2ratio (treated/control)| ≥ 1 (see Methods).
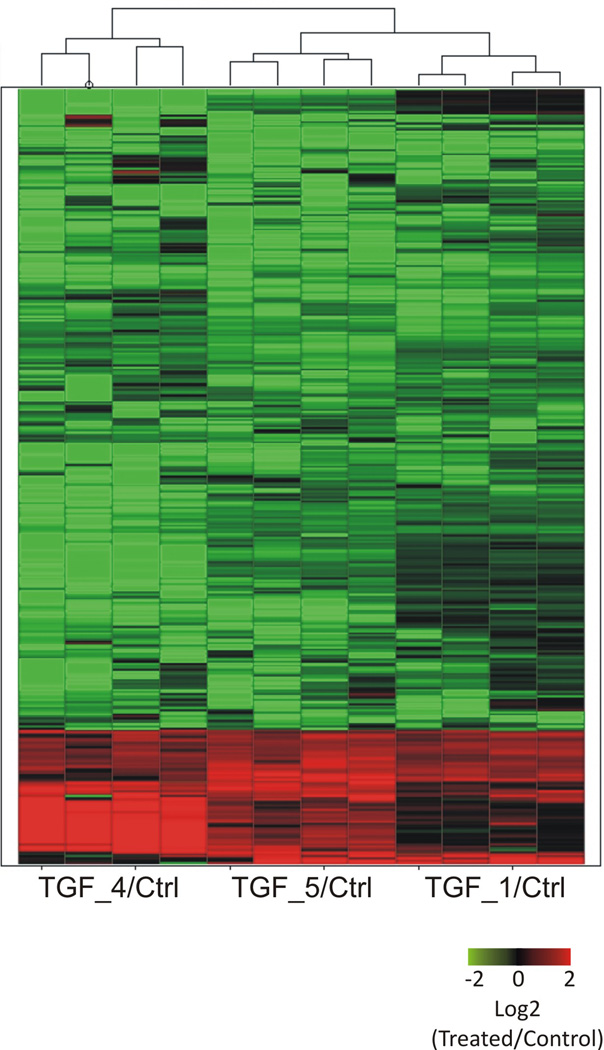
We used SAM statistical approach combined with Leave-one-out approach (see Methods) and a minimum expression change of |log2 ratio [treated/control]| ≥ 1. We identified 381 genes (4.4 % of all expressed genes) with significant changes in expression level between TGF-β treated and control samples (q-value < 0.05 in all leave-one-out analysis). Among these genes, 316 are repressed (down-regulated) by human TGF-β and 65 are induced (up-regulated) by the human cytokine (Fig. 1).
Fig. 1. Heat map of 381 differentially expressed genes between hTGF-β treated and non-treated adult worms.
Each line represents a gene and each column represents a replica (4 technical replicas for each one of three biological replicas). Color is proportional to expression levels of the gene, computed as the log 2 of the expression ratio between treated and control intensities (log2 [Treated/Control]); color intensity is proportional to the log-ratio according to the color range indicated in the figure.
Among the 316 TGF-β repressed genes, 200 were predicted in the S. mansoni genome (Table 1). Among the remaining 116 down-regulated genes (36 %) that were not predicted in the genome, 27 have similarity to S. japonicum predicted genes, 48 genes have homologs from other species in GenBank and 41 have no match to genes from other species in GenBank. In the opposite scenario, among the 65 TGF-β up-regulated genes, 40 were S. mansoni genes predicted in the genome (Table 1). From the remaining 25 up-regulated genes (33 %) that were not predicted in the genome, 5 have homology to S. japonicum predicted genes, 16 genes encode protein homologs from other species available in GenBank and 4 genes have no match to genes from other species in GenBank (Table 1). The list of differentially expressed genes is available in Supplementary Table 2.
We performed Gene Ontology (GO) Analysis with the group of differentially expressed genes. The GO categories identified as significantly enriched (adjusted p-value <0.05, using Benjamini-Hochberg correction) are listed in Table 2; the detailed list of genes belonging to enriched GO categories is available in Supplementary Table 3.
Table 2.
Gene Ontology categories enriched in differentially expressed genes down-regulated by hTGF-β
| Ontology | GO ID | GO Description | Counts | adjusted p- value |
|---|---|---|---|---|
| Biological Process | GO:2000112 | regulation of cellular macromolecule biosynthetic process | 30/524 | 3.5 × 10−3 |
| GO:0010468 | regulation of gene expression | 31/527 | 2.0 × 10−2 | |
| GO:0034656 | nucleobase, nucleoside and nucleotide catabolic process | 21/309 | 2.0 × 10−2 | |
| GO:0032774 | RNA biosynthetic process | 22/410 | 3.9 × 10−2 | |
| Celular Component | GO:0005634 | nucleus | 62/1345 | 1.4 × 10−4 |
| GO:0044449 | contractile fiber part | 9/75 | 3.8 × 10−4 | |
| GO:0016459 | myosin complex | 10/84 | 4.8 × 10−3 | |
| Molecular Function | GO:0016787 | hydrolase activity | 53/1055 | 7.3 × 10−3 |
| GO:0032559 | adenyl ribonucleotide binding | 50/756 | 3.4 × 10−2 | |
Here we highlight among enriched GO categories with down-regulated genes, those related to Cellular Component such as nucleus and myosin complex (Table 2); in Biological Process categories it is worth mentioning categories such as RNA biosynthetic process, nucleobase, nucleoside and nucleotide catabolic process and regulation of gene expression; among the categories of Molecular Function hydrolase activity, acting on acid anhydrides and adenyl ribonucleotide binding are interesting ones (Table 2). We did not find any enrichment of GO categories among the up-regulated genes.
We performed a putative functional annotation using Ingenuity Pathway Analysis (IPA) software. Essentially, IPA provides the biological context for gene expression changes by integrating available literature information on model organisms (human, mouse, rat) regarding molecular and chemical interactions, cellular phenotypes as well as about signaling and metabolic pathways. IPA provides pre-computed libraries of canonical pathways, which are well-characterized metabolic and cell signaling pathways that are based on curated literature, have a directionality of flow, have been generated prior to our data input, and do not change upon our data input; in this case IPA computes the canonical pathways enriched with up- or down-regulated genes. In addition, IPA computes what are called enriched networks, which are generated de novo based on our input data, do not have directionality, and typically contain molecules involved in several pathways. For these analyses we annotated S. mansoni gene homologs to human genes, as described in Materials and Methods, and uploaded them to IPA along with information on the respective gene expression changes.
We found that S. mansoni genes altered by parasite treatment with hTGF-β could be modeled into canonical pathways described for the human gene homologs. The most significantly enriched (p-value = 1.3 × 10−5) canonical pathway is calcium signaling (shown in Supplementary Figure 2) and its genes are listed in Supplementary Table 4.
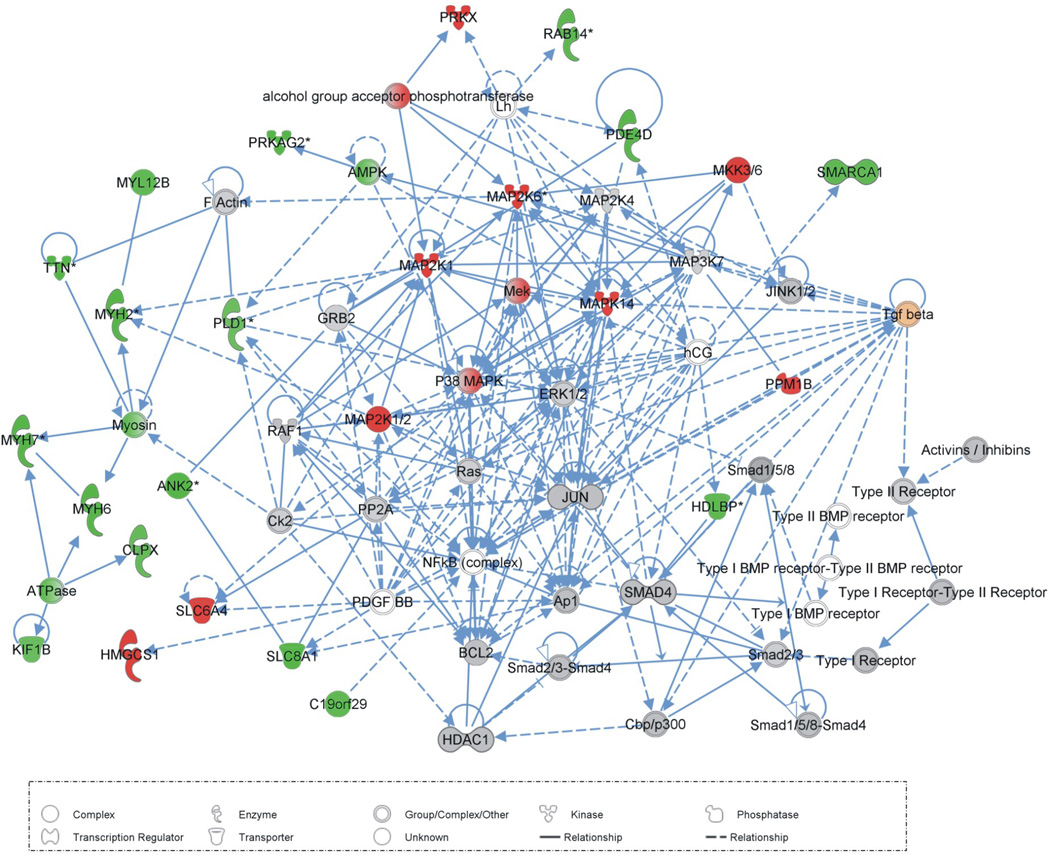
Using the same approach we looked for gene interaction networks that were statistically enriched with genes showing hTGF-β induced changes in expression, and here we highlight the five most significant (p-values from 10−54 to 10−19). A list of differentially expressed genes that comprise these five networks is in Supplementary Table 5. The first network (p-value = 10−54) is associated to functions such as muscular system development and function, tissue morphology, cellular assembly and organization; the second network (p-value = 10−31) is associated with cellular assembly and organization and cell cycle; the third network (p-value = 10−31) is related to DNA replication, recombination and repair, gene expression; the fourth network (p-value = 10−23) is related to functions such as cell death, cellular compromise and cellular development, and the fifth network (p-value = 10−19) is related to cellular assembly and organization, cell-to-cell signaling and interaction, connective tissue development and function. In Figure 2, we highlight the first network, which is related to muscular system development and function, tissue morphology, cellular assembly and organization.
Fig. 2. Gene interaction network related to muscular system development and function, tissue morphology, cellular assembly and organization.
The shapes of elements correspond to different types of molecules, as indicated in the lower box; arrows represent the relationships between elements: dashed lines = indirect interaction, filled lines = direct interaction; color intensity is proportional to expression value, computed as log2 [Treated/Control]; green means negative log-ratios, i.e. down-regulation in treated parasites, and red means positive log-ratios, i.e. up-regulation in treated parasites. The human TGF-β ligand is highlighted in orange.
Using IPA we were able to find biological functions that were statistically enriched with genes showing hTGF-β induced changes in expression; all but two genes are common to the five enriched networks described above. These biological functions are: muscular system development and function (adjusted p-value: 3.6 × 10−2 – 8.2×10−10), tissue morphology (adjusted p-value: 3.6 ×10−2 – 8.5×10−10), cellular assembly and organization (adjusted p-value: 3.6 ×10−2 – 2.9 ×10−5), cell cycle (4.4 × 10−2 × 2.7 ×10−4), organ development (3.1 × 10−2 – 3.4 × 10−4), tissue development (2.7 × 10−2 – 4.5×10−2) and cellular grown and proliferation (4.9 × 10−2 – 3.4 × 10−4). The list of genes related to these biological functions is available in Supplementary Table 6
3.2. Validation of differentially expressed protein-coding genes by Reverse Transcription- quantitative Real Time PCR
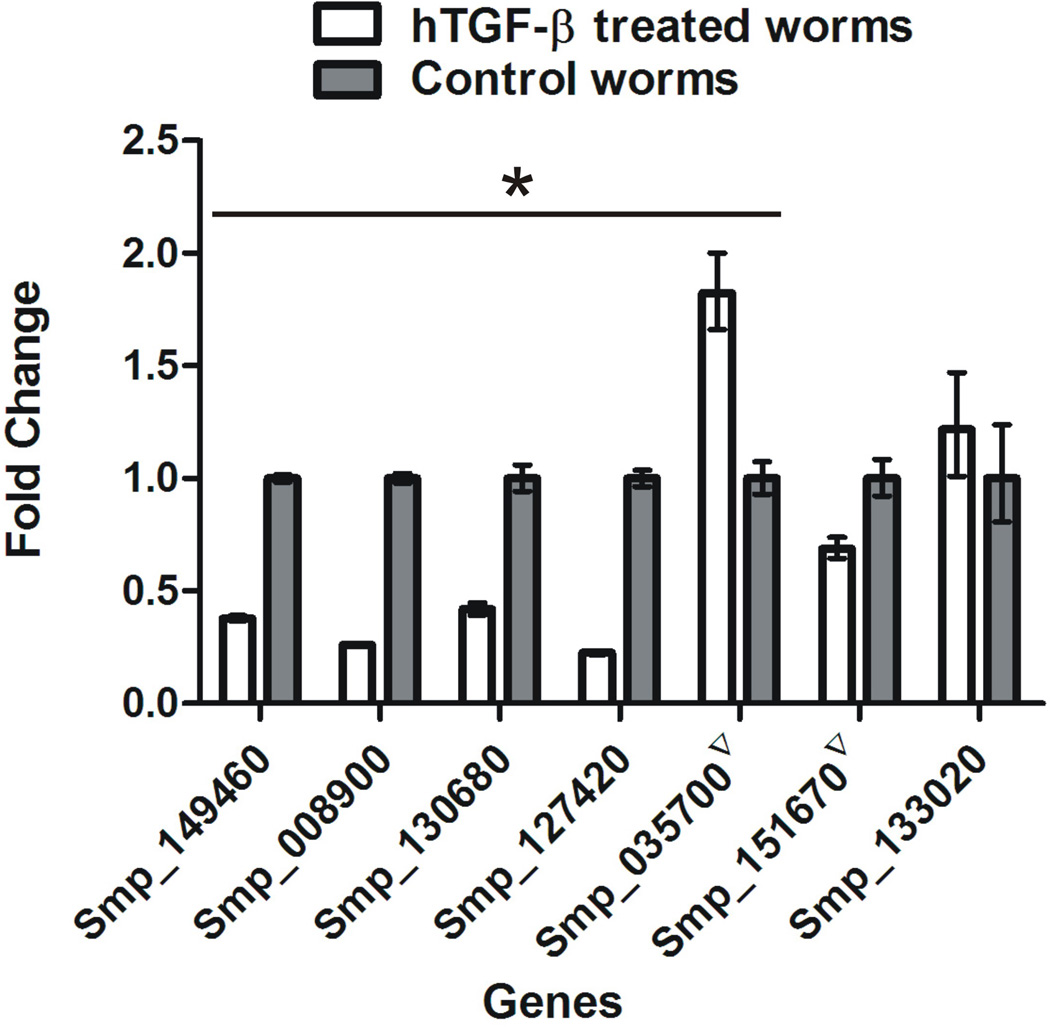
From all of the protein coding genes differentially expressed upon hTGF-β treatment of parasites, we selected seven genes according to their biological relevance for validation of expression changes by Reverse Transcription- quantitative Real Time PCR (RT-qPCR). They were: Smp_149460 (tyrosine kinase), Smp_008900 (eukaryotic translation initiation factor 4 gamma, putative), Smp_130680 (DNA-directed RNA polymerase II subunit, putative), Smp_127420 (AMP-activated protein kinase, gamma regulatory subunit, putative), Smp_035700 (adenosine deaminase putative, identified in the array by gi|82697983); Smp_151670 (Serine/Threonine Kinase_STE Group_STE7 Family_MEK1 Subfamily, identified in the array by Sjc_0093560); and Smp_133020 (serine/threonine kinase).
Figure 3 shows the RT-qPCR results; validated genes with significant expression changes (p-value < 0.05) are marked with asterisks (see line across the genes at top). It can be seen that five out of the seven selected genes (71.4%) were successfully validated, whereas Smp_151670 showed an opposite expression pattern by RT-qPCR when compared with array data and Smp_133020 showed RT-qPCR change in expression in the same direction as that measured by the array, however with no statistical significance. The Pearson’s correlation of RT-qPCR expression data with array expression data is 0.86, estimated as described in [38].
Fig. 3. Validation by Reverse Transcription Real Time PCR of expression changes induced by hTGF-beta in S. mansoni adult worms and detected by microarray.
White bars represent the average value of hTGF-β treated worm samples and gray bars represent the average value of control worms for each gene as identified in the X axis. Y axis represents the expression values in Fold change of treated relative to control, as measured by qPCR. A t-test was used to calculate the significance; genes with significant difference (p < 0.05) are marked with asterisk (see top line across genes). Smp gene names marked with inverted triangles have different identifiers in the microarray tables, as described in the text.
3.3. Non protein-coding RNAs and hTGF-β
We identified a set of 43 gene loci with significant changes in the transcription level of non-coding RNAs (ncRNAs) transcribed from the opposite strand of the known protein coding genes (antisense strand); it is worth noting that 2 out of the 43 genomic loci had significant expression changes in the messages transcribed from both strands (sense and antisense), whereas in the remaining 41 loci we detected expression changes only in the ncRNA from the opposite strand of the protein-coding gene (Table 3). Regarding the 2 loci with expression changes in both strands, both showed a concordant pattern of expression changes induced by hTGF-β: one locus showed down-regulated messages from both strands (protein-coding sense message and non-coding antisense RNA message), whereas the other locus showed up-regulated messages from both strands. Out of the 41 genomic loci with changes only in the antisense ncRNA, 25 are down-regulated and 16 are up-regulated by hTGF-β.
Table 3.
Differentially expressed anti-sense ncRNA transcripts induced by hTGF-β in S. mansoni adult worms
| Pattern of change in expression | gene loci with differential expression in both sense and antisense messages |
gene loci with differential expression in the antisense message only |
|---|---|---|
| Gene loci with differentially expressed antisense ncRNAs | 2 | 41 |
| Up-regulated | 16 | |
| Down-regulated | 25 | |
| sense and antisense messages with the same pattern of expression changes by hTGF-beta | 2 | |
| messages from the same locus both up-regulated | 1 | |
| messages from the same locus both down-regulated | 1 | |
| sense and anti-sense messages with opposite patterns of expression changes by hTGF-beta | 0 | |
| Gene category locus (total) | ||
| S. mansoni predicted gene | 2 | 24 |
| Similar to S. japonicum predicted gene | 0 | 2 |
| GenBank Homolog gene | 0 | 15 |
We mapped all these genomic loci and we observed that for the 2 with expression changes detected in both strands, the antisense message of 1 of them maps to regions opposite to the intron and 1 to regions opposite to an exon of the respective protein-coding gene. Among the 41 loci with changes in expression of messages from the opposite strand of protein-coding genes, 11 map to regions opposite to introns and 30 to regions opposite to exons.
4. Discussion
The goal of this work was to identify at the molecular level the changes induced by in vitro exposure of S. mansoni to hTGF-β. TGF-β is known as a global regulator, affecting numerous biological pathways [15]; microarrays are a powerful tool to identify large-scale transcriptional changes in different organisms and in the most diverse biological situations, and to achieve our goal we have used a custom-designed S. mansoni oligo-microarray with 44-thousand unique elements [31]. Identification of S. mansoni genes that are affected by hTGF-β is an important first step towards understanding the molecular targets and mechanisms that are responsible for the biological effects of hTGF-β on the parasite.
It is important to note that all expression changes observed were measured using RNA extracted from the entire parasite, containing all types of tissues; we should keep in mind that some changes could happen in specific tissues while other changes could happen in all tissues. Actually, at present it is extremely difficult to evaluate a tissue specific gene expression response of the parasite; a very recent work just established the microdissection of S. mansoni and applied it to gene expression studies [39]. In addition, it is possible that some of the down-regulated messages are a secondary effect of TGF-β, as our present experiments cannot distinguish between very early direct and somewhat late secondary events.
Interestingly, we found 141 S. mansoni genes that were regulated by hTGF-β and were not previously predicted in the S. mansoni genome. Most of the 381 differentially expressed genes were down-regulated (316 genes) by the host cytokine and just 65 genes were up-regulated by hTGF-β.
The literature points to a number of parasite physiological processes in which TGF-β receptor ligands play a role, such as host-parasite interactions, development, male-female interaction, mitotic activity, egg embryogenesis [11,24,27–29,40]. It should be pointed out that, at the molecular level TGF-β induces activated cytoplasmic SMAD complexes that translocate to the nucleus where co-activators or co-repressors will be recruited, thus leading to activated or repressed gene expression [18,19]. Thus, it is not inconceivable that a large number of genes were down-regulated in our dataset. Moreover, TGF-β can trigger an immense diversity of responses, favoring or retarding biological processes depending on the genetic makeup and environment of target cells/tissues [18]. At this point, our experiments with whole-worm gene expression cannot pinpoint any particular physiological response. We believe that the GO categories, as well as the canonical pathways and non-canonical networks identified here and discussed below, could serve as a guide to further direct experimentation with parasites, possibly using tissue-specific targeted approaches such as FISH, vector-regulated conditional RNAi, measurements with tissue-micro-dissected samples, etc.
Significant enrichment of gene categories comprised by the differentially expressed genes is an important approach to the understanding of biological functions and molecular processes affected by hTGF-β on the parasite. Gene Ontology annotation describes gene products according to a controlled vocabulary of terms [41], which permits a consistent statistical enrichment analysis.
Among the hTGF-β downregulated genes, we observed that GO categories belonging to biological processes such as RNA biosynthetic process, regulation of gene expression and nucleobase, nucleoside and nucleotide catabolic process were detected with enriched counts. These functions are interesting because they point to regulation at the transcriptional level.
Again, among the hTGF-β down-regulated genes, we observed in the cellular component GO category the enrichment of contractile fiber and myosin complex ontologies and in the molecular function there was an enrichment of genes related to hydrolase activity and adenyl ribonucleotide binding. It is interesting because it is already described that TGF-β induces cytoskeleton remodeling through myosin chain and Rho GTPase (Smp_176920 that is down-regulated in S. mansoni by hTGF-β) [42,43]; now we are documenting in the parasite a significant change in the expression of cytoskeletal proteins, and a study of the signaling mechanism is warranted.
Another powerful approach to perform functional annotation is the use of Ingenuity Pathway Analysis (IPA) tool (http://www.ingenuity.com/). This is a curated database of extensively compiled literature information regarding data at the molecular level for protein-protein interactions, for protein-protein enzymatic modifications and for biological functions and associated gene networks, in human, rat and mouse models. As described above, we annotated S. mansoni protein coding genes with homology to human protein coding genes and we used IPA database to search for gene interaction networks; the underlying hypothesis is that sequence conservation probably means a conservation of function.
We were able to identify S. mansoni elements that can be modeled into a human canonical pathway of calcium signaling, which was significantly enriched (p-value = 1.3 × 10−5) with genes affected by hTGF-β (Supplementary Figure 2 and Supplementary Table 4); it is already described that in human osteoblasts there is induction of calcium signaling by TGF-β [44], suggesting a possible conserved mechanism throughout evolution.
Five gene interaction networks were identified as significantly enriched in genes affected by hTGF-β. We highlight the elements that comprise network 1 (see Figure 2 and Supplementary Table 5) that are related to muscular system development and function, tissue morphology, cellular assembly and organization. It is noteworthy that MEK1, MKK6 and p38 MAPK (belonging to the non-canonical TGF-β signaling pathway) are up-regulated upon treatment with hTGF-β, showing that activation of the pathway, which is known to occur by phosphorylation of these kinases, can additionally be enhanced by increasing the expression levels of the kinases.
The literature already indicates that TGF-β is important for parasite development, mitotic activity and egg embryogenesis [24,27–29], however the exact molecular mechanisms behind these biological events is not clear; here we suggest the possible molecules responsible for these biological events in Supplementary Table 6, comprising functions such as: muscular system development and function, tissue morphology, cellular assembly and organization, cell cycle, organ development, tissue development and cellular growth and proliferation. This opens new avenues for researchers to explore the conserved molecules that could play a role in the mechanisms related to these biological functions.
Five out of the seven protein coding genes that were selected for validation by RT-Real Time PCR had their expression changes confirmed. Selection was based on the fact that most of these genes were highlighted by the functional analyses mentioned above. Specifically, Smp_149460 (tyrosine kinase) is a SYK homolog (spleen tyrosine kinase), and it was selected for validation because it has been described as involved in the regulation of spermatogenesis and oogenesis in worms [45,46]; Smp_008900 (eukaryotic translation initiation factor 4 gamma, putative or eIF4G), which is highly associated with the RNA binding GO function (and is related with the control of translation process); Smp_130680 (DNA-directed RNA polymerase II subunit, putative) transcribes all mRNA in the cell and is present in network number 5; Smp_133020 (serine/threonine kinase), is the MAPK14 homolog involved in cell migration, proliferation and differentiation and is present in network number 1; Smp_127420 (AMP-activated protein kinase , gamma regulatory subunit, putative) is also in network number 1; Smp_035700 (adenosine deaminase putative), identified in the array as gi|82697983 is present in network number 5 and Smp_151670 (Serine/Threonine Kinase_STE Group_STE7 Family_MEK1 Subfamily), identified in the array as Sjc_0093560, that belongs to TGF-beta signaling pathway and is present in network 1 [47]. The Pearson correlation between microarray data and RT-qPCR data for the seven tested genes mentioned above was found to be 0.86, showing a good concordance between the two methods. We consider it to be a successful validation rate (71%) because it is already known that both experimental techniques have inherent pitfalls that cannot be fully controlled [38].
In the last years knowledge about biological regulation and gene networking is changing because of the advances in molecular biology techniques, which allowed the discovery of many classes of non-protein coding RNAs that regulate several processes in a cell, through a set of distinct mechanisms. After publication of the schistosome genome and transcriptome sequencing data, a new window has been opened to explore ncRNAs in schistosomes (reviewed in [48]). It is interesting to note that a considerable number of antisense ncRNAs (43) were detected with significant expression changes induced by hTGF-β (Table 3).
In humans it is known that several microRNAs are regulated by TGF-β [49] and that microRNAs interact with TGF-β signaling elements (SMADs) to change the canonical role of these signaling elements [50]. Another perspective that recently has been explored is the role of SMADs in the post-transcriptional processing of miRNAs [51]; data suggest that coordinately-regulated miRNAs cooperate to regulate specific signaling pathways (such as TGF-β). Perhaps ncRNAs cooperate with TGF-β elements in S. mansoni signaling.
A considerable number of ncRNAs (43) transcribed from the same genomic loci of known protein coding genes was detected with significant expression changes (Table 3). These ncRNAs are really interesting because they are potentially a source of Natural Antisense Transcripts (NATs) under the regulation of hTGF-β that can act in the cell not only as source of microRNAs but also operate through other mechanisms already described in eukaryotes [48]; further investigation is necessary to explore this hypothesis.
We conclude that this work offers a repository of S. mansoni genes with changes in their expression level in response to hTGF-β, and that these genes are potentially responsible for the effects of hTGF-β that are observed in the parasite. These identified molecular targets may lead to elucidation of the mechanisms involved in the role of crosstalk in the biology of schistosome host-parasite interactions.
Highlights.
381 genes in S. mansoni worms treated with hTGF-β exhibited changes in expression
From these genes, 316 genes are downregulated and 65 are upregulated
We list statistically enriched Gene Ontology categories and genes in each category
We model S. mansoni genes altered by treatment with hTGF-β into canonical pathways
We identified 43 genes with changes in transcription level of non-coding RNAs
Supplementary Material
qPCR was performed with total RNA and primers described in Supplementary Table 1, using 3 technical replicas for each one of the five biological samples (three hTGF-β test samples, TGF_1, TGF_4 and TGF_5; two Control samples, Ctrl_2 and Ctrl_3). The horizontal lines show the mean ± standard deviation. There are no significant changes (p-value > 0.15).
The shapes of elements correspond to different types of molecules, as indicated in the lower box; arrows represent the relationships between elements: dashed lines = indirect interaction, filled lines = direct interaction; color intensity is proportional to expression value, computed as log2 [Treated/Control]; green means negative log-ratios, i.e. down-regulation in treated parasites, and red means positive log-ratios, i.e. up-regulation in treated parasites.
Acknowledgements
Supported in part by a grant from Fundaçao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) to SVA and by NIH AI 46762 to PTL. KCO and MLPC received postdoctoral and Master fellowships from FAPESP, respectively, and SVA is the recipient of an established investigator fellowship from CNPq. Schistosome material was obtained from the Schistosome Supply Contract (NIAID Contract No. HHSN272201000009I) to Dr. Fred Lewis.
Abbreviations
- AMPK
5' AMP-activated protein kinase
- BMP
Bone Morphogenic Protein
- CBP
CREB binding protein
- eIF
eukaryotic translation initiation factor
- Erk
Extracellular signal-regulated kinases
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- hTGF-β
Human Transforming Growth Factor-β
- miRNA
microRNA
- ncRNAs
non-coding RNAs
- R-SMAD
Receptor-mediated SMAD
- RT-qPCR
Reverse Transcription- quantitative Real Time PCR
- Sm
Schistosoma mansoni
- SYK
Spleen Tyrosine Kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katia C. Oliveira, Email: katiacpo@iq.usp.br.
Sergio Verjovski-Almeida, Email: verjo@iq.usp.br.
Philip LoVerde, Email: loverde@uthscsa.edu.
References
- 1.Doumenge JP, Mott KE. Global distribution of schistosomiasis: CEGET/WHO atlas. World Health Stat Q. 1984;37:186–199. [PubMed] [Google Scholar]
- 2.Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, Ritter D, et al. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 4.Davies SJ, Lim KC, Blank RB, Kim JH, Lucas KD, Hernandez DC, et al. Involvement of TNF in limiting liver pathology and promoting parasite survival during schistosome infection. Int J Parasitol. 2004;34:27–36. doi: 10.1016/j.ijpara.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han ZG, Brindley PJ, Wang SY, Chen Z. Schistosoma genomics: new perspectives on schistosome biology and host-parasite interaction. Annu Rev Genomics Hum Genet. 2009;10:211–240. doi: 10.1146/annurev-genom-082908-150036. [DOI] [PubMed] [Google Scholar]
- 6.Wolowczuk I, Nutten S, Roye O, Delacre M, Capron M, Murray RM, et al. Infection of mice lacking interleukin-7 (IL-7) reveals an unexpected role for IL-7 in the development of the parasite Schistosoma mansoni. Infect Immun. 1999;67:4183–4190. doi: 10.1128/iai.67.8.4183-4190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu W, Niles EG, Loverde PT. Thyroid hormone receptor orthologues from invertebrate species with emphasis on Schistosoma mansoni. BMC Evol Biol. 2007;7:150. doi: 10.1186/1471-2148-7-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira KC, Carvalho ML, Venancio TM, Miyasato PA, Kawano T, DeMarco R, et al. Identification of the Schistosoma mansoni TNF-alpha receptor gene and the effect of human TNF-alpha on the parasite gene expression profile. PLoS Negl Trop Dis. 2009;3:e556. doi: 10.1371/journal.pntd.0000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman A, Niles EG, Verjovski-Almeida S, LoVerde PT. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathog. 2006;2:e54. doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies SJ, Shoemaker CB, Pearce EJ. A divergent member of the transforming growth factor beta receptor family from Schistosoma mansoni is expressed on the parasite surface membrane. J Biol Chem. 1998;273:11234–11240. doi: 10.1074/jbc.273.18.11234. [DOI] [PubMed] [Google Scholar]
- 11.You H, Gobert GN, Jones MK, Zhang W, McManus DP. Signalling pathways and the host-parasite relationship: putative targets for control interventions against schistosomiasis: signalling pathways and future anti-schistosome therapies. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:203–214. doi: 10.1002/bies.201000077. [DOI] [PubMed] [Google Scholar]
- 12.You H, Zhang W, Moertel L, McManus DP, Gobert GN. Transcriptional profiles of adult male and female Schistosoma japonicum in response to insulin reveal increased expression of genes involved in growth and development. International journal for parasitology. 2009;39:1551–1559. doi: 10.1016/j.ijpara.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Escobedo G, Roberts CW, Carrero JC, Morales-Montor J. Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol. 2005;21:588–593. doi: 10.1016/j.pt.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Haseeb MA, Solomon WB, Palma JF. Schistosoma mansoni: effect of recombinant tumor necrosis factor alpha on fecundity and [14C]-tyrosine uptake in females maintained in vitro. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:265–269. doi: 10.1016/s0742-8413(96)00137-5. [DOI] [PubMed] [Google Scholar]
- 15.Davies SJ, McKerrow JH. Developmental plasticity in schistosomes and other helminths. Int J Parasitol. 2003;33:1277–1284. doi: 10.1016/s0020-7519(03)00161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dissous C, Khayath N, Vicogne J, Capron M. Growth factor receptors in helminth parasites: signalling and host-parasite relationships. FEBS Lett. 2006;580:2968–2975. doi: 10.1016/j.febslet.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 17.Freitas TC, Pearce EJ. Growth factors and chemotactic factors from parasitic helminths: molecular evidence for roles in host-parasite interactions versus parasite development. Int J Parasitol. 2010;40:761–773. doi: 10.1016/j.ijpara.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 19.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 20.Beall MJ, McGonigle S, Pearce EJ. Functional conservation of Schistosoma mansoni Smads in TGF-beta signaling. Mol Biochem Parasitol. 2000;111:131–142. doi: 10.1016/s0166-6851(00)00307-8. [DOI] [PubMed] [Google Scholar]
- 21.Carlo JM, Osman A, Niles EG, Wu W, Fantappie MR, Oliveira FM, et al. Identification and characterization of an R-Smad ortholog (SmSmad1B) from Schistosoma mansoni. FEBS J. 2007;274:4075–4093. doi: 10.1111/j.1742-4658.2007.05930.x. [DOI] [PubMed] [Google Scholar]
- 22.Osman A, Niles EG, LoVerde PT. Identification and characterization of a Smad2 homologue from Schistosoma mansoni, a transforming growth factor-beta signal transducer. J Biol Chem. 2001;276:10072–10082. doi: 10.1074/jbc.M005933200. [DOI] [PubMed] [Google Scholar]
- 23.Osman A, Niles EG, LoVerde PT. Expression of functional Schistosoma mansoni Smad4: role in Erk-mediated transforming growth factor beta (TGF-beta) down-regulation. J Biol Chem. 2004;279:6474–6486. doi: 10.1074/jbc.M310949200. [DOI] [PubMed] [Google Scholar]
- 24.Freitas TC, Jung E, Pearce EJ. TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni. PLoS Pathog. 2007;3:e52. doi: 10.1371/journal.ppat.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freitas TC, Jung E, Pearce EJ. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni. Int J Parasitol. 2009;39:281–287. doi: 10.1016/j.ijpara.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertin B, Oger F, Cornette J, Caby S, Noel C, Capron M, et al. Schistosoma mansoni CBP/p300 has a conserved domain structure and interacts functionally with the nuclear receptor SmFtz-F1. Mol Biochem Parasitol. 2006;146:180–191. doi: 10.1016/j.molbiopara.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Loverde PT, Osman A, Hinck A. Schistosoma mansoni: TGF-beta signaling pathways. Exp Parasitol. 2007;117:304–317. doi: 10.1016/j.exppara.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LoVerde PT, Andrade LF, Oliveira G. Signal transduction regulates schistosome reproductive biology. Curr Opin Microbiol. 2009;12:422–428. doi: 10.1016/j.mib.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knobloch J, Beckmann S, Burmeister C, Quack T, Grevelding CG. Tyrosine kinase and cooperative TGFbeta signaling in the reproductive organs of Schistosoma mansoni. Exp Parasitol. 2007;117:318–336. doi: 10.1016/j.exppara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Duvall RH, DeWitt WB. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967;16:483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 31.Verjovski-Almeida S, Venancio TM, Oliveira KC, Almeida GT, DeMarco R. Use of a 44k oligoarray to explore the transcriptome of Schistosoma mansoni adult worms. Exp Parasitol. 2007;117:236–245. doi: 10.1016/j.exppara.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Quackenbush J. Microarray data normalization and transformation. Nat Genet. 2002;32(Suppl):496–501. doi: 10.1038/ng1032. [DOI] [PubMed] [Google Scholar]
- 33.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukunaga K, Hummels DM. Leave-One-out Procedures for Nonparametric Error-Estimates. Ieee Transactions on Pattern Analysis and Machine Intelligence. 1989;11:421–423. [Google Scholar]
- 35.Robinson PN, Wollstein A, Bohme U, Beattie B. Ontologizing gene-expression microarray data: characterizing clusters with Gene Ontology. Bioinformatics. 2004;20:979–981. doi: 10.1093/bioinformatics/bth040. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online. 2006;8:175–193. doi: 10.1251/bpo126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nawaratna SS, McManus DP, Moertel L, Gobert GN, Jones MK. Gene Atlasing of digestive and reproductive tissues in Schistosoma mansoni. PLoS Negl Trop Dis. 2011;5:e1043. doi: 10.1371/journal.pntd.0001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Yan Q, Shen DK, Liu F, Zhu ZD, Song HD, et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nature genetics. 2003;35:139–147. doi: 10.1038/ng1236. [DOI] [PubMed] [Google Scholar]
- 41.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, et al. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- 43.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Smad7 is required for TGF-beta-induced activation of the small GTPase Cdc42. J Cell Sci. 2004;117:1835–1847. doi: 10.1242/jcs.01036. [DOI] [PubMed] [Google Scholar]
- 44.Nesti LJ, Caterson EJ, Wang M, Chang R, Chapovsky F, Hoek JB, et al. TGF-beta1 calcium signaling increases alpha5 integrin expression in osteoblasts. J Orthop Res. 2002;20:1042–1049. doi: 10.1016/S0736-0266(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 45.Stricker SA, Smythe TL. Differing mechanisms of cAMP- versus seawater-induced oocyte maturation in marine nemertean worms I. The roles of serine/threonine kinases and phosphatases. Mol Reprod Dev. 2006;73:1578–1590. doi: 10.1002/mrd.20597. [DOI] [PubMed] [Google Scholar]
- 46.Beckmann S, Buro C, Dissous C, Hirzmann J, Grevelding CG. The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000769. e1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira KC, Carvalho MLP, Maracaja-Coutinho V, Kitajima JP, Verjovski-Almeida S. Non-coding RNAs in schistosomes: an unexplored world. An Acad Bras Cienc. 2011;83:673–694. doi: 10.1590/s0001-37652011000200026. [DOI] [PubMed] [Google Scholar]
- 49.Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. Transforming growth factor-beta and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–161. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 50.Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta} J Biol Chem. 2010;285:41328–41336. doi: 10.1074/jbc.M110.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis-Dusenbery BN, Hata A. Smad-mediated miRNA processing: A critical role for a conserved RNA sequence. RNA Biol. 2011;8 doi: 10.4161/rna.8.1.14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qPCR was performed with total RNA and primers described in Supplementary Table 1, using 3 technical replicas for each one of the five biological samples (three hTGF-β test samples, TGF_1, TGF_4 and TGF_5; two Control samples, Ctrl_2 and Ctrl_3). The horizontal lines show the mean ± standard deviation. There are no significant changes (p-value > 0.15).
The shapes of elements correspond to different types of molecules, as indicated in the lower box; arrows represent the relationships between elements: dashed lines = indirect interaction, filled lines = direct interaction; color intensity is proportional to expression value, computed as log2 [Treated/Control]; green means negative log-ratios, i.e. down-regulation in treated parasites, and red means positive log-ratios, i.e. up-regulation in treated parasites.