Abstract
We hypothesize that normal aging implies neuronal durability, reflected by age-independent concentrations of their marker - the amino acid derivative N-acetylaspartate (NAA). To test this we obtained the whole-brain and whole-head NAA concentration (WBNAA and WHNAA), with proton MR spectroscopy; and the fractional brain parenchyma volume (fBPV) – a metric of atrophy, by segmenting the MRI from 42 (18 male) healthy young (31.9±5.8 years-old) and 100 (64 male, 72.6±7.3 years-old) cognitively-normal elderly. The 12.8±1.9 mM WBNAA of the young was not significantly different from the 13.1±3.1 mM in the elderly (p>0.05). In contrast, both fBPV (87.3±4.7% versus 74.8±4.8%) and WHNAA (11.1±1.7 mM versus 9.8±2.4 mM) were significantly higher in the young (~14%, p<.0001 for both). The similarity in mean WBNAA between two cohorts 4 decades of normal aging apart suggests that neuronal integrity is maintained across the lifespan. Clinically, WBNAA could be used as a marker for normal (hence, also abnormal) brain aging. In contrast, WHNAA and fBPV seem age-related suggesting that brain atrophy may occur without compromising the remaining tissue.
Keywords: Aging, Brain volumetry, Elderly, Healthy human brain, MRI, N-acetylaspartate (NAA), Proton MR Spectroscopy, Whole-brain
1. Introduction
The estimated 40 million Americans currently 65 or older comprise the fastest growing segment of the US population; expected to increase to 70 million by 2030 (Hoyert et al., 2005). They will also live longer, given that the average life expectancy at birth increased from 65.6 years for men and 71.4 for women in 1950, to 74.8 and 80.1 in 2003 (Hoyert et al., 2005). This demographic is susceptible to several chronic neurological disorders, e.g., 5 – 40% to Alzheimer’s and 0.5% to Parkinson's Diseases (Hebert et al., 2003; NIH, 2000) and 3 – 6% to a spectrum of vascular dementias (Bowler, 2005). Brain imaging in up to 50% of them also reveals white matter (WM) hyperintensities reflecting ischemic damage (Meyer et al., 1992); microbleeds in about 25% (Vernooij et al., 2008); and lacunar and silent infarcts in approximately 9% (Vernooij et al., 2008) and 15% (Das et al., 2008). The size of this population underscores a need for markers and metrics of normal aging in order to facilitate early detection when preemptive treatment may be most effective and to monitoring its progress.
Among the candidate markers of neuronal health none so far has yielded more diagnostic information than the amino-acid derivative N-acetyl-L-aspartate (NAA) (Baslow, 2003; Benarroch, 2008). Even though its exact role(s) are still unknown (Moffett et al., 2007), since in the mature brain NAA is almost exclusive to neurons and their processes (Baslow, 2003), it is considered a putative marker of their integrity (Benarroch, 2008). Yielding the most intense peak in non-invasive proton MR spectroscopy (1H-MRS), regional (or global) NAA concentration declines have been shown in disorders of the central nervous system e.g., in multiple sclerosis, HIV, cancer, brain trauma and Alzheimer’s disease (Mountford et al., 2010; Schuff et al., 2006).
Unfortunately, most studies employ either single-voxel or 2D multi-voxel 1H-MRS that cover relatively small (0.1–10%) fractions of the brain. Their volumes-of-interest (VOI), therefore, must be image-guided, introducing two implicit assumptions that: i) metabolic changes occur only at MRI-visible abnormalities; and ii) for diffuse disorders these small VOIs represent the entire brain (De Stefano et al., 2002). In addition, these methods also require: iii) long acquisition time to yield sufficient sensitivity; iv) VOIs placed distal to the skull in order to prevent contamination with lipids signals from the bone marrow and subcutaneous adipose tissue, thereby missing most of the cortex; and v) knowledge of the T1 and T2 relaxation times (that are often unknown, especially in pathologies) for absolute quantification. These may explain the conflicting reports of NAA in healthy aging, showing both decreasing (Charles et al., 1994; Christiansen et al., 1993; Fukuzako et al., 1997; Lim and Spielman, 1997) and stable levels (Chang et al., 1996; Meyerhoff et al., 1994; Saunders et al., 1999; Soher et al., 1996).
All these issues can be addressed by obtaining the global NAA signal from the entire brain. In this study we used non-localizing 1H-MRS (Gonen et al., 1998) to compare the whole-brain NAA concentration (WBNAA) in healthy young (under 45 years old) and cognitively normal elderly (60 years and older) in order to test the hypothesis that the hallmark of normal brain aging is neuronal health and density preservation, reflected by unchanged NAA concentration.
2. Methods
2.1. Human subjects
Forty-two young: 18 men, 24 women, 20 – 44 (31.9±5.8) years old; and 118 cognitively normal elderly: 74 men, 44 women, 58 – 89 (72.3±7.7) years old, were prospectively enrolled. “Healthy” in the young was established based on a questionnaire to exclude 20 medical and neurological conditions before the scan and an “unremarkable” MRI afterwards. In the elderly major depression was ruled out by an interview assessing the criteria according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (WHO, 1992). Cognitive abilities were assessed with a comprehensive neuropsychological assessment, including the Mini Mental State Examination [MMSE (Folstein et al., 1983)], the California Verbal Learning Test (Delis et al., 1987), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)-Neuropsychological Assessment Battery (Morris et al., 1989); the Rey-Osterrieth Complex Figure (Schreiber et al., 1999), Digit Span and Corsi Blocks (Wechsler, 1987), a computerized test of attention (Zimmermann and Fimm, 1992), the Stroop Color-Word Interference Test, (Perret, 1974), the Trail Making Test, part A & B (Army, 1944), a phonemic fluency test (S-words) (Thurstone, 1938), the Clock Drawing Test (Thalmann et al., 2002), and the Five-Point Test for measuring design fluency (Regard et al., 1982). To confirm subjects’ preserved cognitive status in daily activities a knowledgeable informant was questioned using the 16- item version of the modified Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE) (Ehrensperger et al., 2010). For inclusion in the study, subjects’ demographically adjusted standard scores had to be within normal limits. In addition, all elderly subjects were visually rated for vascular WM lesions using the Wahlund (0 – 15) scores (Wahlund et al., 2001). Few of the elderly participants were taking medications that could potentially influence NAA levels: Two were on stable small doses of antidepressants: one was taking citalopram 10 mg, the other trimipramine 25 mg for sleep problems. Four others were taking benzodiazepines for sleep problems on “as need basis.” None was taking cognition enhancing drugs, although herbal preparations and vitamins were allowed. Given the frequency and dosage of the aforementioned drugs and small number of patients taking them, we do not believe that they could bias or alter our results. The study was approved by the Institutional Review Boards of both the New York University School of Medicine and University Hospital Basel and all participants gave written informed consent.
2.2. MRI
All experiments were done in a 3 T head-only MR scanner (Allegra, Siemens AG, Erlangen, Germany) using its circularly-polarized transmit-receive head-coil. After placing a subject into the scanner, its magnetic field homogeneity was adjusted over the head using our proton chemical shift imaging automatic shim procedure, for a consistent 27±4 Hz water linewidth in under 5 minutes (Hu et al., 1995). For brain segmentation we acquired sagittal T1-weighted Magnetization Prepared RApid Gradient Echo (MP-RAGE) MRI: TE/TR/TI: 3.5/2150/1000 ms, 7° flip angle, 144 slices 1.1 mm thick, 256×224 matrix and 256×256 mm2 field-of-view (FOV). For vascular WM lesion scoring in the elderly we also obtained FLuid Attenuated by Inversion Recovery (FLAIR) T2- and proton density-weighted MRI: TE/TI/TR=103/2300/9000 ms; same FOV, 384×384 matrix, 28 slices 5 mm thick, aligned with the inferior border of the corpus callosum. The proton-density FLAIR used the same parameters except for TE/TR=37/2700 ms.
WM hyperintensive lesions were scored (only in the elderly, since in the young presence of such lesions would constitute an exclusion) according to Wahlund et al. (Wahlund et al., 2001). Specifically, they were rated from 0 – 3 in each of the following five regions: frontal, temporal, parieto-occipital, infratentorial and basal ganglia for a total vascular lesion score range of 0 – 15.
2.2.1 Brain parenchyma volume - VB
Subjects’ brain parenchyma volume, VB, was segmented semi-automatically from the MP-RAGE images using our FireVoxel package (Mikheev et al., 2008). The process starts by placing a seed region in periventricular WM to obtain its average signal intensity, IWM. Following selection of all pixels at or above 0.55 (but below 135% to exclude fat) of IWM, a brain mask is formed for each slice in three steps: (i) morphological erosion; (ii) recursive region growth retaining pixels connected to the seed; (iii) morphological inflation to reverse the effect of erosion. Pixels of intensity below 0.55 of IWM were defined as cerebrospinal fluid (CSF). The masks were truncated at the foramen magnum to include the brain stem and cerebellum but not the cord (Fig. 1). The masks are each visually inspected to ensure proper inclusion of brain and exclusion of “non brain,” e.g., skull, air-spaces and dura. Finally, VB is the pixel volume × their number in the masks. The precision of this approach was recently established at 3.4% (Mikheev et al., 2008).
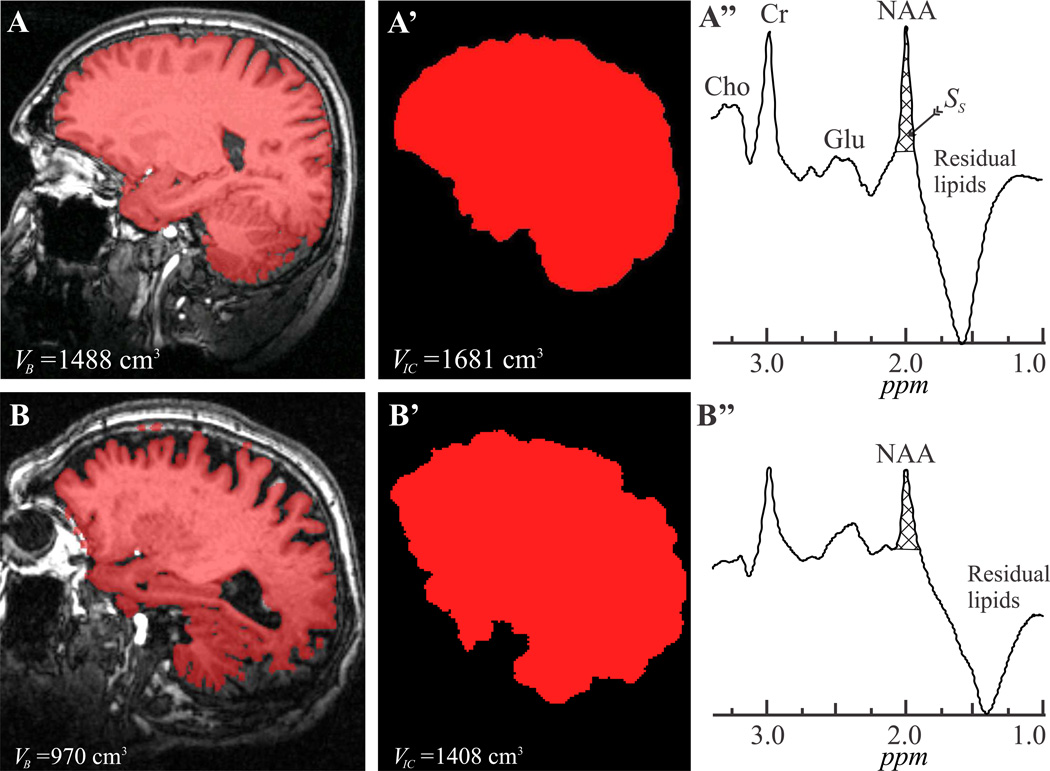
Fig. 1.
Left: Sample T1-weighted MP-RAGE images from 41 (A) and 86 (B) year old males overlaid with their brain parenchyma masks (red). Note the mask correspondence with the underlying anatomy and, for the elderly brain, the relative atrophy incurred in 4 decades of normal aging.
Center, A’, B’: Intracranial volume segmentation of A and B with MRIcro.
Right, A’’, B”: Whole-head 1H-MRS spectra from these subjects (not normalized for VB). Note the NAA peak at 2 ppm, effective lipids suppression performance and that of the other metabolites peaks [most notably the glutamate (Glu), creatine (Cr) and choline (Cho)] only NAA is implicitly localized by its biochemistry to just the brain. Subject NAA peak area, SS (cross-hatched), for Eq. [1] was obtained by integration.
2.2.2 Intracranial volume - VIC
Intracranial volumes, VIC, were obtained semi-automatically from the MP-RAGE images using MRIcro, a free downloadable software package: http://www.mricro.com (Rorden and Brett, 2000). It employs the brain extraction tool to skull-strip the intracranial surface using a deformable model (Smith, 2002). The process estimates the threshold between the brain and CSF, determines the head’s center of gravity C, constructs a small tessellated surface F (initially a sphere) centered at C and incrementally adjusts the vertices of F to balance its smoothness and the desired signal intensity criteria. A volume-of-interest masking the extracted brain (Fig. 1) was then used to calculate the intracranial volume, VIC. These brain masks are also each visually inspected to ensure complete skull stripping and proper inclusion of all intracranial volume. The fractional brain parenchyma volume, fBPV (in %), was the defined as VB /VIC × 100.
2.2.3 MR spectroscopy - global NAA quantification
The global amount of brain NAA, QNAA, was obtained with a non-localizing. TE/TI/TR=0/940/104 ms 1H-MRS sequence (Gonen et al., 1998), that relies on the implicit localization of the NAA by its biochemistry to neurons, i.e., to just the brain (Gonen et al., 1998). Its long, TR≫T1 and short TE≈0 ensure insensitivity to possible T1 and T2 variations , that are typically unknown, especially in the elderly (Hovener et al., 2008).
Absolute quantification was done against a reference 3 L sphere of 1.5×10−2 mole NAA in water. Subject and reference NAA peak areas, SS and SR, were obtained by manual phasing and selection of the NAA peak limits of integration (Fig. 1) by four blinded operators. A result more than two standard deviations (average for the four readers’ over all the subjects, ~8%) from the mean for that patient, was rejected. If more than one was rejected the set was excluded. The results were then averaged into and and QNAA estimated as (Gonen et al., 1998),
| [1] |
where are the transmitter voltages for non-selective 1 ms 180° inversion pulses on the reference and subject, reflecting relative coil sensitivity.
It is noteworthy that although other metabolites, e.g., macromolecules and other N-acetyl bearing species also resonate about 2.01 ppm, their contribution to the peak area is estimated at less than 10% (Baslow, 2003). Furthermore, although several other metabolites are also visible in the whole-head spectrum (see Fig. 1A”), since the sequence is non-localizing, only the NAA is implicitly localized to just the brain.
To normalize for differences in brain size among subjects, QNAA was divided by the brain parenchyma volume, VB, to yield the whole-brain NAA concentration:
| [2] |
a specific, brain size-independent metric. Its inter- and intra-subject variability in younger healthy individuals has been shown at better than ±8% (Benedetti et al., 2007).
The whole head NAA (WHNAA) concentration, a marker of NAA atrophy since unlike the brain, the intracranial cavity volume does not change throughout adult life, was estimated as,
| [3] |
which is sensitive to the fBPV as well as the NAA concentration in the remaining tissue.
2.3. Statistical analyses
Analysis of covariance (ANCOVA) was used to compare the two groups in terms of the mean of each measure (fBPV, WBNAA, and WHNAA) adjusting for gender. The groups (young, elderly) are characterized in terms of each measure as the mean ± standard deviation. Between-group percentage difference was computed relative to the mean of the young. In the elderly, the relationships between the WBNAA, WHNAA, fBPV and total vascular lesion score were examined using Spearman correlation coefficients. Relationships between cognitive tests and brain metric were checked with Spearman correlation coefficient.
3. Results
Eighteen of the 118 elderly subjects’ data sets had to be excluded from the analysis due to data quality criteria described above but none from the young. The mean ± standard deviation of their fBPV, WBNAA and WHNAA defined by age and gender are given in Table 1. The WBNAA concentration of the young was not significantly different from the elderly (p>0.05), as shown in Table 2 and Fig. 2A. Their difference was only 2.6% on average and the 95% confidence interval implies it is no greater than 10%, supporting our hypothesis that the two groups are bioequivalent in terms of their WBNAA. Gender accounted for less than 1% of the variation in WBNAA both with and without accounting for the association of age with WBNAA.
Table 1.
Mean ± standard deviation of each metric within each subject group defined by age (Elderly versus Young) and gender.
| Metric | Female | Male | ||
|---|---|---|---|---|
| Elderly (n=36) | Young (n=24) | Elderly (n=64) | Young (n=18) | |
| fBPV (%) | 76.9±3.6 | 87.4±4.0 | 73.6±4.9 | 87.3±5.6 |
| WBNAA (mM) | 13.3±3.2 | 12.7±1.3 | 13.0±3.0 | 12.9±2.5 |
| WHNAA (mM) | 10.2±2.4 | 11.1±1.2 | 9.6±2.4 | 11.2±2.3 |
Table 2.
Estimate ± standard error of the mean estimate of each metric adjusted for gender among subjects in each age group.
| Metric | Elderly | Young | p*, a | 95% CI: as % of Control Meanb |
|---|---|---|---|---|
| fBPV (%) | 75.1±0.5 | 87.2±0.8 | <0.0001 | −15.87% to −11.83% |
| WBNAA (mM) | 13.1±0.3 | 12.8±0.3 | 0.4405 | −4.15% to 9.47% |
| WHNAA (mM) | 9.8±0.2 | 11.1±0.3 | 0.0008 | −18.02% to −4.86% |
Significance level set at p<0.001 and is indicated in bold.
Each p value is from analysis of covariance (ANCOVA) to compare age groups in terms of the mean of each measure adjusted for gender.
95% confidence interval (CI) is provided for the mean difference between age groups in terms of each measure adjusted for gender. The limits of the intervals are expressed as a percentage of the gender-adjusted mean for the young subjects.
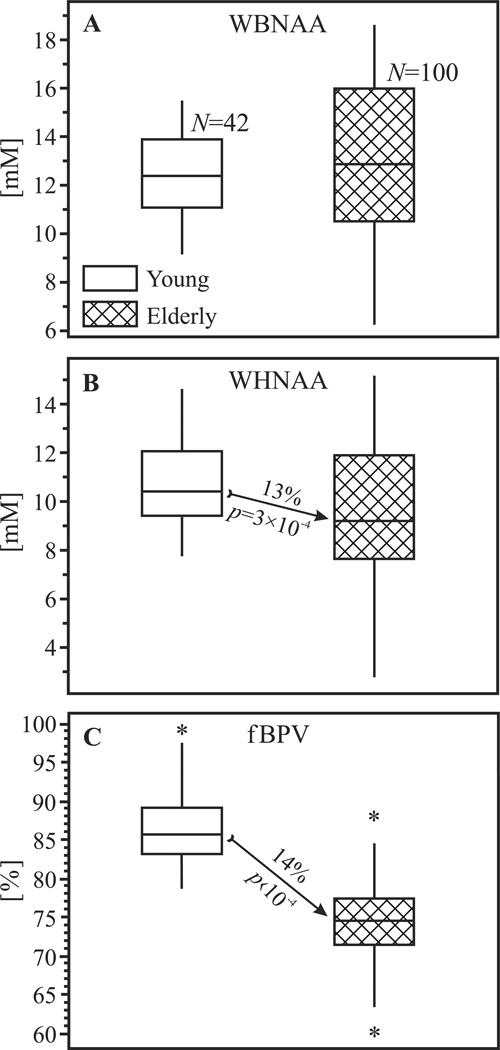
Fig. 2.
Box plots showing the first, second (median) and third quartiles (box) and ±95% (whiskers) and outliers (*) of the WBNAA (top, A), WHNAA (center, B) and fBPV (bottom, C) distributions in healthy young and elderly. Note the WBNAA similar medians as opposed to the significantly lower WHNAA and fBPV in elderly (denoted by the arrows).
The mean WHNAA in the young, however, was significantly higher (13%, p=3×10−4) than in the elderly, as shown in Table 2 and Fig. 2B. fBPV in the young were also significantly higher (14%, p<10−4) than in the elderly, as shown in Fig. 2C.
Although the mean WBNAA concentrations between the two age groups were not statistically different, its variance was significantly (±1.2 mM, 61%, p<10−4) greater in elderly than in young, as shown in Fig. 2A. Similarly, the elderly WHNAA distribution was significantly (±0.7 mM, 38%) broader (p=3×10−4). No significant difference was found between the fBPV distributions of the young and elderly. The median WM MRI hyperintensities lesion score was 3 (interquartile range 2) in our elderly cohort. Its minimum value was 0, and the maximum 8 out of a possible 15. There was no significant correlation between the total vascular lesion score and WBNAA (R=−0.03, p=0.8), WHNAA (R=−0.05, p=0.7), or fBPV (R=−0.04, p=0.7). Consequently, total vascular lesion score was not included as a covariate into the ANCOVA. No correlations were found between neuropsychological measures and NAA metrics.
4. Discussion
An unequivocal connection between NAA deficit and axonal injury/loss has been established by immunopathology and immunocytochemistry on post mortem spinal cord samples from chronic multiple sclerosis (MS) patients and matched deceased controls, showing that the former had lower axonal density and proportionally lower NAA than the latter as well as lower NAA/axonal-volume in demyelinated axons and myelinated axons of normal-appearing white matter (Bjartmar et al., 2000). Two previous studies have also shown that axonal loss in acute and chronic cerebral MS lesions correlated with NAA reduction (Bitsch et al., 1999; Trapp et al., 1998). In addition, WBNAA reduction has also been reported in elderly subjects with suspected Alzheimer’s disease (AD), a condition known to target and destroy neurons (Gomez-Isla et al., 1997; Price et al., 2001), compared with their cognitively normal contemporaries (Falini et al., 2005). Given this established NAA – neuron connection, our WBNAA observation in two relatively large cohorts, is consistent with the hypothesis that normal aging is characterized by the preservation of neuronal health and density in the intact brain parenchyma from adolescence throughout old age.
It is important to note that this absence of difference between the young and cognitively normal elderly does not represent a lack of sensitivity of the WBNAA method. Specifically, given that significant WBNAA differences between mild cognitively impaired individuals and their matched controls have already been reported in much smaller cohorts (Falini et al., 2005), our finding of no WBNAA difference between large cohorts of young and cognitively intact elderly, is consistent with the premise that such changes with normal aging are smaller than encountered even in early dementia. This premise is also supported by a post mortem studies of cognitively normal deceased elderly that show no significant changes in neuronal density between the sixth and ninth decade (Gomez-Isla et al., 1997; Price et al., 2001).
The similar declines in tissue volume (fBPV; ~13%) and in whole-head NAA (WHNAA; ~14%) are consistent with our hypothesis that NAA loss results almost exclusively from physiological brain atrophy during aging and that its concentration in the remaining intact parenchyma (reflected by WBNAA) remains unchanged. These (interrelated) fBPV and WHNAA declines coincide with the main hypothesis, given that the intra-cranial volume, VIC, does not change throughout life whereas the global NAA amount, QNAA, and brain-parenchymal volume (VB) both do, apparently by equal amount. Furthermore, the ~13% fBPV difference between the cohorts corresponds to a mean annual atrophy rate of 0.33%, consistent with 0.32% reported in a recent longitudinal whole-brain volumetric study and cross-sectional data showing a steady 0.33% decline from the fourth through the seventh decades (Scahill et al., 2003).
It is noteworthy that although the means of the WBNAA distributions of the two cohorts are within 3%, the variance for both, WBNAA and WHNAA is ~60% larger in the elderly (Fig. 2). This may be due to age-associated changes, e.g., axonal thinning (or loss) as well as brain water content decline (Chang et al., 1996). Indeed, stereological analyses have shown total myelinated fiber length decreases of up to 45% in the white matter of elderly compared with the young (Marner et al., 2003; Tang et al., 1997). Both age-associated changes in fiber length and decline in water content may lower brain volume in elderly while their neurons are preserved, leading to higher packing (density) reflected by increased WBNAA levels in some elderly and to the variance in their cohort’s upper WBNAA scores. On the other (lower) extreme of the WBNAA distribution, subclinical pathology such as periventricular WM MRI hyperintensities may decrease the NAA in some elderly; likely the ones with cerebrovascular risk factors. Yet that assumption was not confirmed in our study. The overall WM hyperintensity lesion burden in our normal elderly cohort, which was (expectedly) low: median score of 3 from a range of 0 – 15, did not correlate with WBNAA. Some other subclinical abnormalities that are T1 and FLAIR MRI-occult could, therefore, be responsible for the observed increased WBNAA variance. It is noteworthy that due to its long TR and very-short TE the WBNAA sequence is insensitive to (possible) changes in T1 or T2 relaxation times that may occur with aging and that could otherwise affect other, echo-based, 1H-MRS methods (Kirov et al., 2008; Rigotti et al., 2007).
Finally, we did not find significant relationships between the neuropsychological measures in these normal elderly and their NAA metrics. We believe that this can be attributed to the ordinal nature of the scores of most of these tests and the fact that our subjects all had normal cognition. Consequently, the range of their tests scores was both (i) narrow, and (ii) congested at the top of the full range of the possible scores for these tests. The relationships we did find disappeared upon a correction for multiple comparisons that is necessary given the number tests conducted.
We investigated two rather large groups of individuals in order to obtain statistically robust data and to compensate for inherent methodological measurement noise: First, the 6–8% sensitivity of WBNAA that precludes detection of smaller changes, e.g., early, focal or regional pathology (Benedetti et al., 2007). Non-localizing, WBNAA and WHNAA therefore yield no information on potential regional variability (such as between gray and white matter) or whether the larger variances of the global NAA in the elderly represent specific regional or global phenomena (e.g. individual differences between gray than white matter atrophy). Finally, this is a cross-sectional study with consequent inter-individual variations. Although a longitudinal study would yield more precise information on normal individual variations, in the manner of one that was done in healthy young subjects [showing WBNAA to be stable over 4 years (Rigotti et al., 2007)], none to our knowledge has been undertaken so far in cognitively intact elderly.
5. Conclusions
In conclusion, we found no significant difference in the global tissue concentration of the MR marker of neuronal integrity, between young adults and the elderly. When accounting for normal (age appropriate) brain atrophy, the decrease of NAA and brain parenchyma in aging are closely related, indicating that the concentration of NAA (and presumably of the numbers and integrity of the neuronal cells this metabolite reflects) in the remaining brain tissue of the elderly is similar to that metric in much younger subjects. These results characterize for the first time the WBNAA and WHNAA metrics associated with healthy brain aging to provide a baseline for future normative studies as well as a surrogate non-invasive instrumental marker for normal versus pathological aging.
Acknowledgements
This work was supported by NIH Grants NS050520 and EB01015. We gratefully acknowledge the financial support for this study from GlaxoSmithKline and the Novartis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors state that there are no actual or potential conflicts of interest related to this paper.
References
- Army . Army Individual Test Battery Manual of Directions and Scoring. Washington DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70:1353–1357. doi: 10.1212/01.wnl.0000311267.63292.6c. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Rigotti DJ, Liu S, Filippi M, Grossman RI, Gonen O. Reproducibility of the Whole-Brain N-Acetylaspartate Level across Institutions, MR Scanners, and Field Strengths. AJNR Am J Neuroradiol. 2007;28:72–75. [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Annals of Neurology. 2000;48:893–901. [PubMed] [Google Scholar]
- Bowler JV. Vascular cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v35–v44. doi: 10.1136/jnnp.2005.082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Patterson LJ, Doraiswamy PM, McDonald WM. Proton spectroscopy of human brain: effects of age and sex. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:995–1004. doi: 10.1016/0278-5846(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Toft P, Larsson HB, Stubgaard M, Henriksen O. The concentration of N-acetyl aspartate, creatine + phosphocreatine, and choline in different parts of the brain in adulthood and senium. Magn Reson Imaging. 1993;11:799–806. doi: 10.1016/0730-725x(93)90197-l. [DOI] [PubMed] [Google Scholar]
- Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, Yoshita M, D'Agostino RB, Sr, DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis SJ, Smith S, Mortilla M, Tartaglia MC, Bartolozzi ML, Guidi L, Federico A, Arnold DL. Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Arch Neurol. 2002;59:1565–1571. doi: 10.1001/archneur.59.10.1565. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Ehrensperger MM, Berres M, Taylor KI, Monsch AU. Screening properties of the German IQCODE with a two-year time frame in MCI and early Alzheimer's disease. Int Psychogeriatr. 2010;22:91–100. doi: 10.1017/S1041610209990962. [DOI] [PubMed] [Google Scholar]
- Falini A, Bozzali M, Magnani G, Pero G, Gambini A, Benedetti B, Mossini R, Franceschi M, Comi G, Scotti G, Filippi M. A whole brain MR spectroscopy study from patients with Alzheimer's disease and mild cognitive impairment. Neuroimage. 2005;26:1159–1163. doi: 10.1016/j.neuroimage.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Hashiguchi T, Sakamoto Y, Okamura H, Doi W, Takenouchi K, Takigawa M. Metabolite changes with age measured by proton magnetic resonance spectroscopy in normal subjects. Psychiatry Clin Neurosci. 1997;51:261–263. doi: 10.1111/j.1440-1819.1997.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gonen O, Viswanathan AK, Catalaa I, Babb J, Udupa J, Grossman RI. Total brain N-acetylaspartate concentration in normal, age-grouped females: quantitation with nonecho proton NMR spectroscopy. Magn Reson Med. 1998;40:684–689. doi: 10.1002/mrm.1910400506. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hovener JB, Rigotti DJ, Amann M, Liu S, Babb JS, Bachert P, Gass A, Grossman RI, Gonen O. Whole-brain N-acetylaspartate MR spectroscopic quantification: performance comparison of metabolite versus lipid nulling. AJNR Am J Neuroradiol. 2008;29:1441–1445. doi: 10.3174/ajnr.A1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- Hu J, Javaid T, Arias-Mendoza F, Liu Z, McNamara R, Brown TR. A fast, reliable, automatic shimming procedure using 1H chemical-shift-imaging spectroscopy. J Magn Reson B. 1995;108:213–219. doi: 10.1006/jmrb.1995.1126. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Fleysher R, Patil V, Liu S, Gonen O. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 2008;60:790–795. doi: 10.1002/mrm.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Spielman DM. Estimating NAA in cortical gray matter with applications for measuring changes due to aging. Magn Reson Med. 1997;37:372–377. doi: 10.1002/mrm.1910370313. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, MacKay S, Constans JM, Norman D, Van Dyke C, Fein G, Weiner MW. Axonal injury and membrane alterations in Alzheimer's disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging. 2008;27:1235–1241. doi: 10.1002/jmri.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chem Rev. 2010;110:3060–3086. doi: 10.1021/cr900250y. [DOI] [PubMed] [Google Scholar]
- NIH. Parkinson's Disease: Hope Through Research. In: Stroke NIoNDa., editor. Stroke. Bethesda, MD: NIH; 2000. [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Regard M, Strauss E, Knapp P. Children's production on verbal and non-verbal fluency tasks. Percept Mot Skills. 1982;55:839–844. doi: 10.2466/pms.1982.55.3.839. [DOI] [PubMed] [Google Scholar]
- Rigotti DJ, Inglese M, Babb JS, Rovaris M, Benedetti B, Filippi M, Grossman RI, Gonen O. Serial whole-brain N-acetylaspartate concentration in healthy young adults. AJNR Am J Neuroradiol. 2007;28:1650–1651. doi: 10.3174/ajnr.A0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999;9:711–716. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Schreiber HE, Javorsky DJ, Robinson JE, Stern RA. Rey-Osterrieth Complex Figure performance in adults with attention deficit hyperactivity disorder: a validation study of the Boston Qualitative Scoring System. Clin Neuropsychol. 1999;13:509–520. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT509. [DOI] [PubMed] [Google Scholar]
- Schuff N, Meyerhoff DJ, Mueller S, Chao L, Sacrey DT, Laxer K, Weiner MW. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–262. doi: 10.1007/0-387-30172-0_17. discussion 361-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Thalmann BRS, Stähelin H, Brubacher D, Ermini-Fünfschilling D, Bläsi S, Monsch A. Dementia screening in general practice: optimised scoring for the Clock Drawing. Test Brain Aging. 2002;2:36–43. [Google Scholar]
- Thurstone LL. Primary Mental Abilities. Chicago: University of Chicago Press, Chicago; 1938. [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- Wechsler DA. Wechsler Memory Scale-Revised manual. New York: Psychological Corporation; 1987. [Google Scholar]
- Zimmermann P, Fimm B. Testbatterie zur Aufmerksam keits prufung (TAP) Wörselew: Psy. text; 1992. [Google Scholar]