Abstract
Lipidomics is a branch of the field of metabolomics. Although only about a decade since its inception, lipidomics has already had a major influence on the way in which questions about lipid metabolism and signaling are posed. The field is intertwined in the culture and rich history of mass spectrometry. Early work emphasized analytical issues such as limits of detection and numbers of molecular species quantitated in single injections. Increased sophistication in applications of lipidomic analysis and emerging technologies, such as imaging mass spectrometry, are facilitating the study of lipid metabolism and signaling species in cellular functions and human diseases. In the coming years we anticipate a richer understanding of how specific lipid species mediate complex biological processes and interconnections between cellular pathways that were thought to be disparate.
Background and History
Lipidomics is the systematic identification of the lipid molecular species of a cell, organelle, globule, or whole organism with emphasis on the quantitative determination of composition changes in response to a perturbation, such as stimulation of a receptor-mediated signaling pathway or alteration in metabolism. Since the first use of the term over a decade ago there has been controversy as to what truly constitutes “lipidomic” analysis, but most of the early contributors agree that the origins emerged from instrumentation advances in mass spectrometry, and computational biology. The ability to identify lipid molecular species and track changes in the composition of a cell membrane or biopsied tissue are rooted in the technological revolution of mass spectrometry instrumentation, particularly electrospray ionization mass spectrometry. Development of next generation instruments that could be used beyond the confines of advanced analytical chemistry laboratories, allowed the technology to be increasingly used by researchers in the biological sciences. Several investigators made major contributions to the methodology that has led to mass spectrometry as the preferred method of lipid identification (see reviews [1,2]) but in the late 1990s a transition began that engaged the power of systems biology in the quantitative analysis of lipids. As advances in the sensitivity and resolving power of mass spectrometry progressed, investigators pursued systematic identification of the lipomes of various cell types. An influential paper by McLafferty and colleagues [3] demonstrated the use of positive and negative mode Fourier-transform ion cyclotron resonance mass spectrometry (FTICR-MS) to identify the glycerophospholipid composition of a mucosal mast cell line. Subsequently, Ivanova et al [4] expanded this capability by tracking a greater number of species and measuring changes in multiple species during the process of regulated exocytosis. In 2001 the National Institute of General Medicine (NIGMS) formally sponsored the creation of a Lipidomics Core as part of the large scale collaborative initiative program, The Alliance for Cellular Signaling. This program was designed to define the components of signaling systems and describe the complex overlapping physical and regulatory interactions between these components in a quantitative manner [5] and quantitation of lipid species was acknowledged as critical element necessary for a comprehensive systems analysis of cellular signaling networks.
The early goals in lipidomics were oriented on technology development and building the infrastructure for the analytical and computational challenges [6,7]. The field has rapidly progressed and increasingly is being used to address questions in diverse biological systems. From its somewhat esoteric origins lipidomics has gained acceptance as previously unappreciated roles of lipid species in cellular processes are being discovered and molecular mechanism described. Among the contributions of the NIGMS-supported Lipid Metabolites and Pathways Strategy (LIPID MAPS) project has been the reorganization of lipid classification to facilitate bioinformatic organization and making databases more compatible with search functions [8]. A review on the structural organization of the Lipidomics database and online tools provided in the LIPID MAPS database (http://www.lipidmaps.org/) was recently contributed by Subramaniam and colleagues [9]. This includes an in-depth discussion of the various issues related to classification, ontology, nomenclature, and structural representation of lipid molecules that were considered in the creation of the database.
Current state-of-the-art Lipidomics
Lipid molecular species are a heterogeneous category of cellular metabolites. Historically, lipids were functionally defined as molecules that were soluble in certain kinds of organic solvents (e.g., chloroform) or extracted from aqueous solutions. We have come to appreciate that all lipids found in nature derive from one of two different biosynthetic pathways involving either the condensation of acyl carrier protein intermediates derived from malonyl-CoA and acyl-CoA esters and the intermediacy of a carbanion structure. This mechanism leads to lipids that include fatty acids, phospholipids, and glycerolipids. The second route is the carbocation pathway that involves condensation of branched chain 5-carbon pyrophosphate intermediates, including steroids and polyisoprenoids [10]. Owing to this chemical diversity of species, it has been pragmatic to divide lipid species further into subcategories based on metabolic pathways. The six functional divisions of lipid categories are shown in TABLE 1. References provided cite the best current practices for methodologies on the extraction and analysis of species in these respective lipid categories.
Table 1. Major Lipid Classes.
Major lipid classes (with representative species shown) as organized by the LIPID Metabolites And Pathways Strategy (LIPID MAPS) consortium. This consortium has defined the mouse macrophage lipidome (an inventory of thousands of lipid molecular species) and worked to develop state-of-the-art lipid analysis protocols, developed website based tools (www.lipidmaps.org.), and increase access of the broader scientific community to lipidomics [see further details in Ivanova et al., 2007 Methods in Enzymology; Myers et al., 2011 Biochim Biophys Acta (17,18)].
| LIPID CLASSIFICATION | REPRESENTATIVE SPECIES |
|---|---|
| Glycerolipids |
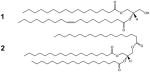 [1] Diacylglycerol (DAG) and [2] Triacylglycerol (TAG) |
| Glycerophospholipids |
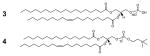 [3] Phosphatidic Acid (PA) and [4] Phosphatidylcholine (PC) |
| Sphingolipids |
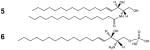 [5] Ceramide (Cer) and [6] Sphingosine-1-Phosphate (S1P) |
| Sterols |
[7] 25-Hydroxycholesterol and [8] Zymosterol |
| Fatty Acids / Eicosanoids |
[9] Arachidonic Acid (20:4) and [10] Oleic Acid (18:1) |
| Prenols |
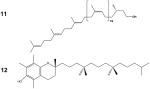 [11] Dolichol and [12] Vitamin E |
Recently, a thematic issue of Chemical Reviews (2011) [11] provided a timely set of contributions on various topics in lipid biochemistry, metabolism, and signaling. The reader is directed to this issue for a more comprehensive overview of this topic. A central theme running through these scholarly reviews is the way that lipidomic analysis is shaping the direction of the field. The sequencing of genomes, proteomics, and metabolomics are increasingly integrating once seemingly disparate areas of research. In the first ten years of lipidomics, we have met several challenges that were technical in nature, including development of the proper workflow for the handling, extraction, separation, and analysis of lipid species. As noted, the methodologies differ in part due to the chemical heterogeneity of lipid species. However, successful workflows are described for each of the lipid classes in cited special issues [12, 13]. The workflow for glycerophospholipid analysis is illustrated in FIGURE 1. Several important features are shown including the choice of a profiling approach (i.e., shotgun lipidomics) or a prior separation of classes using liquid chromatography (LC) followed by mass spectrometry detection and analysis. There has been considerable debate over the relative merits of these two approaches. Our experience has been that shotgun protocols are particularly useful for initial identification of differences between two conditions (i.e., profiling). Stimulation of a cell by an extracellular ligand or knockdown of a gene product are common challenges well suited to initial characterization using a direct infusion or shotgun approach. By contrast, LC-MS approaches are preferred when absolute quantitation is needed. The lack of linearity in ionization of hydrocarbon chains of different lengths has been well documented [14–17]. This lack of uniformity in ionization between extracted cellular metabolites can satisfactorily be addressed by strategic inclusion of chemically relevant internal standards and the rigorous generation of standard curves using defined amounts of the chemical constituents. Thus, as illustrated in FIGURE 1, we frequently use a shotgun approach to profile changes between two or more conditions subsequently followed by a rigorous, quantitative analysis using LC-MS/MS approaches with standard curves for absolute quantitation. The initial profiling helps to identify the types and amounts of chemically defined internal standards that are needed for absolute quantitation. The details of the computational analysis and data handling approaches are discussed in reviews elsewhere [18,19].
Figure 1. Lipidomics Overview.
The workflow begins with the extraction of lipids from a biological source. Tissues, cells, subcellular organelles or globules, bacteria, purified virions (among others) have been used in the determinations of lipid compositions. Depending on the specific class of lipid being analyzed different extraction and analytical separation procedures are used. Two citations provide comprehensive descriptions of these procedures (12,13). In the case of glycerophospholipids, the species may be profiled using a direct injection or “shotgun” protocol (upper). This is particularly effective at the initial identification of changes in lipid species between two or more conditions (e.g., challenge with extracellular ligand or gene knockout). Subsequently, absolute quantitation of lipid species is obtained by a more detailed analysis using LC-MS/MS in combination with appropriate selection of chemically defined lipid standards. This approach gives more reliable absolute quantitation by taking into account the nonlinear ionization of lipid species with differences in hydrocarbon chain lengths and other chemical dissimilarities. Lastly, the tracking of low abundance lipid metabolites and flux analysis is facilitated by use of an alkyne-labeled lipid precursor. This allows the separation of alkyne-cobalt complexes from native lipid pools using a modification of click chemistry.
The identification of novel enzymes involved in lipid metabolism and signaling offers a new set of challenges for the second decade of lipidomics/metabolomics. Some exciting examples of identification of novel enzymes that modulate known lipid species [20] have recently been described. This serves to highlight where the field is heading. Four major challenges are outlined below that although vexing, clearly represent the maturation of the field as we move beyond technical issues. The first challenge is better establishment of substrate-product relationships in lipid metabolic and signaling pathways. Classical approaches to measure flux have typically labelled cells with a stable isotope containing lipid precursor and followed the generation of lipid products through identification of species containing the isotopic label. This classic approach has elucidated many biochemical pathways and continues to be widely utilized. However, we are to some extent victims of our own success. As the number of molecular species has multiplied the spectra of some regions have grown dense and a slight shift in a labelled species may cause it to overlap the mass to charge (m/z) atop another lipid species. This was the motivation for creating a type of “lipid affinity chromatography,” where species could be followed and resolved from the bulk of native species. Milne et al., (2010) [21] described the capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. This technique allows alkyne labelled precursor lipids (e.g., fatty acyls or glycerophospholipids) to be incorporated into cells and metabolism is followed by capture of the alkyne-labelled products. Unlike other types of click-chemistry the covalent attachment is reversible and generated probe lipids can be released. This technique offers a unique series of probes for defining the biotransformations of relatively low abundance signaling lipids.
A second major challenge is to go beyond the identification of lipid biomarkers in human diseases and to discern whether any of the changes described represent actual mechanistic causes of pathophysiologies. Several reports have demonstrated that lipid molecular species are altered in human diseases. For example, lipid changes have been examined in clinical biopsies and animal models of various diseases, including Alzheimer’s disease [22, 23], Parkinson’s disease [24], hepatic liver diseases [25], and diabetes [26]. Moreover, the lipomes of pathogenic organisms have been analyzed to attempt to identify novel targets, including Mycobacterium tuberculosis [27,28] and human cytomegalovirus virions [29]. While these and other exciting discoveries are emerging, the field has yet to reach its full potential by identification of novel mechanisms that truly underlie human diseases. Indeed, we have made great strides from the early days where studies were more descriptive and observational. Recent studies are increasingly pursuing the identification of specific enzymes with altered activities and how these aberrations in metabolite concentrations affect cellular processes. This leads to the third major challenge, namely translating these discoveries into new small molecule therapeutics. Progress in the use of mass spectrometry-based OMICS to discover targets and accelerate development of novel chemical modulators has been long anticipated. Recently, isoenzymes-selective inhibitors of phospholipase D [30,31] have been developed using mass spectrometry-based assays to screen large numbers of compounds generated by technology-enabled synthesis approaches. Increasingly discoveries made through the integration of genomics, proteomics, and lipidomics/metabolics will accelerate the discovery of new therapeutic targets.
The final challenge looks ahead to subsequent technology development. The advances in mass spectrometry instrumentation over the last twenty years have roots in the genesis of lipidomics and metabolomics, but it was the emergence of the interdisciplinary field of chemical biology that has empowered chemists to use analytical tools to answer fundamental questions in biology. The biomedical sciences have been a primary beneficiary of this renaissance in lipidology [32]. Lipidomics/Metabolomics has largely been used to described temporal changes in lipid species content, but new approaches will provide spatial information on lipid localization within tissues and cells [33,34]. Advances will largely depend on the next generation of instrumentation being built [35,36] to resolve subcellular details of lipid composition.
Further improvements in the resolution and identification of lipid molecular species are emerging from other approaches as well. Blanksby and colleagues [37,38] have used ozone-induced dissociation to identify the positions of double bonds in unsaturated fatty acids. This advancement has the potential to vastly expand key molecular level detail between species that differ by only in the position of a double bond. Another technology that appears to hold great promise in advancing lipidomic capabilities is ion-mobility mass spectrometry [39]. The inclusion of cross-sectional areas of lipid molecules will potentially add yet another dimension of information that will facilitate identification of molecular species. More systematic analysis of a broader range reference standards need to be reported, but the approach appears well positioned to contribute to more facile identification of complex molecular species. These and other advances will undoubtedly lead to new discoveries about the roles of lipid species in cell biology and pathophysiology as the OMICS revolution continues to transition from the obscure to a mainstream research tool.
Acknowledgments
The author thanks Pavlina Ivanova and Michelle Armstrong for helpful conversations and assistance with this review. We acknowledge partial support for work described from the National Institutes of Health (U54 GM069338, U54 MH084659, P01 ESO13125) and the McDonnell Foundation for Brain Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Murphy RC, Fiedler J, Hevko J. Analysis of nonvolatile lipids by mass spectrometry. Chem Rev. 2001;101(2):479–526. doi: 10.1021/cr9900883. [DOI] [PubMed] [Google Scholar]
- 2.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts from biological samples by ESI-mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Fridriksson EK, Shipkova PA, Sheets ED, Holowka DA, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- **4.Ivanova PT, Cerda BA, Horn DM, Cohen JS, McLafferty FW, Brown HA. Electrospray ionization mass spectrometry analysis of changes in phospholipids in RBL-2H3 mastocytoma cells during degranulation. Proc Natl Acad Sci USA. 2001;98(13):7152–7157. doi: 10.1073/pnas.131195098. This paper describes the systematic determination of lipid composition changes during regulated exocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilman AG, et al. Overview of the alliance for cellular signaling. Nature. 2002;420:703–706. doi: 10.1038/nature01304. [DOI] [PubMed] [Google Scholar]
- 6.Forrester JS, Milne SB, Ivanova PT, Brown HA. Computational lipidomics: a multiplexed analysis of dynamic changes in membrane lipid composition during signal transduction. Mol Pharmacol. 2004;65:813–821. doi: 10.1124/mol.65.4.813. [DOI] [PubMed] [Google Scholar]
- 7.Ivanova PT, Milne SB, Forrester JS, Brown HA. New tools in the understanding of membrane dynamics and lipid signaling. Mol Interventions. 2004;4:86–96. doi: 10.1124/mi.4.2.6. [DOI] [PubMed] [Google Scholar]
- 8.Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witzum J, Dennis EA. A comprehensive classification system for lipids. J Lipid Res. 2005;46:1796–1802. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam S, Fahy E, Gupta S, Sud M, Byrnes RW, Cotter D, Dinasarapu AR, Maurya MR. Bioinformatics and systems biology of the lipidome. Chem Rev. 2011;111(10):6452–6490. doi: 10.1021/cr200295k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Brown HA, Murphy RC. Working towards exegesis for lipids in biology. Nat Chem Biol. 2009;5(9):602–606. doi: 10.1038/nchembio0909-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown HA, Marnett LJ, editors. Chemical Reviews. 2011. Lipid biochemistry metabolism and signaling; p. 111. [DOI] [PubMed] [Google Scholar]
- 12.Merrill AH, Murphy RC, editors. Biochim Biophys Acta- Mol Cell Biol Lipids. 2011. Lipidomics and imaging mass spectrometry; p. 1811. [DOI] [PubMed] [Google Scholar]
- 13**.Brown HA, editor. Meth Enzymol. 2007. Lipidomics and bioactive lipids; pp. 432–434. Detailed protocols on extractions, separations, and mass spectrometry analysis of different lipid classes. [DOI] [PubMed] [Google Scholar]
- 14.Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharju P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res. 2001;42:663–672. [PubMed] [Google Scholar]
- 16.Hermansson M, Uphoff A, Käkelä R, Somerharju P. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Anal Chem. 2005;77:2166–2175. doi: 10.1021/ac048489s. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Meth Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- 18.Myers DS, Ivanova PT, Milne SB, Brown HA. Quantitative analysis of glycerophospholipids by LC-MS: acquisition, data handling, and interpretation. Biochim Biophys Acta. 2011;1811:748–757. doi: 10.1016/j.bbalip.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanova PT, Milne SB, Myers DS, Brown HA. Lipidomics: a mass spectrometry based, systems level analysis of cellular lipids. Curr Opin Chem Biol. 2009;13:526–531. doi: 10.1016/j.cbpa.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long JZ, Cisar JS, Milliken D, Niessen S, Wang C, Trauger SA, Siuzdak G, Cravatt BF. Metabolomics annotates ABHD3 as a physiologic regulator of medium chain phospholipids. Nat Chem Biol. 2011;7(11):763–765. doi: 10.1038/nchembio.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Milne SB, Tallman KA, Serva R, Rouzer CA, Armstrong MA, Marnett LJ, Lukehart CM, Porter NA, Brown HA. Capture and release of alkyne-derivatized glycerophospholipids using cobalt chemistry. Nat Chem Biol. 2010;6(3):205–207. doi: 10.1038/nchembio.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer’s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE. 2011;6(7):e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, Wenk MR, Arancio O, Di Paolo G. Phospholipase d2 ablation ameliorates Alzheimer’s disease-linked synaptic dysfunction and cognitive deficits. J Neurosci. 2010;30(49):16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappley I, Myers DS, Milne SB, Ivanova PT, LaVoie MJ, Brown HA, Selkoe DJ. Lipidomics profiling in mouse brain reveals differences between ages and genders with smaller changes associated with α-synuclein genotype. J Neurochem. 2009;111(1):15–25. doi: 10.1111/j.1471-4159.2009.06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorden DL, Ivanova PT, Myers DS, McIntyre JO, VanSaun MN, Wright JK, Matrisian LM, Brown HA. Increased diacylglycerol characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS ONE. 2011;6(8):e22775. doi: 10.1371/journal.pone.0022775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord G, Betters JL, Ivanova PT, Milne SB, Myers DS, Madenspacher J, Chung S, Liu M, Davis MA, Lee RG, Crooke RM, Graham MJ, Parks JS, Brasaemle DL, Fessler M, Brown HA, Brown MJ. CGI-58/ABHD5- derived signaling lipids regulate systemic inflammation and insulin action. Diabetes. 2012 Jan 6; doi: 10.2337/db11-0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow ED, Cox JS. TB Lipidomics-the final frontier. Chem Biol. 2011;18(12):1517–1518. doi: 10.1016/j.chembiol.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madigan CA, Cheng TY, Layre E, Young DC, McConnell MJ, Debono CA, Murry JP, Wei JR, Barry CE, 3rd, Rodriguez GM, Matsunaga I, Rubin EJ, Moody DB. Lipidomic discovery of deoxysiderophores reveals a revised mycobactin biosynthesis pathway in Mycobacterium tuberculosis. Proc Natl Acad Sci. 2012 Jan 9; doi: 10.1073/pnas.1109958109. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu STH, Sharon-Friling R, Ivanova P, Milne SB, Myers DS, Rabinovitz JD, Brown HA, Shenk T. Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role for SNARE machinery in virion egress. Proc Natl Acad Sci. 2011;108(31):12869–12874. doi: 10.1073/pnas.1109796108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5(2):108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavieri R, Scott SA, Selvy PE, Kim K, Jadhav S, Morrison RD, Daniels S, Brown HA, Lindsley CW. Design, synthesis and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4. 5]decan-8-yl)ethylbenzamides:discovery of an isoform selective small molecule phospholipase D2 (PLD2) inhibitor. J Med Chem. 2010;53(18):6706–6719. doi: 10.1021/jm100814g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11(8):593–598. doi: 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- 33.Hankin JA, Barkley RM, Murphy RC. Sublimation as a method of matrix application for mass spectrometry imaging. J Am Soc Mass Spectrom. 2007;18(9):1646–1652. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Berry KA, Hankin JA, Barkley RM, Spraggins JM, Caprioli RM, Murphy RC. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem Rev. 2011;111(10):6491–6512. doi: 10.1021/cr200280p. Review of advances in mass spectrometry-based imaging technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winograd N, Garrison BJ. Biological cluster mass spectrometry. Annu Rev Phys Chem. 2010;61:305–322. doi: 10.1146/annurev.physchem.040808.090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Passarelli MK, Winograd N. Lipid imaging with time-of-flight secondary ion mass spectrometry (ToF-SIMS) Biochim Biophys Acta. 2011;1811:976–990. doi: 10.1016/j.bbalip.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanksby SJ, Mitchell TW. Advances in mass spectrometry for lipidomics. Annu Rev Anal Chem (Palo Alto Calif) 2010;3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 38*.Brown SH, Mitchell TW, Blanksby SJ. Analysis of unsaturated lipids by ozone-induced dissociation. Biochim Biophys Acta. 2011;1811:807–817. doi: 10.1016/j.bbalip.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Kliman M, May JC, McLean JA. Lipid analysis and lipidomics by structurally selective ion mobility mass spectrometry. Biochim Biophys Acta. 2011;1811:935–945. doi: 10.1016/j.bbalip.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]



