Abstract
Urolithiasis remains a very common disease in Western countries. Seventy to eighty percent of kidney stones are composed of calcium oxalate, and minor changes in urinary oxalate affect stone risk. Intestinal oxalate secretion mediated by anion exchanger SLC26A6 plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate urolithiasis. Using the relatively selective PKC-δ inhibitor rottlerin, we had previously found that PKC-δ activation inhibits Slc26a6 activity in mouse duodenal tissue. To identify a model system to study physiologic agonists upstream of PKC-δ, we characterized the human intestinal cell line T84. Knockdown studies demonstrated that endogenous SLC26A6 mediates most of the oxalate transport by T84 cells. Cholinergic stimulation with carbachol modulates intestinal ion transport through signaling pathways including PKC activation. We therefore examined whether carbachol affects oxalate transport in T84 cells. We found that carbachol significantly inhibited oxalate transport by T84 cells, an effect blocked by rottlerin. Carbachol also led to significant translocation of PKC-δ from the cytosol to the membrane of T84 cells. Using pharmacological inhibitors, we observed that carbachol inhibits oxalate transport through the M3 muscarinic receptor and phospholipase C. Utilizing the Src inhibitor PP2 and phosphorylation studies, we found that the observed regulation downstream of PKC-δ is partially mediated by c-Src. Biotinylation studies revealed that carbachol inhibits oxalate transport by reducing SLC26A6 surface expression. We conclude that carbachol negatively regulates oxalate transport by reducing SLC26A6 surface expression in T84 cells through signaling pathways including the M3 muscarinic receptor, phospholipase C, PKC-δ, and c-Src.
Keywords: SLC26A6, PKC-δ, phospholipase C, c-Src kinase
urolithiasis is one of the most common urologic diseases in industrialized societies (3). Seventy to eighty percent of kidney stones are composed of calcium oxalate (3). Urinary oxalate is an important determinant of the level of calcium oxalate supersaturation, and minor changes in the levels of urinary oxalate affect the risk for stone formation (67). In patients with recurrent calcium oxalate stones, mild hyperoxaluria is a common finding (67). Moreover, in males with recurrent calcium oxalate stones, the amount and size of calcium oxalate crystals, as well as the severity of the disease, were shown to be highly related to their urinary excretion of oxalate (67).1
The mammalian intestinal tract plays a major role in oxalate homeostasis by acting as a site for dietary oxalate absorption as well as an avenue, together with the kidneys, for oxalate excretion. The amount excreted in the urine depends on dietary intake, net intestinal absorption, endogenous production, and renal clearance. Intestinal transport of oxalate takes place either passively through the paracellular pathway, or actively through the transcellular (transepithelial) pathway (38). The transcellular pathway is mediated by integral membrane proteins (anion exchangers and channels) located at the apical and basolateral poles of the enterocyte and is of significant importance as it is potentially regulated (38).
Anion exchanger SLC26A6 is expressed in the apical membranes of many tissues including enterocytes. Studies in Slc26a6- null mice revealed that Slc26a6 plays an essential role in transcellular intestinal oxalate transport (28, 45). These mice were found to have a critical defect in intestinal oxalate secretion, resulting in enhanced net absorption of ingested oxalate, hyperoxalemia, hyperoxaluria, and a high incidence of calcium oxalate urolithiasis (45). Thus, intestinal oxalate secretion mediated by SLC26A6 plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate urolithiasis. Defects in the function or regulation of this key transporter are potential molecular mechanisms predisposing to calcium oxalate urolithiasis in humans. Therefore, elucidating the molecular mechanisms regulating SLC26A6 is of potential physiologic and therapeutic importance.
We had previously found that PKC-δ activation negatively regulates Slc26a6 activity in mouse duodenal tissue under physiological conditions (37). Given the essential role of Slc26a6 in intestinal oxalate secretion, it was of great interest to determine the physiological factors that may regulate Slc26a6 through PKC-δ activation. Therefore, to search for a model to screen for such potential physiological factors, we examined the human intestinal cell line, T84, which endogenously expresses SLC26A6 (80). We find that carbachol (carbamyl choline) negatively regulates oxalate transport by reducing SLC26A6 surface expression in T84 cells through signaling pathways including the M3 muscarinic receptor, phospholipase C, PKC-δ, and c-Src.
MATERIALS AND METHODS
Cell culture.
T84 cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in DMEM-F-12 culture medium supplemented with 5–10% fetal bovine serum, 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C in 5% CO2-95% air. Oxalate flux and other studies described below were performed using confluent cells grown for 6–14 days postplating on 24-well plastic supports and/or on 0.4-μm collagen-coated polystyrene transwell membrane filters (Corning, Corning, NY) in 12- and/or 24-mm inserts, following overnight serum starvation (7, 9, 69). For Ussing chamber studies, T84 cells were similarly grown on 0.4-μm collagen-coated 12-mm Snapwell inserts (Corning). Maturation of the monolayers grown on transwell-permeable supports was monitored by measuring the transepithelial resistance (TER) using EVOM2 Epithelial Voltohmmeter (World Precision Instruments). Differentiation of the monolayers was also monitored by assessing villin protein expression, which is a marker of epithelial maturation (2, 25).
Radioactive flux studies.
T84 cells were grown as described above. After aspiration of the culture medium, the cells were incubated in an isotonic NaCl solution (in mM: 120 NaCl, 2 CaCl2, 1 MgCl2, 20 HEPES, 5 glucose, titrated with Tris base to pH 7.4) at room temperature for 30 min. This solution was then aspirated and replaced with a Cl-free solution (in mM: 130 K-gluconate, 5 glucose, 20 HEPES, pH 7.4) or a Cl− solution (in mM: 130 KCl, 5 glucose, 20 HEPES, pH 7.4) containing 9 μM [14C]oxalate for 6 min. This 6-min period was chosen because it falls within the linear range of oxalate uptake by these cells. The influx of radiolabeled oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl-free solution. The cells were then solubilized in 0.2 N NaOH followed by neutralization with an equivalent amount of 0.2 N HCl. The solubilized cells were then transferred to vials with scintillation fluid (Opti-Fluor, Packard), and the radioactivity was measured by scintillation spectrometry. Flux studies on cells grown on transwells were similarly performed as above, with the flux solution added to the apical side (top) or the basolateral side (bottom) of the transwell when assessing unidirectional apical or basolateral influx, respectively. The influx of radiolabeled oxalate was similarly terminated, and the transwells were placed upside down and were allowed to dry for several minutes. Membrane filters containing the cells were cut from the support and placed into vials with scintillation fluid, and radioactivity was similarly measured following overnight solubilization.
PKC-δ translocation.
For the translocation studies, T84 cells were grown and serum starved as described above. Cells were treated with vehicle (control) or 150 μM carbachol (CCH) for 90 min (60 min in the culture medium and 30 min in the NaCl solution) followed by washing × 3 in ice-cold phosphate-buffered saline (PBS). The cells were then scraped into 400 μl ice-cold homogenization buffer (in mmol/l: 250 sucrose, 20 Tris·HCl, 4 ethylenediaminetetraacetic acid, 2 ethylene glycol-bisiph-N,N,N,N′,N′-tetraacetic acid, titrated with NaOH to pH 7.5) containing complete protease inhibitor cocktail tablets (Complete; Roche Applied Science), and subsequently homogenized on ice with 25 strokes of a glass tissue homogenizer. The homogenate was then ultracentrifuged (123,589 g, 4°C, 50 min) (Optima TLX Ultracentrifuge; Beckman, Fullerton, CA), and the supernatant was saved as the cytosolic fraction (31). The pellet was resuspended in 150 μl of the homogenization buffer containing 0.5% (vol/vol) Triton X-100, incubated on ice for 30 min, followed by centrifugation (18,300 g, 4°C, 20 min), and the resulting supernatant was saved as the membrane fraction (31). PKC-δ immunoreactivity in these fractions was tested by immunoblotting using a commercially available PKC-δ specific antibody (Santa Cruz).
SLC26A6 knockdown in T84 cells.
To knock down SLC26A6 expression in T84 cells, predesigned SureSilencing shRNA plasmids were prepared by Superarray Bioscience (Frederick, MD). Each vector expresses a short hairpin RNA, or shRNA, under control of the U1 promoter and neomycin resistance gene. Transfection-grade plasmid DNA was prepared by transforming these plasmids into competent Escherichia coli cells following Stratagene's protocol. To accomplish SLC26A6 knockdown in T84 cells, 2 × 106 cells were untransfected (UT) or nucleofected with 12 μg of shRNA plasmid DNA (NC = negative control shRNA or S1 and S2 = shRNAs targeting SLC26A6) using the Cell Line Nucleofector Kit T (Amaxa: catalog no. VCA-1002; Program T-005). The shRNA sequences for S1 and S2 are 5′-CAACGTTGAGGACTGCAAGAT-3′ and 5′-CAATCGGGCGGATCTGCTTAT-3′, respectively. The negative control shRNA is a scrambled artificial sequence that does not match any human, mouse, or rat gene. Stable shRNA-expressing cells were selected using neomycin (G418: 1,000 μg/ml; Invitrogen), with the medium being changed every 2–3 days. The minimum G418 concentration necessary to kill untransfected cells (the effective concentration) was determined by generating a dose-response curve and was found to be 1,000 μg/ml. The selection process was continued until enough cells were available for the generation of a frozen stock and for isolation of total RNA. After a frozen stock was made, the cells (polyclonal population) were continued to be grown in media containing a maintenance G418 concentration (50% of the effective concentration).
SDS-PAGE, Western blotting, and immunoprecipitation.
T84 cells were scraped and lysed in lysis buffer (in mM: 150 NaCl, 50 Tris·HCl, 5 EDTA, 50 sodium fluoride, 15 sodium pyrophosphate, 0.5% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, pH 7.4) supplemented with Complete Protease Inhibitor Cocktail (Roche Diagnostics), the lysate was then centrifuged (14,000 g, 4°C, 10 min), and the supernatant was retained for gel electrophoresis. Total protein levels were determined, and equal amounts of protein lysates were separated by SDS-PAGE using 7.5% polyacrylamide gels, with subsequent electro-transfer to polyvinylidene difluoride (PVDF, Immobilon-P, Millipore). Following transfer, all immunoblots were stained with Ponceau S Solution [0.1% Ponceau S (wt/vol) in 5% acetic acid (vol/vol)]. For Western blot analysis, PVDF strips were incubated first in blotto (5% nonfat dry milk and 0.1% Tween 20 in PBS) for 1 h to block nonspecific binding. Immunoblots were then incubated for 1 h with anti-PKC-δ antibody (1:200 dilution; catalog no. sc-937, Santa Cruz), or overnight at 4°C with anti-SLC26A6 antibody (1:100 dilution; catalog no. sc-26728, Santa Cruz), or anti-GAPDH antibody (1:1,000–5,000 dilution; catalog no. sc-32233, Santa Cruz). The strips then were washed in blotto and incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000 dilution; donkey anti-rabbit, anti-mouse, or anti-goat IgG, Jackson Laboratory). Antibody reactivity was detected with the enhanced chemiluminescence system (Western Lightning Plus-ECL, Perkin Elmer, and SuperSignal West, Thermo Scientific) according to the manufacturer's protocol.
To define the specific Src kinase(s) involved in the observed regulation, we examined whether carbachol stimulates phosphorylation of the Src family kinases c-Src and/or Fyn, which are known to be expressed in T84 cells (77). To this end, T84 cells were treated with vehicle (control) or 150 μM carbachol for 90 min (60 min in the culture medium and 30 min in the NaCl solution), washed with PBS, and total protein lysates were then prepared as described above. Equal amounts of total protein lysates were incubated with monoclonal anti-c-Src or anti-Fyn antibody (catalog no. 05-184 and 06-133; Upstate Biotechnology) overnight at 4°C. The immune complexes were then precipitated using protein G-agarose beads (GE Healthcare). The immunoprecipitates were collected by centrifugation and washed five times with the lysis buffer. After separation by SDS-PAGE, proteins were transferred to immunoblots as above. To detect the phosphorylation of c-Src and/or Fyn, immunoblots were incubated for 1 h at room temperature in blocking buffer [0.1% Tween 20 and 5% nonfat dry milk in Tris-buffered saline (TBS)]. Immunoblots were then incubated overnight at 4°C with anti-phospho-Src Tyr416 (1:1,000 dilution; catalog no. 2101; Cell Signaling Technology) in the dilution buffer (0.1% Tween 20 and 5% bovine serum albumin in TBS). The membranes were washed for 5 min × 3 with the wash buffer (0.1% Tween 20 in TBS). Immunoblots were then incubated for 1 h with HRP-conjugated donkey anti-rabbit secondary antibody, and antibody reactivity was detected as described above.
Surface biotinylation.
For the surface biotinylation studies, T84 cells were grown and serum starved as described above. Cells were treated with vehicle (control) or 150 μM carbachol for 90 min (60 min in the culture medium and 30 min in the NaCl solution) followed by washing × 1 in ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS + CM). To biotinylate apical membrane proteins (with all steps performed on ice and/or in a cold room), T84 cells were first incubated in PBS + CM containing 5 mM dithiothreitol (DTT) for 10-min × 2 with gentle shaking to remove the accumulated surface mucus as previously reported (71). T84 cell were washed for 5–10 min × 3 with PBS + CM to remove all excess DTT. Subsequently, cells were incubated with 1–2 mg/ml of the biotinylation reagent Sulfo-NHS-SS-Biotin (Thermo Scientific) in PBS + CM (pH 8) for 30 min × 2 (37), followed by washing × 2 in ice-cold PBS + CM. The cells were then incubated in PBS + CM containing 100 mM glycine for 15 min to quench excess biotin, and subsequently washed × 2 with PBS + CM. Total protein lysates were prepared as described above, and normalized samples were incubated overnight with 150–200 μl of streptavidin agarose beads (Thermo Scientific). Biotinylated proteins were dissociated from the beads with sample buffer (10% SDS, 2% β-mercaptoethanol, 20% glycerol, 5 mM Tris·HCl, pH 6.8) containing 100 mM DTT, as well as by boiling for 2 min. After separation by SDS-PAGE, proteins were transferred to immunoblots and probed with the anti-SLC26A6 antibody as above.
Short-circuit current measurement.
For short-circuit current (Isc) measurement, T84 cell monolayers, prepared as described above, were mounted in modified Ussing chambers (Physiological Instruments, San Diego, CA). The mucosal and serosal surfaces of the monolayer were bathed with 4 ml of warmed (37°C), Ringer buffer (140 mM Na+, 119.8 mM Cl−, 5.2 mM K+, 1.2 mM Mg2+, 1.2 mM Ca2+, 25 mM HCO3−, 2.4 mM HPO42−, 0.4 mM H2PO4−, pH 7.4, gassed with 95% O2-5% CO2) containing 10 mM mannitol or glucose on the mucosal or serosal side, respectively. Transepithelial short-circuit current (Isc), resistance, and total tissue conductance (GT) across monolayers were continuously recorded (at 20-s intervals) using Acquire and Analyze software (Physiological Instruments). Following a 15-min equilibration period, vehicle or the chloride channel blocker CFTR inhibitor-172 (10 μM) was added to the mucosal side of matched pairs of monolayers (GT ≤ 20%) for 15 min, followed by the addition of carbachol (150 μM) to the serosal side to elicit chloride secretion.
Materials.
Carbachol, U73122, U73343, PP2, SB 202190, phorbol 12-myristate 13-acetate (PMA), CFTR inhibitor-172, Gö6976, and Gö6983 were purchased from Calbiochem. Atropine, 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), rottlerin, and DIDS (4,4′diisothiocyanostilbene-2,2′-disulfonic acid) were purchased from Sigma. PD98059 was purchased from Upstate Cell Signaling Solutions. PD98059, PMA, PP2, U73343, CFTR inhibitor-172, Gö6983, and SB 202190 were dissolved in DMSO and stored at −20°C. Gö6976 was dissolved in DMSO and stored at +4°C. U73122 and 4-DAMP were dissolved in DMSO and made fresh before use. Carbachol was dissolved in H2O and stored at −20°C. Atropine and DIDS were dissolved in H2O and made fresh before use. [14C]oxalate was purchased from Amersham and Vitrax (specific activities: 117 mCi/mmol and 54 mCi/mmol, respectively). Equivalent volumes of DMSO (0.1–0.2%) or H2O were added to control media. Of note is that the concentration of each drug was chosen on the basis of the published literature (references provided in results), where for some drugs a wide range of concentration has been reported. For example, a range of use of largely between 100 nM and 10 μM and between 5 nM and 2 μM has been reported for Gö6983 and Gö6976, respectively. We routinely test several concentrations in pilot experiments. For Gö6976, we have tested up to 2 μM and we did not see an effect.
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni or Student-Newman-Keuls post hoc test, or by Student's t-test for unpaired samples when comparing two groups. P values < 0.05 were considered statistically significant.
RESULTS
To functionally characterize T84 cells, we examined whether these cells are capable of transporting oxalate measured as influx of radioactive [14C]oxalate in exchange for intracellular Cl (Cli). To this end, after incubation in the isotonic NaCl solution described above, radioactive [14C]oxalate influx into these cells was measured by replacing the solution with a Cl-free solution [Cli > extracellular Cl (Clo)] or a Cl solution (Clo > Cli) containing 9 μM [14C]oxalate for 6 min. Shown in Figure 1, imposing an outward Cl gradient by removing extracellular Cl (Cli > Clo) caused significant stimulation of [14C]oxalate uptake (gluconate), which is greatly inhibited by external Cl (Clo > Cli), consistent with Cl-oxalate exchange.
Fig. 1.
Functional characterization of T84 cells. T84 cells were first incubated as described in materials and methods in NaCl solution for 30 min, which then was replaced with a Cl-free solution [intracellular Cl (Cli) > extracellular Cl (Clo)] in the absence of (gluconate) or presence of 100 μM of the anion exchange inhibitor DIDS (gluconate + DIDS), or a chloride solution (Clo > Cli) containing [14C]oxalate for 6 min. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control (gluconate) value ([14C]oxalate uptake rate = 3.04 ± 0.75 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. Both DIDS and the presence of chloride (Clo > Cli) significantly inhibited [14C]oxalate uptake (P < 0.001, by ANOVA).
The addition of the anion exchange inhibitor DIDS (100 μM) during the influx period led to significant inhibition of oxalate uptake by T84 cells (gluconate + DIDS). These findings of a Cl-oxalate exchange activity which is DIDS-sensitive indicate transport characteristics described for SLC26A6 when expressed in Xenopus oocytes (50). Of note is that T84 cells express the anion exchanger SLC26A3 in addition to SLC26A6 (80). However, SLC26A3 transports oxalate poorly and it is relatively DIDS-insensitive when expressed in Xenopus oocytes (15). Accordingly, it is most likely that Cl-oxalate exchange activity (i.e., [14C]oxalate uptake in the presence of an outward Cl gradient) in T84 cells is mediated by SLC26A6.
To characterize T84 cells as a valid model to study SLC26A6 regulation, it is critical to confirm that the observed oxalate transport is indeed mediated by SLC26A6. To this end, SLC26A6 knockdown studies were performed. T84 cells were transfected with two shRNAs (S1 and -2) targeting SLC26A6, as well as a negative control shRNA (NC), and cells stably expressing these shRNAs were selected using neomycin. Using immunoblotting and shown in Fig. 2, A and B, S2 shRNA significantly reduced SLC26A6 protein expression level by ∼64%. Equal loading was verified by probing the lower half of the same blot with an anti-GAPDH antibody and observing no significant difference (Fig. 2A). Importantly, and as shown in Fig. 3, S2 shRNA significantly reduced Cl-oxalate exchange activity on the apical side [where SLC26A6 is expressed (80)] by >57%, with no effect on the Cl-oxalate exchange activity measured on the basolateral side. It should be noted that S1 shRNA, which did not significantly reduce SLC26A6 expression, also had no significant effect on oxalate influx (in pmol·cm−2·min−1: UT = 1.16 ± 0.19; NC = 1.35 ± 0.03; S1 = 1.12 ± 0.01; S2 = 0.38 ± 0.01). Collectively, these results confirm that the apical Cl-oxalate exchange activity observed in T84 cells is indeed largely mediated by SLC26A6 and thus strongly support the use of oxalate transport measurements in T84 cells as a valid model to study SLC26A6 regulation.
Fig. 2.
SLC26A6 (A6) knockdown in T84 cells using short hairpin RNA (shRNA). A: representative Western blot analysis of SLC26A6 protein expression. SLC26A6 protein expression was evaluated in T84 cell lysate (30 μg protein/lane: UT, untransfected cells; NC, T84 cells transfected with the negative control shRNA; S1 and S2, T84 cells transfected with shRNAs targeting SLC26A6). The lower half of the same blot was probed with an anti-GAPDH antibody to normalize loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software [National Institutes of Health (NIH, Bethesda, MD)]. Values are means ± SE of 7 different experiments of relative SLC26A6 abundance to GAPDH and are presented as a percentage of the respective control (UT) value. shRNA knockdown of SLC26A6 (S2) significantly reduced SLC26A6 total protein expression (P < 0.001 compared with UT and NC, by ANOVA).
Fig. 3.
Effect of SLC26A6 knockdown on [14C]oxalate uptake by T84 cells. Apical (A) and basolateral (B) [14C]oxalate uptake by UT, NC, and S2 was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was normalized to the respective control (UT) value ([14C]oxalate uptake rate = 2.14 ± 0.57 and 1.53 ± 0.47 pmol·cm−2·min−1, apical and basolateral, respectively). All experiments were performed on transwell-grown cells. SLC26A6 knockdown significantly reduced apical [14C]oxalate uptake (P < 0.05 compared with UT and NC, by ANOVA), but had no effect on basolateral [14C]oxalate uptake.
We next examined whether PKC activation will inhibit oxalate transport by T84 cells as previously observed for Slc26a6 expressed in Xenopus oocytes and for Slc26a6 endogenously expressed in mouse duodenal tissue (37). For this purpose, T84 cells were preincubated with the PKC activator PMA for 45 min before [14C]oxalate uptake was measured in the presence of an outward Cl gradient. Illustrated in Fig. 4, preincubation with PMA caused significant inhibition of [14C]oxalate uptake by T84 cells. To characterize the PKC signaling pathway mediating PMA inhibition of oxalate transport in T84 cells, we tested the effects of the PKC inhibitors Gö6983 [5 μM (26, 61, 72)] and Gö6976 [100 nM (53, 61, 62, 69)] on the PMA-induced suppression of [14C]oxalate. As seen in Fig. 4A, Gö6983 completely blocked the PMA-induced inhibition of [14C]oxalate uptake by T84 cells, whereas it had no significant effect on baseline transport measured in the absence of PMA. On the other hand, Gö6976 had no significant effect on the PMA-induced inhibition of [14C]oxalate uptake by T84 cells or on baseline transport. As indicated in materials and methods, we have tested up to 2 μM Gö6976 and we did not see an effect. These results indicate that the PMA-induced inhibition of [14C]oxalate uptake by T84 cells is due to activation of a Gö6983-sensitive PKC signaling pathway, just as in the case of Slc26a6 expressed in Xenopus oocytes (37). Of note is that PMA [as reported before during the initial 60 min (74)] and the PKC inhibitors (alone or in combination with PMA) were found not to affect the TER (mean TER in Ω·cm2 = 1,338.6 ± 36.3) under the conditions of these experiments, indicating that they do not affect the paracellular permeability.
Fig. 4.
Effect of PKC activation on [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control) or 200 nM PMA (PMA) for 45 min (× 15 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in materials and methods. [14C]Oxalate uptake was also measured in the presence of Gö6976 (100 nM) or Gö6983 (5 μM) for 60 min followed by 200 nM PMA with continued presence of Gö6976 (PMA + Gö6976) or Gö6983 (PMA + Gö6983), or 100 nM Gö6976 (Gö6976) or 5 μM Gö6983 (Gö6983) alone for 105 min. Values are means ± SE of 5 independent experiments (3 on plastic- and 2 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.09 ± 0.60 pmol·cm−2·min−1). Gö6983 significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA). B: effect of rottlerin on PMA-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control) or 200 nM PMA (PMA) for 45 min (× 15 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in materials and methods. [14C]Oxalate uptake was also measured in the presence of rottlerin (10 μM) for 60 min followed by 200 nM PMA with continued presence of rottlerin (PMA + rottlerin), or 10 μM rottlerin alone for 105 min (rottlerin). Values are means ± SE of 5 independent experiments (3 on plastic- and 2 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.64 ± 0.29 pmol·cm−2·min−1). Rottlerin significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA).
Gö6976 has highest affinity for the classical calcium-dependent PKC isozymes (cPKC) and the novel isozyme PKC-μ (35, 57). Thus, our observation that Gö6976 had no effect on the PMA-induced inhibition of [14C]oxalate uptake by T84 cells suggests that PMA does not mediate its suppressive effect on oxalate transport by activating cPKC isoforms or PKC-μ. In contrast, Gö6983 is a high-affinity inhibitor of not only cPKC isozymes, but also PKC-δ and PKC-ζ (35, 66). Therefore, our finding that PMA-induced suppression of [14C]oxalate uptake by T84 cells was completely blocked by Gö6983 suggests a potential role for PKC-δ since PKC-ζ is an atypical PKC that is not activated by phorbol esters (44).
To provide additional evidence for the role of PKC-δ in mediating inhibition of oxalate transport in T84 cells, we tested the effect of rottlerin, a relatively selective inhibitor of PKC-δ-sensitive processes in intact cells (20, 36, 43, 65, 74, 76). Interestingly, as reported for Slc26a6 expressed in Xenopus oocytes and endogenous Slc26a6 activity in mouse duodenal tissue (37), rottlerin completely blocked the PMA-induced inhibition of [14C]oxalate uptake by T84 cells, whereas it had no significant effect on baseline transport measured in the absence of PMA (Fig. 4B). Importantly, rottlerin or PMA + rottlerin were found not to affect the TER (mean TER in Ω·cm2 = 1,197.6 ± 13.5) under the conditions of these experiments. Collectively, these results suggest that PKC-δ activation similarly regulates oxalate transport activity in T84 cells as observed with murine Slc26a6 expressed in Xenopus oocytes and with Slc26a6 endogenously expressed in mouse duodenal tissue. These findings thus support use of the T84 intestinal cell line as a valid model to study regulation of SLC26A6 in a manner similar to the behavior of the endogenous transporter in native tissues under physiological conditions.
Cholinergic signaling has been reported to modulate intestinal ion transport through signaling pathways including PKC activation (27, 40). We therefore examined whether cholinergic stimulation with carbachol modulates [14C]oxalate transport by T84 cells. Shown in Fig. 5A, preincubation of T84 cells grown on transwell inserts with carbachol [150 μM (5, 24, 33); apically or basolaterally] for 90 min caused significant inhibition of apical [14C]oxalate uptake. Carbachol similarly inhibited [14C]oxalate uptake by T84 cells grown on plastic supports (Fig. 5B). Under the same conditions carbachol had no significant effect on the TER (mean TER in Ω·cm2 = 1,471.7 ± 18.4), as previously reported (74).
Fig. 5.
Effect of carbachol (CCH) on [14C]oxalate uptake by T84 cells. A: T84 cells grown on transwell inserts were preincubated with vehicle (control) or 150 μM carbachol (apically = CCH-A or basolaterally = CCH-B) for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 4 independent experiments each of which was normalized to the respective control value ([14C]oxalate uptake rate = 2.42 ± 0.61 pmol·cm−2·min−1). Carbachol significantly reduced [14C]oxalate uptake (P < 0.001 apically and basolaterally, by ANOVA). B: T84 cells grown on plastic supports were preincubated with vehicle (control) or 150 μM carbachol for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 4 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.04 ± 0.15 pmol·cm−2·min−1). Carbachol significantly reduced [14C]oxalate uptake (P < 0.0007, two-tailed t-test).
The most straightforward interpretation of the observation that carbachol inhibits Cl-oxalate exchange is that this inhibition results from a direct effect on expression or function of SLC26A6 itself. However, since carbachol is known to stimulate electrogenic chloride secretion in T84 cells (22), it may have accelerated the dissipation of the outwardly directed Cl gradient driving uptake of [14C]oxalate, thereby accounting for the observed inhibition. To rule out this possibility, we tested the effect of the chloride channel blocker CFTR inhibitor-172 [10 μM (1)]. Shown in Fig. 6A, preincubation of T84 cells with CFTR inhibitor-172 (CFTR-172) before incubation with carbachol (CCH + CFTR-172) had no significant effect on the inhibitory action of carbachol on [14C]oxalate uptake by T84 cells. We similarly tested the effect of CFTR inhibitor-172 on carbachol-induced changes in short-circuit current (ΔIsc) across T84 cell monolayers under the same conditions (seeded at the same time and studied concurrently as in Fig. 6A) mounted in Ussing chambers. T84 cells secrete chloride and changes in Isc are reflective of chloride secretion (10). Illustrated in Fig. 6B, carbachol greatly stimulated Isc across T84 monolayers, and CFTR inhibitor-172 reduced carbachol-induced chloride secretion by >70%. The finding that a Cl channel inhibitor that greatly reduced carbachol-induced Cl secretion failed to diminish the effect of carbachol to inhibit Cl-oxalate exchange strongly argues that cholinergic activation with carbachol must directly inhibit SLC26A6 activity.
Fig. 6.
Effect of CFTR inhibitor-172 (CFTR-172) on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM CFTR inhibitor-172 for 15 min followed by 150 μM carbachol for 90 min (CCH + CFTR-172), or 10 μM CFTR inhibitor-172 alone for 15 min (CFTR-172), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was done in duplicate or triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 0.94 ± 0.12 pmol·cm−2·min−1). All experiments were performed on transwell-grown cells. CFTR inhibitor-172 has no significant effect on the inhibition induced by carbachol. B: effect of CFTR inhibitor-172 on carbachol-induced chloride secretion [measured as changes in short-circuit current (ΔIsc)] across T84 cells. T84 cell monolayers (seeded at the same time and studied concurrently as in A) were mounted in Ussing chambers as described in materials and methods. Following a 15-min equilibration period, vehicle (control) or 10 μM CFTR inhibitor-172 was added to the mucosal side of matched pairs of monolayers for 15 min, followed by the addition of carbachol (150 μM) to the serosal side to elicit chloride secretion. Shown here is the peak Isc elicited by carbachol. Values are means ± SE of 7 monolayers per group. CFTR inhibitor-172 significantly inhibited carbachol-induced chloride secretion (ΔIsc) (P < 0.001, by ANOVA).
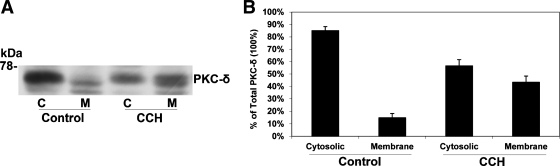
To evaluate whether carbachol negatively regulates [14C]oxalate uptake by T84 cells through PKC-δ activation, we tested the effect of rottlerin. Shown in Fig. 7, rottlerin completely blocked carbachol-induced inhibition of [14C]oxalate uptake by T84 cells, whereas it had no significant effect on baseline transport measured in the absence of carbachol. These data suggest that carbachol negatively regulates [14C]oxalate uptake by T84 cells through PKC-δ activation. Since a hallmark of PKC activation is the translocation of the activated PKC isoform(s) from the soluble (cytosolic) to the particulate (membrane) fraction (59), we evaluated whether carbachol, under the same conditions leading to inhibition of oxalate transport, induces PKC-δ translocation from the cytosol to the membrane of T84 cells. Shown in Fig. 8, carbachol under these conditions led to significant translocation of PKC-δ from the cytosol to the membrane fraction of T84 cells. These results demonstrate the association between the observed suppression of oxalate transport in T84 cells with the biochemical translocation of PKC-δ and provide further evidence that PKC-δ can be the involved isoform.
Fig. 7.
Effect of rottlerin on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min (× 60 min in the culture medium and × 30 min in the NaCl solution), and then [14C]oxalate uptake was measured as described in materials and methods. [14C]Oxalate uptake was also measured in the presence of 10 μM rottlerin for 60 min followed by 150 μM carbachol with continued presence of rottlerin for 90 min (CCH + rottlerin), or 10 μM rottlerin alone for 150 min (rottlerin). Values are means ± SE of 5 independent experiments (4 on plastic- and 1 on transwell-grown cells) each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.17 ± 0.43 pmol·cm−2·min−1). Rottlerin significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA).
Fig. 8.
Effect of carbachol on PKC-δ translocation in T84 cells. A: representative Western blot analysis of PKC-δ (60 μg protein/lane). T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min before cytosolic (C) and membrane (M) fractions were collected as described in materials and methods. B: densitometry of immunoblot results. Western blot band density was quantified using NIH Scion Image software. Values are means ± SE of 4 different experiments each of which was normalized to total blotted PKC-δ (C + M) under each condition (control or CCH). All experiments were performed on plastic-grown cells. Carbachol led to significant translocation of PKC-δ from the cytosolic to the membrane fraction of T84 cells (P < 0.001 compared with the cytosolic and membrane fractions in the control, by ANOVA).
Carbachol modulates epithelial cell function via muscarinic receptors (M1–5), which are linked, through G proteins, to a variety of intracellular second messenger systems (60). M1,3,5 receptors couple preferentially with phospholipase C, leading to activation of PKC and mobilization of intracellular Ca (60). M3 is the predominant receptor mediating the actions of acetylcholine in the gut, and it is known to be expressed in T84 cells (23). Therefore, to characterize the muscarinic receptor(s) mediating the inhibitory action of carbachol on [14C]oxalate uptake by T84 cells, we first tested the effect of the nonspecific muscarinic receptor antagonist atropine [1 μM (8, 33)]. Shown in Fig. 9A, preincubation of T84 cells with atropine before incubation with carbachol completely blocked the inhibitory action of carbachol on [14C]oxalate uptake by T84 cells, whereas atropine had no significant effect on baseline transport. These results indicate the involvement of one or more of the known muscarinic receptors (M1–5) (60).
Fig. 9.
Effect of muscarinic receptor blockade on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 1 μM atropine (ATR: a nonspecific muscarinic receptor antagonist) for 15 min followed by 150 μM carbachol with continued presence of atropine for 90 min (CCH + ATR), or 1 μM atropine alone for 105 min (ATR), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.73 ± 0.30 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. Atropine significantly reduced the inhibition induced by carbachol (P < 0.01, by ANOVA). B: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 200 nM 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP: an M3 muscarinic receptor antagonist) for 15 min followed by 150 μM carbachol with continued presence of 4-DAMP for 90 min (CCH + DAMP), or 200 nM 4-DAMP alone for 105 min (DAMP), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 6 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.17 ± 0.22 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. 4-DAMP significantly reduced the inhibition induced by carbachol (P < 0.001, by ANOVA).
To identify the specific receptor(s) mediating the action of carbachol, we tested the effect of the M3 antagonist 4-DAMP [200 nM (8, 33)], since M3 is known to be expressed in T84 cells (23). Indeed, preincubation of T84 cells with 4-DAMP before incubation with carbachol completely blocked the inhibitory effects of carbachol on [14C]oxalate uptake by T84 cells, while 4-DAMP had no significant effect on baseline transport (Fig. 9B). These data indicate that carbachol negatively regulates [14C]oxalate uptake by T84 cells through activation of the M3 muscarinic receptor.
Since the M3 muscarinic receptor couples preferentially with phospholipase C (60), it was of interest to examine whether phospholipase C is involved in the signaling pathway leading to inhibition of oxalate transport by carbachol. To this end, T84 cells were preincubated with the phospholipase C inhibitor U73122 or its inactive analog U73343 [10 μM (4)] before incubation with carbachol and then [14C]oxalate uptake was measured. Illustrated in Fig. 10, U73122 greatly reduced the inhibitory effect of carbachol on [14C]oxalate uptake by T84 cells, whereas U73343 had no significant effect. Both U73122 and U73343 had no significant effect on baseline transport measured in the absence of carbachol. These results indicate that phospholipase C is involved in the signaling pathway leading to inhibition of oxalate transport by carbachol.
Fig. 10.
Effect of the phospholipase C inhibitor U73122 and its inactive analog U73343 on carbachol-induced inhibition of [14C]oxalate uptake by T84 cells. T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM U73122 or U73343 for 30 min followed by 150 μM carbachol with continued presence of U73122 (CCH + U73122) or U73343 (CCH + U73343) for 90 min, or 10 μM U73122 (U73122) or U73343 (U73343) alone for 120 min, and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 9 independent experiments (6 on plastic- and 3 on transwell-grown cells) each of which was done in duplicate or triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.08 ± 0.34 pmol·cm−2·min−1). U73122 significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA).
PKC activation has been shown to stimulate ERK activation in T84 cells, and carbachol-induced activation of PKC-ε has been shown to mediate ERK activation by a sequential stimulation of Ras, Raf, and MAPK in human neuroblastoma cells (18, 48). Therefore, it is of interest to determine whether MAP kinases lie downstream of PKC-δ in the carbachol-induced signal transduction cascade leading to inhibition of [14C]oxalate uptake by T84 cells. To this end, we tested the effects of the MEK/ERK1/2 inhibitor PD98059 and the p38 inhibitor SB 202190. Shown in Fig. 11, A and B, preincubation of T84 cells with PD98059 [10 μM (63)] and SB 202190 [10 μM (63)] before incubation with carbachol (CCH + PD98059 and CCH + SB) had no significant effect on the inhibitory action of carbachol on [14C]oxalate uptake by T84 cells. These findings indicate that the MAP kinases ERK1/2 and p38 are unlikely to be involved in the carbachol-induced signal transduction cascade leading to inhibition of [14C]oxalate uptake by T84 cells.
Fig. 11.
Effect of ERK1/2 and p38 inhibition on carbachol-induced suppression of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, or 10 μM of the MEK/ERK1/2 inhibitor PD98059 for 15 min followed by 150 μM carbachol with continued presence of PD98059 for 90 min (CCH + PD98059), or 10 μM PD98059 alone for 105 min (PD98059), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.12 ± 0.26 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. B: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, or 10 μM of the p38 inhibitor SB 202190 for 60 min followed by 150 μM carbachol with continued presence of SB 202190 for 90 min (CCH + SB), or 10 μM SB 202190 alone for 150 min (SB), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 3 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 1.57 ± 0.59 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PD98059 and SB 202190 had no significant effect on the inhibition induced by carbachol.
Carbachol had been shown to modulate Cl secretion by T84 cells through pathways involving tyrosine kinases (47). To examine whether tyrosine kinases are involved in carbachol inhibitory regulation of oxalate transport in T84 cells, we tested the effect of the specific Src family kinase inhibitor PP2 [10 μM (13)]. As seen in Fig. 12A, preincubation of T84 cells with PP2 before incubation with carbachol (CCH + PP2) led to significant attenuation of carbachol-induced inhibition of oxalate transport (by ∼48%), whereas it had no significant effect on baseline transport measured in the absence of carbachol. These results indicate that one or more of the Src family kinase(s) is (are) involved in the signaling transduction cascade leading to inhibition of oxalate transport by carbachol in T84 cells. Of note is that we had also tested the effects of the nonspecific tyrosine kinase inhibitors herbimycin A and tyrphostin A25. We found that these inhibitors had no significant effect on the observed regulation but tended to suppress baseline transport (data not shown). It is therefore possible that these nonspecific inhibitors might have a limited inhibitory activity against the involved Src family kinase(s).
Fig. 12.
Effect of the Src family kinase inhibitor PP2 on carbachol- and PMA-induced inhibition of [14C]oxalate uptake by T84 cells. A: T84 cells were preincubated with vehicle (control), 150 μM carbachol for 90 min, 10 μM PP2 for 60 min followed by 150 μM carbachol with continued presence of PP2 (CCH + PP2), or 10 μM PP2 alone for 150 min (PP2), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 8 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 2.88 ± 0.56 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PP2 significantly reduced the inhibition induced by carbachol (P < 0.05, by ANOVA). B: T84 cells were preincubated with vehicle (control), or 200 nM PMA (PMA) for 45 min, 10 μM PP2 for 60 min followed by 200 nM PMA with continued presence of PP2 (PMA + PP2), or 10 μM PP2 alone for 105 min (PP2), and then [14C]oxalate uptake was measured as described in materials and methods. Values are means ± SE of 5 independent experiments each of which was done in triplicate and was normalized to the respective control value ([14C]oxalate uptake rate = 0.90 ± 0.17 pmol·cm−2·min−1). All experiments were performed on plastic-grown cells. PP2 significantly reduced the inhibition induced by PMA (P < 0.01, by ANOVA).
To determine whether the involved Src kinase(s) is (are) upstream or downstream of PKC-δ, we examined the effect of PP2 on PMA-induced inhibition of oxalate transport. To this end, T84 cells were preincubated with PP2 before incubation with PMA (PMA + PP2) and then [14C]oxalate uptake was measured. Illustrated in Fig. 12B, PP2 significantly attenuated PMA-induced inhibition of oxalate transport by T84 cells (by ∼41%), whereas it had no significant effect on baseline transport measured in the absence of PMA. These data suggest that the involved Src kinase(s) lie(s) downstream of PKC-δ in the signaling transduction cascade leading to inhibition of oxalate transport by carbachol in T84 cells.
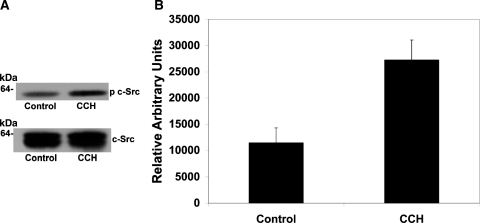
We next examined whether carbachol stimulates phosphorylation of the Src family kinases c-Src and/or Fyn, which are known to be expressed in T84 cells (77). Equal amounts of total protein lysates from vehicle (control)- and carbachol-treated T84 cells were immunoprecipitated with an anti-c-Src or an anti-Fyn antibody, and then immunoblots were prepared and probed with phospho-Src family (Tyr416) antibody (70). We found that under the same conditions leading to inhibition of oxalate transport, carbachol significantly stimulated c-Src phosphorylation in T84 cells (Fig. 13) without an effect on total c-Src expression (Fig. 13A). In contrast, carbachol had no significant effect on Fyn phosphorylation (data not shown). These results suggest that downstream of PKC-δ, the inhibitory effect of carbachol on oxalate transport by T84 cells is partially mediated by the c-Src kinase.
Fig. 13.
Effect of carbachol on c-Src phosphorylation in T84 cells. A: representative Western blot analysis of phospho-c-Src (p c-Src) in T84 cells. T84 cells were preincubated with vehicle (control) or 150 μM carbachol for 90 min before total protein lysates were collected as described in materials and methods. Equal amounts of protein lysates from vehicle (control)- and carbachol-treated T84 cells were immunoprecipitated with an anti-c-Src, and then immunoblots were prepared and probed with phospho-Src family (Tyr416) antibody. The blot was stripped and reprobed with an anti-c-Src (c-Src) antibody to verify equal loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using NIH Scion Image software. Values are means ± SE of 5 different experiments and are expressed as relative arbitrary units. All experiments were performed on plastic-grown cells. Carbachol led to significant stimulation of c-Src phosphorylation in T84 cells (P < 0.02, two-tailed t-test).
As a first step in elucidating the molecular mechanism(s) behind the observed cholinergic-mediated inhibition of SLC26A6 activity in T84 cells, we examined whether carbachol reduces SLC26A6 surface expression, as observed with PKC inhibition of Slc26a6 expressed in Xenopus oocytes (37). To this end, we performed surface biotinylation studies. Control or carbachol-treated cells were exposed to the surface biotinylation reagent Sulfo-NHS-SS-Biotin, biotinylated proteins were precipitated with streptavidin, and then immunoblots were prepared and probed with an anti-SLC26A6 antibody to assess surface SLC26A6 expression. In addition, immunoblots of T84 cell lysates were prepared and probed to assess total SLC26A6 expression. A representative immunoblot is shown in Fig. 14A, and the scanned data from five experiments are illustrated in Fig. 14B. As indicated in Fig. 14, carbachol caused significant reduction of biotin-labeled SLC26A6, which is in general agreement with the observed carbachol-induced inhibition of oxalate transport. However, total SLC26A6 abundance in cell lysate was unaffected by carbachol treatment. The lack of cell penetration of the biotinylation reagent was confirmed by the minimal biotinylation of the intracellular protein GAPDH under the conditions of the experiment (Fig. 14A). Taken together, these findings strongly indicate redistribution of SLC26A6 from the surface membrane without a change in total protein expression as the mechanism by which cholinergic signaling negatively regulates SLC26A6-mediated oxalate transport by T84 cells.
Fig. 14.
Effect of carbachol on SLC26A6 (A6) protein expression assayed by immunoblotting. A: representative Western blot analysis of total and surface biotinylated SLC26A6. T84 cells were preincubated with vehicle (control, CON) or 150 μM carbachol for 90 min, and then SLC26A6 protein expression was evaluated in cell lysate (total: 20 μg protein/lane) and after streptavidin precipitation of surface biotinylated proteins from 1,500 μg of initial cell lysate (Surface) performed as described in materials and methods. The lower half of the same blot was probed with an anti-GAPDH antibody to verify equal loading of protein in each lane (bottom). B: densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SE for five different experiments (2 on plastic- and 3 on transwell-grown cells) each of which was normalized to the respective control value. Carbachol significantly reduced the amount of SLC26A6 protein available to surface biotinylation (P < 0.001, by ANOVA).
DISCUSSION
In the present study we used the human intestinal epithelial cell line T84 as a model to examine the regulation of oxalate transport mediated by anion exchanger SLC26A6. SLC26A6 knockdown in T84 cells using shRNA revealed that the apical Cl-oxalate exchange activity (i.e., [14C]oxalate uptake in the presence of an outward Cl gradient) observed in T84 cells is largely mediated by SLC26A6. It should be noted that, during the process of transepithelial oxalate secretion, SLC26A6 operates in the direction of exchanging intracellular oxalate for mucosal Cl. Nevertheless, SLC26A6 is capable of operating in either direction (46), and we measured its activity by the more convenient assay of cellular oxalate uptake.
We found that [14C]oxalate uptake by these cells is negatively regulated by the cholinergic receptor agonist carbachol. Experiments using different pharmacologic muscarinic receptor antagonists indicated that carbachol negatively regulates [14C]oxalate uptake by T84 cells through stimulation of the M3 muscarinic receptor. Utilizing relatively selective inhibitors as well as translocation studies, we demonstrated that carbachol likely signals through activation of phospholipase C and PKC-δ to negatively regulate [14C]oxalate uptake by T84 cells. We also demonstrated that, downstream of PKC-δ, the inhibitory effect of carbachol on oxalate transport by T84 cells is partially mediated by the c-Src kinase. In addition, assessment of SLC26A6 localization by biotinylation indicated that reduction in surface membrane expression of the anion exchanger is the molecular mechanism underlying cholinergic-mediated inhibition of oxalate transport by T84 cells.
As stated above, the G protein-linked M3 muscarinic receptor is known to couple preferentially with phospholipase C (60). Phospholipase C induces phosphoinositide hydrolysis and generation of diacylglycerol and inositol trisphosphate (60). Diacylglycerol is capable of activating both the classical and the novel (including PKC-δ) PKC isoforms (42, 60). Using an in vitro kinase assay, PKC activation with phorbol ester (PMA) has been shown to significantly stimulate PKC-δ in T84 cells, and this stimulatory effect is completely prevented by 10 μM rottlerin (74). On the other hand, rottlerin has no effect on PMA-induced stimulation of PKC-ε in T84 cells (74). We find in this study that the same dose of rottlerin (10 μM) completely blocks the inhibitory effects of both PMA and carbachol on [14C]oxalate uptake by T84 cells. Of note is that, despite its frequent use as a relatively selective PKC-δ inhibitor (20, 36, 43, 65, 74, 76), rottlerin (in micromolar concentration) has been shown to inhibit the activity of additional unrelated kinases, and might uncouple mitochondrial respiration and reduce ATP levels (19, 58, 75). Moreover, in contrast to the complete blocking effect of rottlerin on PMA-induced stimulation of PKC-δ in T84 cells described above (74), rottlerin (10–20 μM) was shown to have no inhibitory activity against recombinant PKC-δ in vitro (19, 73). Therefore, to provide additional evidence that PKC-δ is the likely involved PKC isoform mediating carbachol inhibition of oxalate transport in T84 cells, we performed studies of translocation, a hallmark of PKC activation (59). Carbachol has previously been shown to translocate PKC-ε from the cytosol to the membrane of T84 cells (74). We observed that, under the same conditions leading to inhibition of oxalate transport, carbachol also induced significant PKC-δ translocation from the cytosol to the membrane of T84 cells, thus providing further evidence that PKC-δ is the likely involved PKC isoform. Of interest in this regard is that PKC-δ mediates carbachol-induced amylase release from pancreas (54), and carbachol-induced tonic contraction of the guinea pig ileum (64).
The major parasympathetic mediator acetylcholine is an important biological regulator of intestinal ion transport (40). Cholinergic activation by acetylcholine and other cholinomimetics (e.g., carbachol) has been shown to stimulate electrogenic Cl secretion, to promote HCO3 secretion, and to inhibit electrogenic Na absorption and coupled NaCl absorption in the intestine (6, 11, 12, 16, 30, 82). We now find that cholinergic stimulation with carbachol negatively regulates SLC26A6-mediated Cl-oxalate exchange activity in T84 cells by reducing SLC26A6 surface membrane expression through signaling pathways including PKC-δ and downstream activation of c-Src. Carbachol has been shown to inhibit rabbit ileal brush-border Na+/H+ exchanger 3 by enhancing its endocytic trafficking in a c-Src-dependent manner (55). Given the fact that T84 cells are well known to exhibit several functional properties resembling the native epithelium, including cell surface receptors (21), we anticipate that similar regulation of oxalate transport by cholinergic stimulation is likely to be active in native tissues in vivo.
As described above, intestinal oxalate secretion mediated by SLC26A6 plays a major constitutive role in limiting net absorption of ingested oxalate, thereby preventing hyperoxaluria and calcium oxalate urolithiasis (45). A role for cholinergic regulation of intestinal oxalate transport had not previously been recognized. Our finding that carbachol negatively regulates SLC26A6-mediated Cl-oxalate exchange activity in T84 cells suggests a potential role for cholinergic regulation of oxalate homeostasis. Most animal models of obesity and hyperinsulinemia are associated with increased parasympathetic (vagal) cholinergic activity (29, 32, 68). Increased tone of the peripheral parasympathetic nerves leads to enhanced release of acetylcholine, which triggers changes in the activity of various effector organs and tissues including the intestine. Obesity is a risk factor for kidney stones and obese stone formers often have mild hyperoxaluria (17, 49). From these observations, it is tempting to speculate that obesity-associated cholinergic activity might lead to acetylcholine-induced inhibition of SLC26A6-mediated intestinal oxalate secretion, thereby potentially contributing to the reported hyperoxaluria and high incidence of kidney stones in obese patients.
It should be emphasized, however, that net absorption of dietary oxalate depends on the balance between oxalate absorption and secretion in the intestine. The identity of the transporter(s) mediating intestinal oxalate absorption remain(s) unknown. However, preliminary studies in Slc26a3-null mice show a significant reduction in mucosal to serosal oxalate flux in the distal ileum and distal colon, which is associated with a significant decrease in urinary oxalate excretion (39), suggesting that Slc26a3 might play an important role in transcellular absorption of oxalate. Whether cholinergic signaling regulates intestinal absorption of oxalate remains to be studied.
Renal clearance of oxalate is largely by glomerular filtration (41), as well as tubular secretion. Oxalate undergoes bidirectional transport in the proximal tubule with overall net secretion (78). Perfusion studies demonstrate net absorption of oxalate by the S1 and S2 segments of the rat proximal tubule and net secretion by the S3 segment (34, 51). Studies in rabbit renal brush-border vesicles have identified Cl-oxalate exchange, oxalate-HCO3 exchange, oxalate-sulfate exchange, and oxalate-OH exchange (52), and depending on the transmembrane gradients for oxalate and the exchanging anion, oxalate secretion is expected with the first exchange mode and absorption with the latter three exchange modes. Slc26a6 is expressed in the apical membrane of proximal tubule cells and is capable of medicating all of the above described modes of anion exchange (14, 46, 56, 79, 81). Cl-oxalate exchange in renal brush-border vesicles from Slc26a6-null mice is completely abolished, indicating that Slc26a6 mediates all of the Cl-oxalate exchange activity in proximal tubule cells, while oxalate-sulfate exchange is partially defective (45). The role of Slc26a6 in mediating the other potentially absorptive modes of oxalate transport in this nephron segment remains unknown. Whether cholinergic signaling regulates renal handling of oxalate also remains to be studied.
In summary, we have demonstrated that cholinergic stimulation with carbachol negatively regulates oxalate transport by reducing SLC26A6 surface expression in T84 cells through signaling pathways that likely include the M3 muscarinic receptor, phospholipase C, PKC-δ, and c-Src. These findings suggest that cholinergic regulation of intestinal oxalate transport may play an important role in oxalate homeostasis and thereby could modify urinary oxalate excretion and stone risk.
GRANTS
This work was supported by National Institutes of Health Grants K08-DK-067245 (H. A. Hassan), P01-DK-17433 (P. S. Aronson), and R37-DK-33793 (P. S. Aronson), and Robert Wood Johnson Foundation Grant 65877 (H. A. Hassan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.A.H. and P.S.A. conception and design of research; H.A.H. and M.C. performed experiments; H.A.H., M.C., and P.S.A. analyzed data; H.A.H., M.C., and P.S.A. interpreted results of experiments; H.A.H. prepared figures; H.A.H. drafted manuscript; H.A.H. and P.S.A. edited and revised manuscript; H.A.H., M.C., and P.S.A. approved final version of manuscript.
Footnotes
This article is the topic of an Editorial Focus by John F. Heneghan and Seth L. Alper (39a).
REFERENCES
- 1. Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl− secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Arpin M, Blair L, Coudrier E, Dudouet B, Finidori J, Carcia A, Huet C, Pringault E, Robine S, Sahuguillo-Merino C, Louvard D. Villin, a specific marker for some epithelia specialized in transport, to study the differentiation of intestinal and kidney cells in vivo and in a human colon adenocarcinoma line HT29 in culture. Mol Aspects Med 10: 257–272, 1988. [DOI] [PubMed] [Google Scholar]
- 3. Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31: 927–949, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta 1773: 483–501, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertrand CA, Laboisse CL, Hopfer U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am J Physiol Cell Physiol 276: C907–C914, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Browning JG, Hardcastle J, Hardcastle PT, Redfern JS. Localization of the effect of acetylcholine in regulating intestinal ion transport. J Physiol 281: 15–27, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182–28190, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cameron HL, Perdue MH. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol Motil 19: 47–56, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Cammisotto PG, Bendayan M, Sane A, Dominguez M, Garofalo C, Levy E. Receptor-mediated transcytosis of leptin through human intestinal cells in vitro. Int J Cell Biol 2010: 928169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest 76: 1837–1842, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandan R, Megarry BH, O'Grady SM, Seybold VS, Brown DR. Muscarinic cholinergic regulation of electrogenic chloride secretion in porcine proximal jejunum. J Pharmacol Exp Ther 257: 908–917, 1991 [PubMed] [Google Scholar]
- 12. Chandan R, O'Grady SM, Brown DR. Modulation of Na+, Cl− and HCO3− transport by carbachol in pig distal jejunum. Eur J Pharmacol 193: 257–264, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooke HJ. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 246: G263–G267, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Curhan GC, Willett WC, Rimm EB, Speizer FE, Stampfer MJ. Body size and risk of kidney stones. J Am Soc Nephrol 9: 1645–1652, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Dabrowski A, Groblewski GE, Schafer C, Guan KL, Williams JA. Cholecystokinin and EGF activate a MAPK cascade by different mechanisms in rat pancreatic acinar cells. Am J Physiol Cell Physiol 273: C1472–C1479, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deb DK, Sassano A, Lekmine F, Majchrzak B, Verma A, Kambhampati S, Uddin S, Rahman A, Fish EN, Platanias LC. Activation of protein kinase C delta by IFN-gamma. J Immunol 171: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204–G208, 1984 [DOI] [PubMed] [Google Scholar]
- 22. Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickinson KE, Frizzell RA, Sekar MC. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol 225: 291–298, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W. Cholinergic-mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol 168: 219–229, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29–18 clones. J Cell Biol 105: 359–369, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ehre C, Zhu Y, Abdullah LH, Olsen J, Nakayama KI, Nakayama K, Messing RO, Davis CW. nPKCε, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol 293: C1445–C1454, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Fondacaro JD. Intestinal ion transport and diarrheal disease. Am J Physiol Gastrointest Liver Physiol 250: G1–G8, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, Li JH, Wess J. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab 4: 363–375, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Geibel JP, Singh S, Rajendran VM, Binder HJ. HCO(3)( −) secretion in the rat colonic crypt is closely linked to Cl(−) secretion. Gastroenterology 118: 101–107, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22: 565–604, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Greenwood JM, Dragunow M. M3 muscarinic receptors promote cell survival through activation of the extracellular regulated kinase (ERK1/2) pathway. Eur J Pharmacol 640: 38–45 2010 [DOI] [PubMed] [Google Scholar]
- 34. Greger R, Lang F, Oberleithner H, Deetjen P. Handling of oxalate by the rat kidney. Pflügers Arch 374: 243–248, 1978 [DOI] [PubMed] [Google Scholar]
- 35. Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase C isoenzymes. FEBS Lett 392: 77–80, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199: 93–98, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS. Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292: C1485–C1492, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 33: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Hatch M, Freel RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 28: 143–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a. Heneghan JF, Alper SL. This, too, shall pass—like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells.” Am J Physiol Cell Physiol (November 2, 2011). doi:10.1152/ajpcell.00389.2011 [DOI] [PubMed] [Google Scholar]
- 40. Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149: 463–479, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holmes RP, Ambrosius WT, Assimos DG. Dietary oxalate loads and renal oxalate handling. J Urol 174: 943–947, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J 291: 329–343, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jain N, Zhang T, Kee WH, Li W, Cao X. Protein kinase C delta associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem 274: 24392–24400, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol 8: 168–173, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Kachintorn U, Vajanaphanich M, Barrett KE, Traynor-Kaplan AE. Elevation of inositol tetrakisphosphate parallels inhibition of Ca2+-dependent Cl− secretion in T84 cells. Am J Physiol Cell Physiol 264: C671–C676, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Kim JY, Yang MS, Oh CD, Kim KT, Ha MJ, Kang SS, Chun JS. Signalling pathway leading to an activation of mitogen-activated protein kinase by stimulating M3 muscarinic receptor. Biochem J 337: 275–280, 1999 [PMC free article] [PubMed] [Google Scholar]
- 49. Kleinman JG. Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney Int 72: 8–10, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98: 9425–9430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Knight TF, Sansom SC, Senekjian HO, Weinman EJ. Oxalate secretion in the rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 240: F295–F298, 1981 [DOI] [PubMed] [Google Scholar]
- 52. Kuo SM, Aronson PS. Pathways for oxalate transport in rabbit renal microvillus membrane vesicles. J Biol Chem 271: 15491–15497, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Lee YM, Li WH, Kim YK, Kim KH, Chung JH. Heat-induced MMP-1 expression is mediated by TRPV1 through PKCalpha signaling in HaCaT cells. Exp Dermatol 17: 864–870, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Li C, Chen X, Williams JA. Regulation of CCK-induced amylase release by PKC-δ in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G764–G771, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284: C769–C779, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 58. McGovern SL, Shoichet BK. Kinase inhibitors: not just for kinases anymore. J Med Chem 46: 1478–1483, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science 268: 247–251, 1995 [DOI] [PubMed] [Google Scholar]
- 60. Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484–496, 1995 [PubMed] [Google Scholar]
- 61. Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol 182: 5800–5809, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Pernas-Sueiras O, Alfonso A, Vieytes MR, Botana LM. PKC and cAMP positively modulate alkaline-induced exocytosis in the human mast cell line HMC-1. J Cell Biochem 99: 1651–1663, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Pham H, Vincenti R, Slice LW. COX-2 promoter activation by AT1R-Gq-PAK-p38beta signaling in intestinal epithelial cells. Biochim Biophys Acta 1779: 408–413, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Poole DP, Furness JB. PKC δ-isoform translocation and enhancement of tonic contractions of gastrointestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 292: G887–G898, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Rahman A, Anwar KN, Uddin S, Xu N, Ye RD, Platanias LC, Malik AB. Protein kinase C-delta regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol Cell Biol 21: 5554–5565, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol Biol Cell 13: 1381–1389, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Robertson WG, Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26: 105–110, 1980 [DOI] [PubMed] [Google Scholar]
- 68. Rohner-Jeanrenaud F. A neuroendocrine reappraisal of the dual-centre hypothesis: its implications for obesity and insulin resistance. Int J Obes Relat Metab Disord 19: 517–534, 1995 [PubMed] [Google Scholar]
- 69. Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKCε. Am J Physiol Cell Physiol 283: C1492–C1500, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(−)/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, Waldron RT, Rozengurt E. Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem 284: 13434–13445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J Biol Chem 276: 37986–37992, 2001 [DOI] [PubMed] [Google Scholar]
- 74. Song JC, Hanson CM, Tsai V, Farokhzad OC, Lotz M, Matthews JB. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am J Physiol Cell Physiol 281: C649–C661, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism. Biochim Biophys Acta 1763: 25–38, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, Malik AB, Fish EN, Platanias LC. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem 277: 14408–14416, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 294: G441–G451, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Verkoelen CF, Romijn JC. Oxalate transport and calcium oxalate renal stone disease. Urol Res 24: 183–191, 1996 [DOI] [PubMed] [Google Scholar]
- 79. Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Worrell RT, Best A, Crawford OR, Xu J, Soleimani M, Matthews JB. Apical ammonium inhibition of cAMP-stimulated secretion in T84 cells is bicarbonate dependent. Am J Physiol Gastrointest Liver Physiol 289: G768–G778, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]
- 82. Zimmerman TW, Dobbins JW, Binder HJ. Mechanism of cholinergic regulation of electrolyte transport in rat colon in vitro. Am J Physiol Gastrointest Liver Physiol 242: G116–G123, 1982 [DOI] [PubMed] [Google Scholar]