Abstract
Influenza virus infection is considered a major worldwide public health problem. Seasonal infections with the most common influenza virus strains (e.g. H1N1) can usually be resolved, but they still cause a high rate of mortality. The factors that influence the outcome of the infection remain unclear. Here we show that deficiency of IL-6 or IL-6 receptor is sufficient for normally sublethal doses of H1N1 influenza A virus to cause death in mice. IL-6 is necessary for the resolution of influenza infection by protecting neutrophils from virus-induced death in the lung and by promoting neutrophil-mediated viral clearance. Loss of IL-6 results in persistence of influenza virus in the lung leading to pronounced lung damage and, ultimately, death. Thus, we demonstrate that IL-6 is a vital innate immune cytokine in providing protection against influenza A infection. Genetic or environmental factors that impair IL-6 production or signalling could increase mortality to influenza virus infection.
INTRODUCTION
Influenza virus infection is a major worldwide public health problem. Without considering the highly pathogenic strains of influenza virus (e.g. H5N1), seasonal infections with the most common influenza virus strains (e.g. H1N1) cause significant mortality with over 40,000 deaths per year and more than 250,000 hospitalizations in the US alone. While seasonal infections with the most common influenza virus strains can usually be resolved by the immune system without major sequelae, in some cases the infection cannot be controlled, virus clearance is impaired and death ensues.1 It remains unclear why certain populations (other than elderly and infants) have serious difficulties in the resolution of influenza virus infection, and what factors influence the outcome of the infection. Thus, during the 2009 H1N1 pandemic it was found that African Americans are more susceptible and had more severe complications than Caucasians.2, 3
Although the adaptive immune response is essential for developing immunological memory, it is not the primary mechanism of defense during primary influenza infection.4, 5 Instead, the innate immune response is considered the major mechanism in the resolution of influenza infection.6 Recent studies have given evidence that macrophages and neutrophils can provide protection against primary influenza infection. Macrophages contribute to the elimination of influenza virus through the release of inflammatory cytokines and IFNα/β, and depletion of macrophages has been shown to increase susceptibility to H3N2 virus infection in mice.7 Although lung neutrophilia is often associated with lung pathology (e.g. acute lung injury),8 a number of recent studies support a protective role for neutrophils in infection with influenza virus by helping with virus clearance.9, 10 The elevated levels of proinflammatory cytokines (TNFα, IL-1β and IL-6) during influenza infection have also been primarily associated with increased lung pathology and worse outcome,11 but it is uncertain whether some of these cytokines could also be protective during seasonal influenza infection.
IL-6 is produced by macrophages, dendritic cells, mast cells and other innate immune cells, and consequently it has long been considered a marker of inflammation. The increased levels of IL-6 found in a number of diseases are mostly defined to be the result of ongoing inflammatory cell activation. However, in addition to immune cells, IL-6 can also be produced by non-immune cells such as epithelial cells, endothelial cells, keratinocytes, and fibroblasts among others, in response to specific stimuli.12, 13 A number of studies have shown that human bronchoepithelial cell lines can produce IL-6 in response to different exposures such as allergens or respiratory viruses.14, 15 We have recently demonstrated that mouse primary lung epithelial cells, but not lung resident immune cells, exhibit a constitutive expression of the IL-6 gene prior to exposure to any environmental insult.16 Thus, the presence of IL-6 may not necessarily correlate with the production of other inflammatory cytokines and it may not be just a marker of ongoing inflammation, but a direct player in the immune response. A number of studies have shown a role of IL-6 in the adaptive immune response, primarily on the differentiation fate of CD4 T cells,17 but IL-6 can also modulate aspects of the innate immune response.12, 13
Elevated levels of IL-6 in the lung as well as in serum have been found in patients infected with influenza virus, including the 2009 H1N1 pandemic influenza.18, 19 However, it is unknown whether IL-6 in these patients contributes to the lung pathology caused by the virus or whether it is elevated as a protective mechanism and eliminating IL-6 could worsen the course of the infection. This study shows that, instead of being pathogenic, IL-6 and IL-6 mediated signals are essential for survival to a non-lethal dose of influenza H1N1 virus infection. Deficiency of IL-6 or IL-6R prevents the clearance of H1N1 virus in association with low numbers of neutrophils present in the lungs of infected mice. We also show that IL-6 provides survival signals to protect neutrophils from influenza virus-triggered apoptosis. Impaired virus clearance caused by the lack of IL-6 or IL-6R signals leads to emphysema-like destruction of the lung and, ultimately, death. Thus, IL-6 is a protective factor against primary infection with influenza H1N1 virus by promoting the innate phase of the immune response and virus clearance.
RESULTS
IL-6 and IL-6R are required for survival to primary infection with H1N1 influenza virus
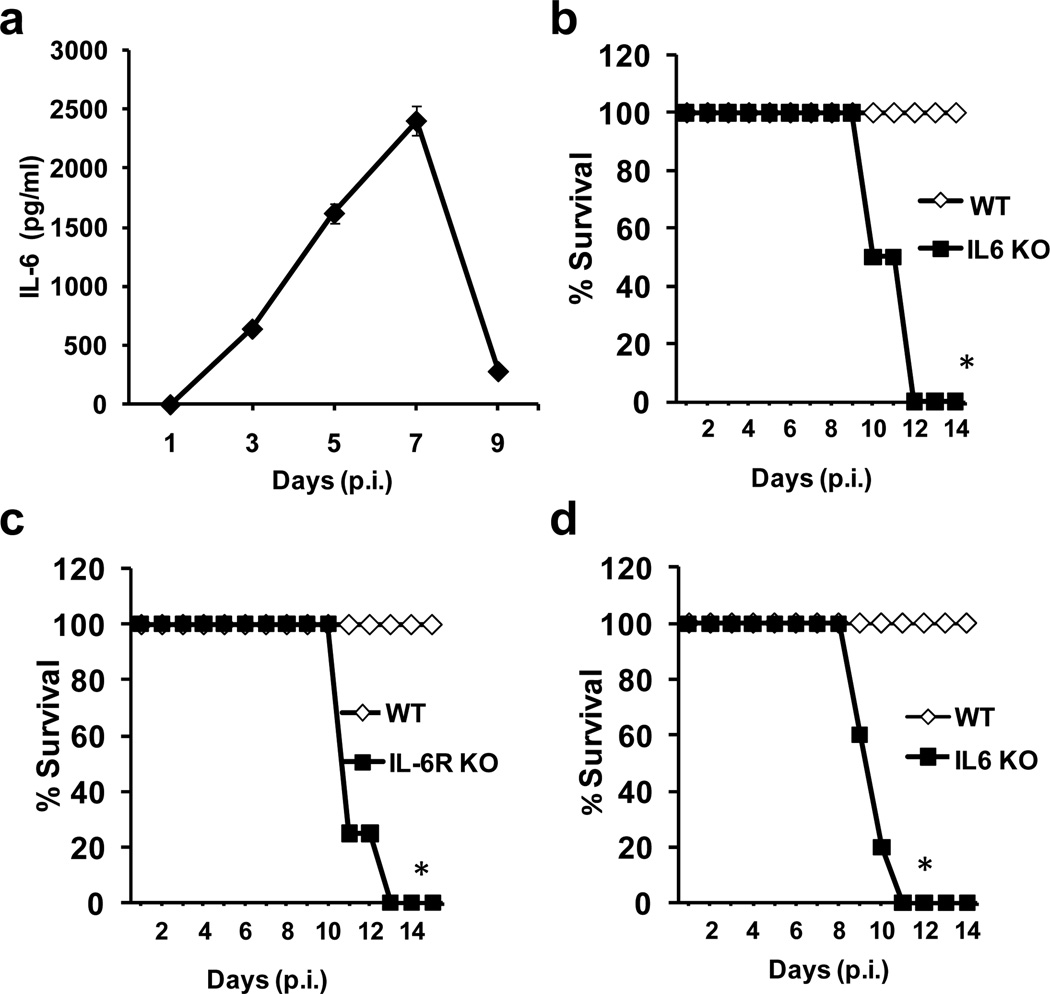
Since elevated IL-6 levels have been found in the lungs of patients during influenza virus infection, we examined the regulation of IL-6 production in the lungs of mice infected with H1N1 influenza. Similar to what has been found in humans, infection of wildtype mice with a sublethal dose of influenza A/Puerto Rico 8/34 (PR8) H1N1 virus promoted the release of IL-6 into the lung airways (Fig. 1a). However, IL-6 accumulation did not follow rapid kinetics since the levels were not high until day 3 postinfection (p.i.), and remained high at least until day 7 p.i. (Fig. 1a). To determine whether IL-6 could play a role in either the pathogenesis or resolution of infection, we examined the response of wildtype and IL-6 deficient mice 20 to infection with sublethal doses of PR8 H1N1 influenza A virus. While wildtype mice recovered from the infection as expected, all IL-6 deficient mice died between days 10–12 p.i. (Fig. 1b). To further demonstrate that IL-6 signaling is necessary for survival from a sublethal dose of PR8 virus, we also infected the recently generated IL-6 receptor (IL-6R) deficient mice 21 with PR8 H1N1 influenza virus. Similar to IL-6 KO mice, IL-6R KO mice succumbed to the sublethal dose of virus between days 10–12 p.i. (Fig. 1c). In 2009, a new subtype of H1N1 influenza (“swine” flu) emerged that was a reassortment of swine, avian, and human H1N1 viruses. It led to a significant increase in influenza-related morbidity and mortality during the 2009 influenza pandemic.22–24 We therefore examined the role of IL-6 during infection with a sublethal dose of the A/California/7/2009 H1N1 isolate of the 2009 pandemic H1N1 influenza virus.25 As with the PR8 H1N1 subtype, virus infection in wildtype mice was followed by 100% recovery, whereas IL-6 KO mice again succumbed to the 2009 H1N1 virus between days 9–11 p.i. (Fig. 1d). Together, these results demonstrate that IL-6 is a key cytokine for survival from primary infection with H1N1 influenza virus.
Figure 1. IL-6 and IL-6R are essential to survive infections with sublethal doses of influenza H1N1 virus.
(a) Wildtype mice (n=4) were infected with a sublethal dose of H1N1 PR8 virus (3×103 EIU) intranasal (i.n.). IL-6 levels in bronchoalveolar lavage fluid (BALF) of infected mice were determined at the indicated periods of time p.i. by Luminex. Values represent mean +/− sd. (b) Kaplan-Meier survival curve of wildtype mice (WT) and IL-6 KO mice (n=4) following i.n. infection with H1N1 PR8 virus (3×103 EIU). (c) Kaplan-Meier survival curve of WT and IL-6R KO mice (n=5) infected with H1N1 PR8 virus as described in (a). (d) Kaplan-Meier survival curve of WT and IL-6 KO mice (n=5) infected with a sublethal dose of A/California/7/2009 H1N1 influenza virus (3×103 EIU). * denotes p<0.05. Statistical significance was determined by log-rank test (b, c and d).
H1N1 influenza virus infection in the absence of IL-6 leads to emphysema-like lung damage
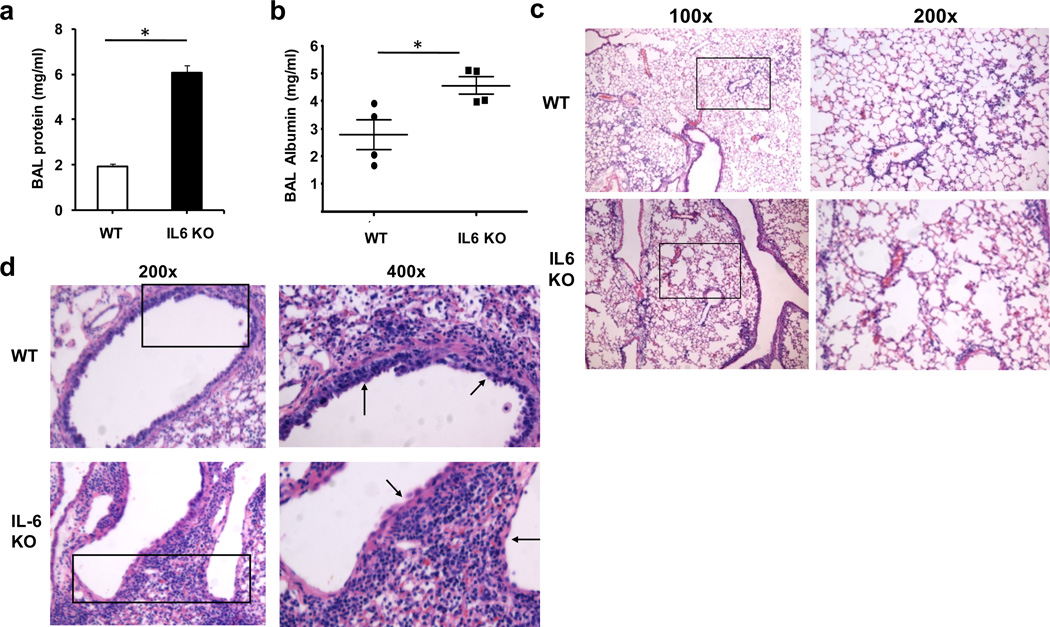
Death due to influenza infection in humans is generally associated with the development of acute respiratory distress syndrome (ARDS) and respiratory failure.26 To address whether mortality caused by H1N1 infection in IL-6 deficient mice could be due to acute lung injury, we examined protein content in bronchoalveolar lavage fluid (BALF) as a parameter indicative of failed barrier function in the lung as well as lung damage. Increased levels of total protein (Fig. 2a) as well as albumin (Fig. 2b) were found in infected IL-6 KO mice compared with wildtype mice at the time the IL-6 deficient mice were moribund (days 9 to 11 p.i.). To further assess lung damage during H1N1 infection, histological examination of lungs from infected mice was performed. No differences in the overall number of inflammatory cells in the lung were observed between wildtype and IL-6 KO mice. However, IL-6 KO mice were noted to have developed emphysema-like enlargement of the alveoli, a finding not present in wildtype mice (Fig. 2c). This phenotype was seen in all examined IL-6 KO mice (Suppl. Fig. S1), discounting a possible non-specific effect of the lung inflation. Furthermore, the enlarged alveoli-phenotype was also found in lungs of virus-infected IL-6R KO mice and absent in wild type controls (Suppl. Fig. S1). This pattern of lung damage was not detected in IL-6 KO mice at day 7 p.i., despite marked inflammation in the lung compared to mice at days 10 to 11 p.i. (Suppl. Fig. S2), suggesting that this phenotype was probably associated with the chronic viral infection instead of acute inflammation. In addition to the damage of the lung parenchyma, most airways in IL-6 KO mice appeared to be denuded of their epithelial lining compared to wildtype mouse airways (Fig. 2d). Thus, mortality caused by H1N1 infection in the absence of IL-6 is associated with capillary leak and emphysematous destruction of the lung parenchyma.
Figure 2. Severe lung damage in IL-6 deficient mice caused by H1N1 influenza virus infection.
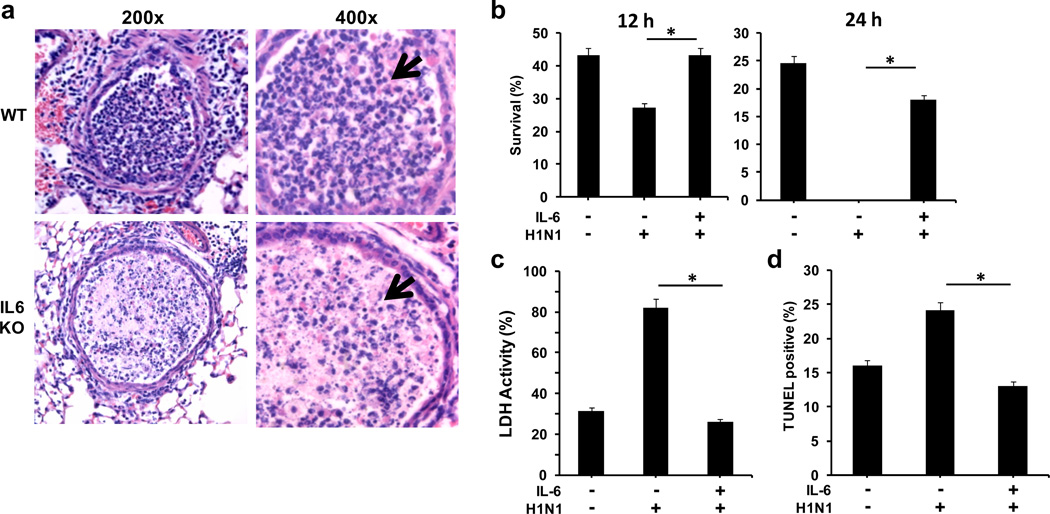
(a & b) Wildtype mice (n=4) and IL-6 KO mice (n=4) were infected with a sublethal dose of H1N1 PR8 virus (3×103 EIU) intranasal (i.n.). Total protein (a) and albumin (b) concentrations in BALF were determined at day 9 p.i.. (c and d) Histopathology of lungs from WT and IL-6 KO mice at day 9 p.i. with PR8 H1N1. 100x and 200x (c) or 200x and 400x (d) magnifications of H&E stained lung sections are shown. Arrows in (d) point to the epithelium of the airways. * denotes p<0.05. Statistical significance was determined by student’s t test (a and b). Results are representative of at least 2 independent experiments.
IL-6 deficiency impairs the presence of neutrophils in the lung and influenza virus clearance
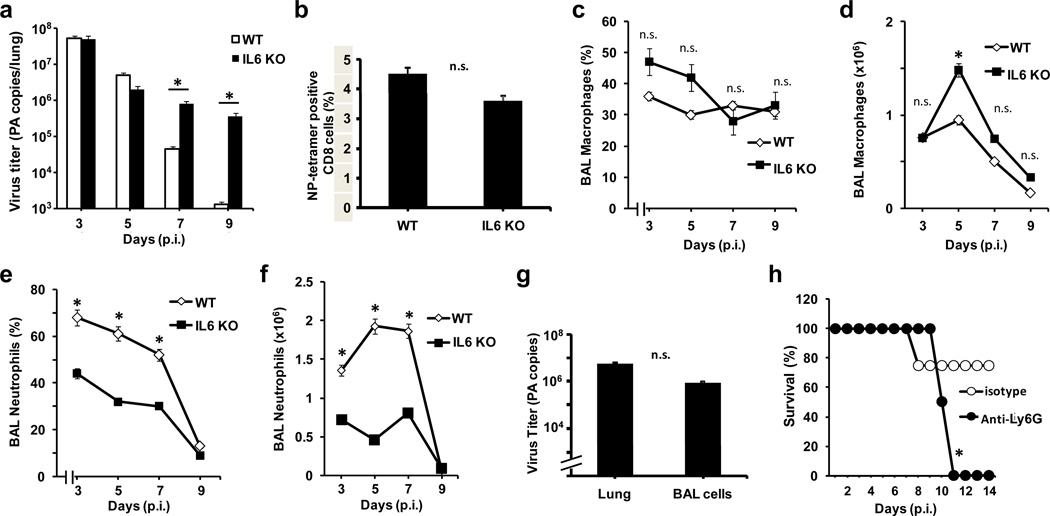
To investigate the potential causes of the increased H1N1-induced lung damage in IL-6 KO mice, we first examined viral titers in the lung during infection. As expected, the peak of virus titer in wildtype mice occurred around day 3, with clearance of virus by day 9 p.i. (Fig. 3a). Viral titers in wildtype and IL-6 KO mice were comparable at days 3 and 5 p.i., indicating that viral replication and infectivity was not affected by the absence of IL-6. However, viral titers in IL-6 KO mice remained high even at day 9 p.i., showing that these mice were unable to clear the virus effectively.
Figure 3. IL-6 deficiency results in insufficient neutrophils in the lung during influenza H1N1 virus infection and inability to clear the virus.
(a) Virus titers determined by the number of influenza polymerase A (PA) RNA copies by real time RT-PCR in total lung of WT and IL-6 KO mice (n=5) at different days p.i. with H1N1 PR8 virus (3×103 EIU). (b) Percentage of CD8 T cells that were positive for nucleoprotein (NP)-tetramers within the mediastinal lymph node from WT and IL-6 KO mice (n=4) 9 days p.i., as determined by flow cytometry analysis. (c-f) Percentage (c and e) and total number (d and f) of macrophages (c and d) and neutrophils (e and f) in BAL from WT and IL-6 KO mice (n=5) at different days p.i. with H1N1 PR8 virus. (g) Virus titers determined by the number of influenza PA RNA copies in total lung and in cells from BAL of WT mice (n=4) 5 days p.i. with PR8 virus. (h) Kaplan-Meier survival curve of WT mice (n=5) infected with H1N1 PR8 virus that received an intraperitoneal administration (500 μg/mouse) of an anti-Ly6G Ab or an isotype control one day prior to infection and another administration at day 3 p.i. * denotes p<0.05. n.s. denotes not significant. Statistical significance was determined by 2-way ANOVA (a, c-f), student’s t test (b and g), or log-rank test (h). Results are representative of 2 to 4 independent experiments.
Since CD8 T cells can contribute but are not essential for influenza virus clearance during primary infection,27 we examined the presence of virus-specific CD8 T cells in the lung draining mediastinal lymph node. We found that IL-6 deficiency did not affect the expansion of virus-specific CD8 T cells in wildtype versus IL-6 KO mice infected with influenza (Fig. 3b). Similarly, expansion of CD4 T cells was not significantly altered in the absence of IL-6 (Suppl. Fig. S3a). B cells have been shown to provide protection against primary influenza virus infection 28 but neither their cell numbers (Suppl. Fig. S3a) nor influenza-specific IgG (Suppl. Fig. S3b) were significantly different between wildtype and IL-6 KO mice. In addition, the levels in BAL of IFNγ, normally associated with a strong antiviral response, were not significantly different between IL-6 KO mice and wildtype mice (Suppl. Fig. 3c). Lymphocyte infiltration into the airways was also not affected by the absence of IL-6 (data not shown). Thus, an impaired adaptive immune response does not seem to underlie the inability of IL-6 KO mice to clear influenza virus infection in the lung.
The innate immune response is a major mechanism for influenza virus clearance during primary infection. Macrophages support influenza virus clearance through the release of inflammatory cytokines as well as IFNα/β, and depletion of macrophages has been shown to increase susceptibility to H3N2 virus infection.7 Nevertheless, analysis of BAL from infected wildtype and IL-6 KO mice showed no statistically significant differences in the percentage of macrophages during the course of infection (Fig. 3c), and only a slight increase in macrophage number in IL-6 KO mice at day 5 p.i. (Fig. 3d). In contrast, there was a significant decrease in the percentage (Fig. 3e) and total number (Fig. 3f) of neutrophils in IL-6 deficient mice compared to wildtype mice as early as day 3 p.i.. Thus, IL-6 deficiency selectively impairs the presence of neutrophils in the lung during influenza virus infection. The greatest difference in the number of neutrophils in the lungs of IL-6 KO versus wildtype mice coincided with the most active period of virus clearance in wildtype mice at days 5 to 7 p.i..
While accumulation of neutrophils in the lung is often associated with lung pathology (e.g. acute lung injury),29 recent studies supported a protective role for neutrophils in infection with influenza virus. Thus, depletion of neutrophils has been shown to be fatal in a model of H3N2 influenza virus infection and to increase severity of disease caused by PR8 virus infection.10, 30 Although neutrophils do not support virus replication, they have the ability to take up virus and they contribute to influenza virus clearance.31, 32 Analysis of H1N1 PR8 in BAL cells from mice 3 days p.i., when the majority of cells are neutrophils, indeed revealed virus copy numbers almost as high as those observed in infected whole lung tissue (Fig. 3g). No difference in virus copy number in BAL cells was observed between wildtype and IL-6 KO mice at this time of infection (Suppl. Fig. S4). To determine the importance of neutrophils in primary infection with a sublethal dose of H1N1 influenza virus, neutrophils from wildtype mice were depleted with an antibody (Ab) that recognizes the neutrophil-specific antigen Ly6G. Similar to IL-6 and IL-6R KO mice, anti-Ly6G treated mice succumbed to the virus between days 10–12 p.i. (Fig. 3h). These results demonstrate that neutrophils are indeed critical for the resolution of primary influenza A virus infection. Thus, impaired H1N1 virus clearance in the absence of IL-6 is likely due to insufficient neutrophil numbers in the lung during infection.
IL-6 is dispensable for neutrophil release and recruitment to the lung during influenza virus infection
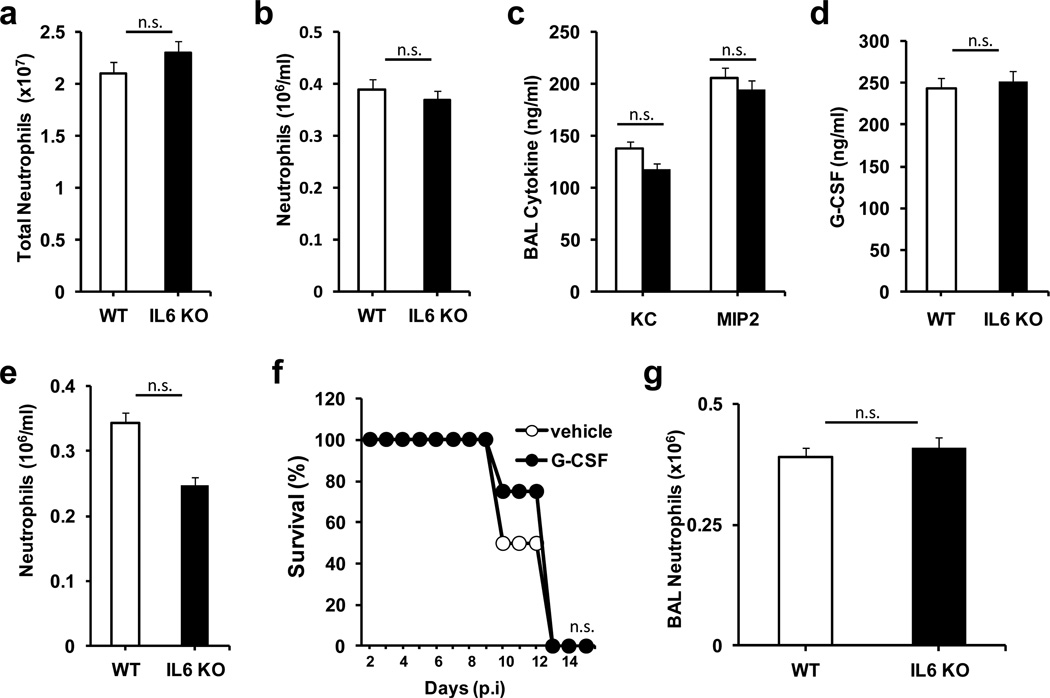
To determine whether IL-6 deficiency could interfere with normal bone marrow generation of neutrophils causing mice to be neutropenic, we examined neutrophil levels in both blood and bone marrow from uninfected wildtype and IL-6 KO mice. No differences in neutrophil levels in bone marrow (Fig. 4a) or peripheral blood (Fig. 4b) were observed. To determine if the absence of IL-6 could cause a decrease in neutrophil recruitment during viral infection, BALF from infected wildtype and IL-6 KO mice was collected at day 3 p.i. and levels of chemokines that mediate neutrophil recruitment were measured. No significant differences in BALF levels of MIP-2 or KC (two critical murine neutrophil chemoattractants) between wildtype and IL-6 KO mice were detected at day 3 p.i. (Fig. 4c) or at days 1 or 5 p.i. (data not shown). G-CSF has been shown to induce the release of neutrophils from the bone marrow to the circulation during ‘stress granulopoiesis’ in response to lung infection.33 G-CSF levels in BALF were comparable between IL-6 KO and wildtype mice day 3 p.i. (Fig. 4d) as well as at day 1 and day 5 p.i. (data not shown). Furthermore, levels of circulating neutrophils in IL-6 KO mice 3 days p.i. did not differ from those found in infected wildtype mice (Fig. 4e). To further address the potential role of G-CSF in influenza pathogenesis in the absence of IL-6, we supplemented IL-6 KO mice with G-CSF during infection. Treatment of IL-6 KO mice with G-CSF did not improve survival in these mice (Fig. 4f), indicating that impaired G-CSF production is not the cause of increased mortality, and that enhanced neutrophil release from the bone marrow does not rescue these mice. Taken together, these results show that the effects of IL-6 deficiency during influenza infection are not due to impaired release of neutrophils from the bone marrow. We also examined neutrophil accumulation to the lungs following exposure to nebulized LPS, known to induce rapid recruitment of neutrophils to the lung.34 No difference in neutrophil recruitment to the lungs was observed between wildtype and IL-6 KO mice following LPS administration (Fig. 4g). Thus, IL-6 deficiency does not appear to interfere with neutrophil release from bone marrow or recruitment to the lung, but interferes with the accumulation of neutrophils in the lung, suggesting an effect in neutrophil survival during influenza infection.
Figure 4. IL-6 deficiency does not interfere with neutrophil release or recruitment to the lung.
(a) Neutrophil numbers in total bone marrow from non-infected WT and IL-6 KO mice (n=3). (b) Frequency of neutrophils (cell number/ml) in peripheral blood of non-infected WT and IL-6 KO mice (n=3). (c) Levels of KC and MIP-2, in BALF from WT and IL-6 KO mice (n=5) 3 days p.i. with H1N1 PR8 virus. (d) Levels of G-CSF in BALF from WT and IL-6 KO mice (n=5) 3 days p.i. with H1N1 PR8 virus. (e) Frequency of neutrophils (cell number/ml) in peripheral blood of WT and IL-6 KO mice (n=4) 3 days p.i. with H1N1 PR8 virus. (f) Survival curve of IL-6 KO mice (n=4) administered i.p. with G-CSF (5 μg/mouse) or PBS control a day prior to infection and at day 3 p.i. (g) Neutrophils in BAL from WT and IL-6 KO mice (n=3) 24 hours after administration of nebulized LPS. * denotes p<0.05, n.s. denotes “not significant”. Statistical significance was determined by student’s t test (a-e and g), or log-rank test (f). Results are representative of at least 2 independent experiments.
IL-6 protects neutrophils from influenza virus-induced death
Histological analysis of the airways of lungs from wildtype mice 5 days p.i. showed the presence of neutrophils in the airways (Fig. 5a). Interestingly, few viable neutrophils were present in the airways of infected IL-6 KO mice (Fig. 5a). Instead, there was an accumulation of pyknotic nuclei characteristic of neutrophil death. To determine if IL-6 provides survival signals to neutrophils in the setting of influenza viral infection, neutrophils were isolated from uninfected wildtype mice, incubated in vitro with PR8 influenza virus in the presence or absence of IL-6, and cell survival was assessed. Influenza virus enhanced neutrophil death at 12 hrs and few cells were recovered intact after 24 hrs (Fig. 5b). However, the presence of IL-6 abrogated virus-induced death of neutrophils (Figs. 5b). A slight decrease in spontaneous neutrophil death by IL-6 was also observed (Suppl. Fig. S5). The effect of IL-6 in preventing neutrophil death caused by H1N1 infection was further delineated by analysis of lactate dehydrogenase (LDH) in the culture supernatants as a parameter of cell lysis. High levels of LDH were detected in the supernatants of neutrophils incubated with PR8 H1N1 virus alone (Fig. 5c), but not in neutrophils incubated with PR8 H1N1 virus in the presence of IL-6 (Fig. 5c). Thus, H1N1 virus promotes neutrophil death, but this effect can be prevented if IL-6 is present. To determine whether virus-induced neutrophil cell death occurred through apoptosis, TUNEL analysis was performed. PR8 H1N1 virus increased the number of apoptotic neutrophils (Fig. 5d), but this effect was abrogated with the addition of IL-6. Thus, the presence of IL-6 can provide survival signals to protect neutrophils from the acute apoptosis triggered by influenza infection in the lung.
Figure 5. IL-6 protects neutrophils from H1N1 virus-mediated apoptosis.
(a) Histopathology of lungs (H&E staining) from WT and IL-6 KO mice at day 5 p.i. with PR8 H1N1 virus. 200x and 400x magnifications of airways are shown. Arrows point to well-defined polymorphonuclear neutrophil in WT airways, and apoptotic nucleus in IL-6 KO airways. (b) Neutrophils from WT mice were cultured in the presence or absence of PR8 H1N1 virus at 1:10 neutrophil:virus (EIU) ratio with or without IL-6 (20 ng/ml). After 12 h and 24 h, live cells were counted by trypan blue staining. Values (mean +/− SD, n=3) represent the frequency of live cells recovered relative to the initial number. (c) LDH activity in supernatants from neutrophils cultured as described in (b). (d) Neutrophils were cultured as in (b) and apoptosis was determined by TUNEL assay and flow cytometry analysis. Values show the percentage of TUNEL positive cells (mean +/− SD, n=3). * denotes p<0.05. Statistical significance was determined by student’s t test. Results are representative of 2–3 independent experiments.
Downregulation of Mcl-1 and Bcl-XL in neutrophils by H1N1 virus is prevented by IL-6
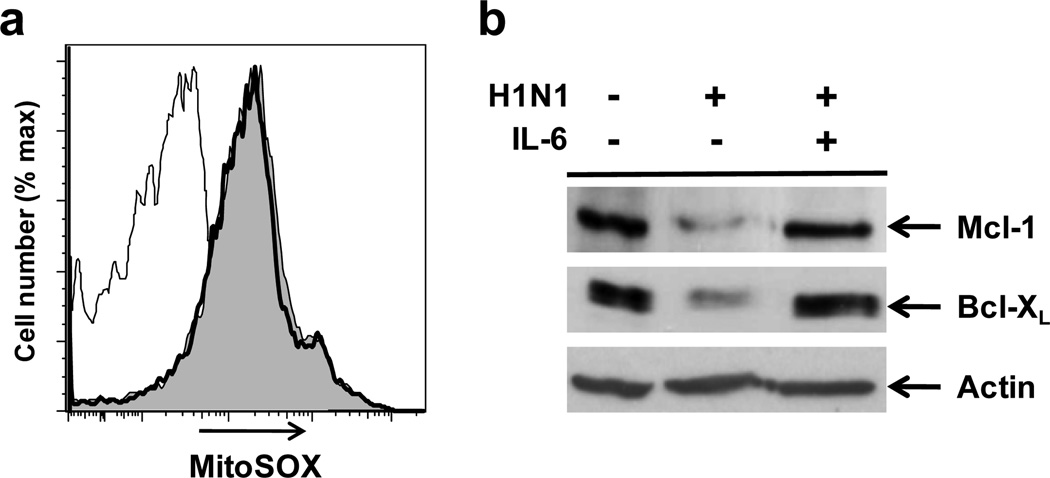
Accumulation of reactive oxygen species (ROS) in the mitochondria in response to stimuli is known to promote neutrophil death.35 We examined whether exposure of neutrophils to influenza virus could cause increased production of mitochondrial ROS (mROS) using MitoSOX staining and flow cytometry analysis. Incubation of neutrophils with PR8 H1N1 virus clearly increased mROS production (Fig. 6a), but the presence of IL-6 had no effect on virus-induced mROS (Fig. 6a). IL6-induced gp130/STAT3 signaling has been associated with chemokine production and trafficking of neutrophils, but not neutrophil apoptosis during acute inflammation.36 Similarly, no effect of IL-6 on phosphoryation of STAT3 in neutrophils during exposure to influenza virus could be detected (data not shown). The balance of anti-apoptotic and pro-apoptotic members of the Bcl2 family plays an essential role in determining cell survival or cell death. Mcl-1 is the most abundant anti-apoptotic member of the family in neutrophils,37 and most stimuli that regulate neutrophil survival or death also modulate Mcl-1 expression. Mcl-1 has been shown to be critical for survival of neutrophils in vitro and in vivo.38 We therefore examined the effect of PR8 H1N1 virus and IL-6 on Mcl-1 levels in neutrophils. Exposure of neutrophils to H1N1 virus caused a marked decrease in the levels of Mcl-1 (Fig. 6b). Interestingly, the addition of IL-6 restored Mcl-1 levels (Fig. 6b). In accordance with previous studies,39 Bcl2 expression could not be detected in neutrophils under any conditions (data not shown). In contrast, the other anti-apoptotic Bcl2 family member Bcl-XL was found to be expressed in resting neutrophils, but was almost undetectable in cells exposed to H1N1 virus (Fig. 6b). The presence of IL-6, however, also prevented the loss of Bcl-XL caused by PR8 H1N1 virus in neutrophils (Fig. 6b). Thus, IL-6 prevents H1N1 virus-induced death of neutrophils by maintaining high levels of essential anti-apoptotic molecules in these cells.
Figure 6. IL-6 prevents downregulation of anti-apoptotic molecules by influenza virus in neutrophils.
(a) Neutrophils from WT mice were cultured in the presence or absence of PR8 H1N1 virus at 1:10 neutrophil:virus (EIU) ratio with or without IL-6 (20 ng/ml). After 18 h, mitochondrial ROS was examined by staining with MitoSox-Red dye and flow cytometry analysis. Histogram profiles for neutrophils cultured in medium (thin line), with H1N1 virus (thick line) or H1N1 virus and IL-6 (filled) are shown. (b) Western blot analysis for Mcl-1 and Bcl-XL in neutrophils from WT mice treated as in (a). Actin was used as loading control.
DISCUSSION
Frequently, increased levels of proinflammatory cytokines in the lung are associated with exacerbated lung pathology (e.g. acute lung injury). We show that, similar to what is seen in humans, infection with H1N1 influenza virus in mice causes a dramatic increase in the levels of pulmonary IL-6 and that these levels remain high during the period of virus clearance. Instead of being pathogenic, our studies here show that this increase in IL-6 levels is protective since IL-6 is essential for H1N1 influenza virus clearance and survival from infection. The emphysema-like lung damage that IL-6 deficient mice develop during H1N1 infection further supports a non-pathogenic, protective role of IL-6 in influenza infection. The role of IL-6 in influenza infection may be dependent on the influenza virus strain and the pathology caused by the virus. Previous studies have shown that IL-6 does not contribute to the pathogenesis of avian H5N1 influenza infection which is primarily mediated by cytokine storm.40, 41 In addition, it does not seem to affect primary infection with a poorly pathogenic H3N2 strain (e.g. H17) that has minimal effect on lung function and host weight.42 The effect of IL-6 on influenza infection differs from other classical proinflammatory cytokines as it is required for viral clearance. In contrast, loss of TNFα does not impact viral clearance while mice deficient in TNFα and IL-1 receptors succumb to H1N1 infection similar to wildtype mice.43, 44 Interferons are another class of cytokines considered vital in the antiviral response. Type I Interferons promote expression of antiviral genes while IFNγ is mainly known for its immunmodulatory activity and is highly upregulated during influenza infection. However, inbred mouse strains are unable to express the antiviral Mx1 gene after interferon stimulation and therefore mice deficient in receptors for both IFN classes clear influenza virus similar to wildtype mice.45 As the C57BL/6 mice used here also lack a functional Mx1 gene the protective role of IL-6 is likely independent of IFN induction. The new finding on the protective role of IL-6 against H1N1 influenza is highly relevant for seasonal influenza virus infection in humans as they indicate that levels of IL-6 may determine the outcome of infection. In light of broadly distributed polymorphisms in IL-6 and IL-6R genes in the human population, these results suggest that genetic variations may affect influenza virus susceptibility and outcome. Interestingly, such polymorphisms in both IL-6 and IL-6R genes have recently been found to associate with susceptibility to respiratory tract infection.46
Together with macrophages, neutrophils are one of the major innate immune cell subsets appearing early in the lung after influenza virus infection. Neutrophils provide a first line of defense through phagocytosis of microbial pathogens and release of reactive oxygen species and antimicrobial enzymes. A relatively recently discovered activity of neutrophils is the formation of neutrophil extracellular traps (NET) for which neutrophils release DNA fibers along with various antimicrobial proteins that attach to bacteria and other pathogens.47 Despite their antimicrobial function, the sustained presence of NETs in the airways has been associated with lung tissue damage and worse disease during influenza infection.48 However, another study demonstrated that mice which are unable to form NETs during influenza infection have similar pathology to wildtype mice.49 In addition, depletion of neutrophils enhances weight loss in influenza infection suggesting they exert a more protective function.7, 48 Our data further support the critical role that neutrophils play in controlling influenza A virus infection. Furthermore, our study shows for the first time that influenza virus can trigger the apoptosis of neutrophils. Although it has been shown that influenza virus can infect and initiate replication in a neutrophil population in vivo,50 neutrophils do not seem capable of producing infectious virus.51 Thus, inducing cell death in neutrophils is possibly an evolutionary adaptation of the virus to eliminate cells that do not support virus replication and can be detrimental for the virus (i.e. through virus clearance). However, we also show that release of IL-6 provides protection against virus by preventing the downregulation of key anti-apoptotic molecules in neutrophils. Thus, the host also appears to have adapted to overcome influenza virus infection by providing IL-6-survival signals to neutrophils and enhancing virus clearance by these cells. It is well known that a large fraction of the healthy African American population has what has been defined as a “benign ethnic neutropenia” that, although considered idiopathic at this point, is associated with the presence of several specific genetic polymorphisms.52 Interestingly, African Americans appeared to be more severely affected by the 2009 influenza pandemic.3 Thus, the IL-6/neutrophil axis may be of critical importance in determining susceptibility to influenza virus infection and subsequent outcome.
MATERIAL AND METHODS
Mice and in vivo treatments
Wild type C57BL/6 mice were from Jackson Laboratories. Null IL-6 KO mice 20 and IL-6R KO mice 21 have been published previously. Mouse procedures were approved by the University of Vermont Institutional Animal Care and Use Committee. 10 to 12 weeks old mice were used in all the experiments.
Mice were infected intranasally with sublethal doses (3×103 EIU) of Puerto Rico A/PR/8/34 H1N1 (PR8) influenza A virus or A/California/7/2009 H1N1. Mice were monitored daily for weight loss and other clinical signs of illness. Animals that lost >25% of their body weight at the day of infection or had become grossly moribund were euthanized. Mice were injected i.p. with 500 µg of rat isotype control Ab or anti-Ly6G Ab (BioXcell, clone 1A8) for the depletion of neutrophils. Mice were injected i.p. with G-CSF (5 μg) or PBS for bone marrow release of neutrophils. Mice were treated with LPS as previously published.34
BAL collection and analysis of cell count, cell differential and cytokines
1 ml cold PBS was instilled into the lungs through the trachea and aspirated back as previously described.53 Cells were centrifuged and counted on an Advia Cell Counter (Siemens). Cells (5 × 104) were cytospun and stained with Hema-3 (Biochemical Sciences). One hundred cells per high power field were counted and classified as macrophages, neutrophils, or lymphocytes by cell morphology and staining. Neutrophils in blood and bone marrow were examined by the Advia Cell Counter. Cytokines and/or chemokines in BALF on indicated days after influenza infection were examined using Luminex Mouse cytokine/chemokine panel I (Millipore) according to the manufacturer's protocol. Total BALF protein was determined by standard Bradford assay (Bio-Rad) as per manufacturer’s instructions. The concentration of albumin in BALF was determined by ELISA (Bethyl Laboratories, Inc.).
Purification of Neutrophils
Purification of Neutrophils from bone marrow was performed as previously published.33 Cells were infected with PR8 virus at a 10:1 virus (EIU) to neutrophil number ratio, with or without 20 ng/ml of mouse IL-6 (R&D Biosystems).
Lactate dehydrogenase (LDH) assay
Supernatants from neutrophils in culture (24 h) were collected at the indicated periods of time and assayed for lactate dehydrogenase activity using the Cytotox 96 LDH assay kit (Promega) as recommended by the manufacturer. % LDH activity in the culture supernatant was calculated relative to the total LDH activity present in the cell pellet, as recommended by the manufacturer.
TUNEL Assay
Apoptosis of neutrophils cultured in vitro in the presence or absence of the virus for 20 h was examined by TUNEL assay as we previously described 54 using the Pharmingen TUNEL kit. FITC-dUTP incorporation was examined by flow cytometry analysis on a LSRII flow cytometer (BD).
Histopathology
Lungs were slowly inflated by instilling 1 ml of formalin intratracheally. The trachea was tightened, the lungs fixed in formalin for 24 h at 4 C and paraffin embedded. Lung tissue sections were stained for H&E according to routine procedures. Images were obtained by the Olympus BX50 light microscope with an Optronics Magnafire digital camera.
Lung viral titers
The number of viral RNA copies per lung was determined by quantitative RT-PCR. RNA was extracted from whole lung tissue using the RNeasy Kit (Qiagen) following manufacturer's instructions and 2 μg of RNA was reverse transcribed into cDNA using random hexamer primers and Superscript II Reverse Transcriptase (Invitrogen Life Technologies). Viral titers were determined by real-time RT-PCR for PR8 virus acid polymerase (PA) gene. This method has been previously described to be equivalent to pfu determination.55
Western blot analysis
Western blot analysis was performed as previously published.54 Anti-Mcl-1, anti-Bcl-XL (Cell Signaling) and anti-actin (Santa Cruz) antibodies were used.
Statistical Analysis
Survival curves were analyzed using the log-rank test. Time courses were analyzed using 2-way ANOVA; all other analysis was performed using the standard student t-test.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH/NIAID grants AI45666 (M.R.), HL84200 (B.S.) and T32-HL076122 (J.R.). We would like to thank Dr. David Woodland (Trudeau Institute) for providing the Puerto Rico/8/34 H1N1 virus and Dr. Daniel Perez (University of Maryland) for providing the A/California/7/2009 H1N1 virus. We would also like to thank Douglas Taatjes and Marilyn Wadsworth (Microscopy Imaging Facility, University of Vermont, Burlington, VT), Timothy Hunter and Mary Lou Shane (DNA Sequencing Facility, University of Vermont, Burlington, VT), Colette Charland (Flow Cytometry Facility, University of Vermont, Burlington, VT) and Koela Ray (Department of Medicine, University of Vermont, Burlington, VT) for technical asistance.
Footnotes
CONFLICT OF INTEREST:
The authors declare no conflict of interest.
REFERENCES
- 1.Monto AS. The risk of seasonal and pandemic influenza: prospects for control. Clin Infect Dis. 2009;48(Suppl 1):S20–S25. doi: 10.1086/591853. [DOI] [PubMed] [Google Scholar]
- 2.Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31(7):676–682. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn SC, Kumar S, Freimuth VS, Musa D, Casteneda-Angarita N, Kidwell K. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health. 2011;101(2):285–293. doi: 10.2105/AJPH.2009.188029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160(1):322–327. [PubMed] [Google Scholar]
- 5.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186(12):2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmolke M, Garcia-Sastre A. Evasion of innate and adaptive immune responses by influenza A virus. Cell Microbiol. 2010;12(7):873–880. doi: 10.1111/j.1462-5822.2010.01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84(15):7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82(6):2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6(3):e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85(2):85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 13.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Stadnyk AW. Cytokine production by epithelial cells. FASEB J. 1994;8(13):1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 15.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98(6 Pt 1):1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 16.Neveu WA, Bernardo E, Allard JL, Nagaleekar V, Wargo MJ, Davis RJ, et al. Fungal Allergen {beta}-glucans Trigger p38 MAPK-mediated IL-6 Translation in Lung Epithelial Cells. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130(1):27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64(3):262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 19.Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES, et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14(6):R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13(5):1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184(12):7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 22.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325(5939):484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, et al. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325(5939):481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460(7258):1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey C, Kumar A. H1N1: viral pneumonia as a cause of acute respiratory distress syndrome. Curr Opin Crit Care. 2011;17(1):64–71. doi: 10.1097/MCC.0b013e3283427259. [DOI] [PubMed] [Google Scholar]
- 27.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174(4):875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175(9):5827–5838. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- 29.Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN. Changes in the inflammatory response of the lung during acute respiratory distress syndrome: prognostic indicators. Am J Respir Crit Care Med. 1996;154(1):76–81. doi: 10.1164/ajrccm.154.1.8680703. [DOI] [PubMed] [Google Scholar]
- 30.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178(4):2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 32.Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178(12):8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 33.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104(2):565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 34.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178(12):8148–8157. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Loison F, Luo HR. Neutrophil spontaneous death is mediated by down-regulation of autocrine signaling through GPCR, PI3Kgamma, ROS, actin. Proc Natl Acad Sci U S A. 2010;107(7):2950–2955. doi: 10.1073/pnas.0912717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 37.Edwards SW, Derouet M, Howse M, Moots RJ. Regulation of neutrophil apoptosis by Mcl-1. Biochem Soc Trans. 2004;32(Pt3):489–492. doi: 10.1042/BST0320489. [DOI] [PubMed] [Google Scholar]
- 38.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109(4):1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487(3):318–322. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 40.Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007;104(30):12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81(6):2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, et al. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog. 2008;4(2):e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damjanovic D, Divangahi M, Kugathasan K, Small CL, Zganiacz A, Brown EG, et al. Negative Regulation of Lung Inflammation and Immunopathology by TNF-alpha during Acute Influenza Infection. The American journal of pathology. 2011;179(6):2963–2976. doi: 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrone LA, Szretter KJ, Katz JM, Mizgerd JP, Tumpey TM. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. The Journal of infectious diseases. 2010;202(8):1161–1170. doi: 10.1086/656365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74(9):3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rantala A, Lajunen T, Juvonen R, Silvennoinen-Kassinen S, Peitso A, Vainio O, et al. Association of IL-6 and IL-6R gene polymorphisms with susceptibility to respiratory tract infections in young Finnish men. Hum Immunol. 2011;72(1):63–68. doi: 10.1016/j.humimm.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Current opinion in microbiology. 2007;10(1):52–56. doi: 10.1016/j.mib.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. The American journal of pathology. 2011;179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6(7):e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A. 2010;107(25):11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cassidy LF, Lyles DS, Abramson JS. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. Journal of clinical microbiology. 1988;26(7):1267–1270. doi: 10.1128/jcm.26.7.1267-1270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, et al. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol. 2009;183(3):1732–1738. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farley N, Pedraza-Alva G, Serrano-Gomez D, Nagaleekar V, Aronshtam A, Krahl T, et al. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol. 2006;26(6):2118–2129. doi: 10.1128/MCB.26.6.2118-2129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178(12):7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.