Abstract
BACKGROUND
The prostate and testis expression (PATE)-like family of proteins are expressed mainly in the male genital tract. They are localized in the sperm head and are homologous to SP-10, the acrosomal vesicle protein also named ACRV1. Our aim was to characterize the expression and functional role of three PATE-like proteins in the testis and ejaculated sperm.
METHODS
The expression and localization of PATE-like proteins in human testis biopsies (n= 95) and sperm cells were assessed by RT–PCR, immunohistochemistry and immunofluorescence staining (at least 600 sperm cells per specimen). The function of the PATE protein was tested by the hemizona assay and hamster egg penetration test (HEPT).
RESULTS
PATE and PATE-M genes and proteins were present almost exclusively in germ cells in the testis: immunoflourescence showed that the percentage of germ cells positive for PATE, PATE-M and PATE-B was 85, 50 and 2%, respectively. PATE and PATE-M proteins were localized in the equatorial segment of the sperm head, while PATE-B protein was localized in the post-acrosomal region. A polyclonal antibody (Ab, at 1:50 and 1:200 dilutions) against the PATE protein did not inhibit sperm–zona binding in the hemizona assay (hemizona index of 89.6 ± 10 and 87 ± 36%, respectively). However, there was inhibition of sperm–oolemma fusion and penetration in the HEPT (penetration index: without Ab 7 ± 3.9; Ab dilution of 1:100, 4 ± 3.5; Ab dilution of 1:20, 0.6 ± 1.2, P < 0.001).
CONCLUSIONS
Our data suggest that PATE protein is involved in sperm–oolemma fusion and penetration but not sperm–zona binding.
Keywords: human sperm, PATE genes, human spermatogenesis, TFP/Ly-6/uPAR proteins, oolemma penetration
Introduction
The first step of fertilization requires binding of sperm to the zona pellucida of the oocyte and penetration, followed by fusion with and penetration of the oolemma plasma membrane. Sperm binding is mediated by a complex of zona pellucida, recognized by carbohydrate-binding proteins on the sperm surface (Nixon et al., 2007). Following sperm penetration into the zona pellucida, the equatorial segment is believed to be the sperm–oolemma fusion site that involves oolemmal microvilli wrapping around the sperm head (Hoshi et al., 1994; Evans et al., 1997). The equatorial segment is formed in the late spermatid stage, and it is maintained until it is incorporated into the oocyte (Toshimori, 1998). The fusion capacity of the equatorial segment has been demonstrated experimentally by fusion with liposomes (Arts et al., 1997).
Much effort has been made to identify the molecules involved in the process of sperm–oocyte binding and fusion. Among those molecules are CD46 (membrane cofactor protein; Anderson et al., 1993; Inoue et al., 2003), mSLLP1 (mouse sperm lysozyme-like protein; Herrero et al., 2005), CRISP-1 (epididymal-derived cysteine-rich secretory protein 1; Cuasnicu et al., 1984; Da Ros et al., 2008), ERp57 (Pdi3a; Ellerman et al., 2006), Izumo (Inoue et al., 2005), the acrosomal vesicle protein SP-10 (Hamatani et al., 2000) and the three ADAMs (a disintegrin and metalloprotease domain) proteins (Evans et al., 1995; Yuan et al., 1997; Primakoff and Myles, 2000).
The prostate and testis expression (PATE) gene was discovered during the search to identify tissue-specific antigens for targeted cancer therapy (Bera et al., 2002). An RT–PCR analysis demonstrated that PATE mRNA is additionally expressed in the epididymis and seminal vesicle (Soler-Garcıá et al., 2005). PATE was identified as a small secreted protein with a signal peptide of 21 amino acids and a molecular weight of 14 kDa. Confocal microscopy of immunostained sperm smears demonstrated that the PATE protein was localized in a band-like pattern in the equatorial segment of the sperm head (Soler-Garcıá et al., 2005).
The human PATE gene was localized on chromosome 11q24.2 and is telomerically juxtaposed to the SP-10 gene. BLASTP analysis of the PATE amino acid sequence against the ‘non-redundant’ National Center for Biotechnology Information database revealed similarities to SP-10. PATE and SP-10 genes encode proteins that contain 10 cysteine residues that conform to the three-fingered protein (TFP) domain, suggesting that the two genes may be part of a single chromosomal locus (Hoshi et al., 1994; Wassarman et al., 2001). Monoclonal antibody (Ab) against the SP-10 peptide dose dependently and significantly decreased sperm binding and penetration to the oolemma in the zona-free hamster egg penetration test (HEPT) of human sperm (Hamatani et al., 2000). The involvement of SP-10 in sperm–oolemma binding was further supported by its localization at the equatorial segment of sperm cells (Hoshi et al., 1994).
Pattern-search techniques revealed three additional PATE-like genes, designated PATE-B, PATE-M and PATE-DJ (Levitin et al., 2008), which were localized to the same 11q24 genomic locus as the PATE gene in a cluster of genes encoding secreted proteins that comprise a typical TFP/Ly-6/uPAR domain. The PATE-B gene was mainly expressed in the prostate, with lower expression levels in the testis. This was in contrast to the PATE-M and PATE-DJ genes that displayed higher expression levels in the testis compared with the prostate (Levitin et al., 2008).
In the present study, the expression and localization of three PATE-like genes and proteins were characterized in the testis and in ejaculated sperm cells. The possible biological role of PATE protein in sperm–zona binding and sperm–oolemma binding and penetration was assessed using the human hemizona assay (HZA) and the HEPT, respectively.
Materials and Methods
Human semen, testis samples and oocyte samples
Semen, testicular tissue and oocyte samples were obtained from volunteers after they provided informed written consent. All human studies were approved by the local Institutional Review Board Committee in accordance with the Helsinki Declaration of 1975.
Animals
Experiments using hamsters were conducted in accordance with the Laboratory Animal Care Regulations of Tel-Aviv Medical Center.
Collection of human sperm cells and testicular tissue samples
Sperm collection
Semen ejaculates were provided by donors with normal semen parameters according to the World Health Organization (WHO, 2010) criteria. Semen was obtained by masturbation after 2–3 days of sexual abstinence. Liquefied semen was centrifuged at 400g for 10 min, washed three times in human tubule fluid (HTF, Irvine Scientific, CA, USA) containing 1% human serum albumin (HSA, Sigma Chemicals, MO, USA), or with phosphate-buffered solution (PBS), depending on the assay protocol (Yogev et al., 1999).
Testicular tissue collection
Men with azoospermia undergoing testicular sperm extraction (TESE) were enrolled in the study. Three biopsies from each testis were taken to extract spermatozoa for ICSI. One biopsy was divided into two pieces and the smallest piece was used for molecular and histological analyses (Yogev et al., 2006).
Histological assessment
The testis biopsies were categorized according to four histological patterns: obstructive azoospermia (normal spermatogenesis), maturation arrest (complete arrest of spermatogenesis at the stage of primary spermatocyte), focal spermatogenesis (some seminiferous tubules with complete spermatogenesis and some tubules deprived of germ cells) and Sertoli cell only (absence of germs cells in all tubules).
RT–PCR
Total RNA from 95 testis biopsies with different pathologies was extracted using the GeneElute™ Mammalian Total RNA Kit (Sigma, MO, USA) following the instructions of the manufacturer. Single-stranded cDNA from RNA samples of each tissue was synthesized using the Revert Aid™ First-strand cDNA Synthesis kit (Fermentas, Burlington, Canada). Forward and reverse oligonucleotide primers were designed using the DNA sequences obtained from the PATE-like sequences and were chosen such that they always spanned an intron (Soler-Garcıá et al., 2005; Levitin et al., 2008). PCR amplification of PATE-like DNA was performed using FAST start enzyme (Roche, Heidelberg, Germany). Whenever no mRNA was detected, the result was reconfirmed by repeating the PCR two more times. All the samples were tested for the expression of β-actin, a control for the RNA isolation, one or more germ cell markers (RBM1, DAZ, CDY2 or BOULE) and spermatid markers (CDY1 transcripts; Kleiman et al., 2001, 2011). As the same PCR product size was obtained for cDNA and genomic DNA with β-actin primers, RNA samples were tested in each case with and without the reverse transcriptase step in order to detect any genomic DNA contamination.
Primary polyclonal Abs
SOL-1 polyclonal Abs (pAbs) raised in rabbit, produced in Dr Pastan's laboratory in the National Institutes of Health, MD, USA, were used to detect the presence of PATE protein. The SOL-1 pAbs were obtained by immunizing rabbits with bacterial full-length recombinant PATE protein (rPATE) that had undergone renaturation and refolding as previously described (Soler-Garcıá et al., 2005). Rabbits were primary immunized with rPATE protein in complete Freund's adjuvant followed by three booster injections of rPATE protein in incomplete Freund's adjuvant. This anti-PATE antisera could detect as little as 50pg of PATE protein in western blot analysis. The rabbit and mouse pAbs against PATE-B and PATE-M, respectively, were produced in Dr Wreschner's laboratory in Tel-Aviv University, Israel. Anti-PATE-B pAbs were prepared by immunizing rabbits with recombinant whole PATE-B protein, synthesized in and secreted by human embryonic kidney cells (HK293) that had been stably transfected with a eukaryotic expression vector comprising DNA coding for the fusion protein PATE-B-hFc. The secreted PATE-B-hFc fusion protein was then purified from the spent medium of these cells by affinity chromatography on protein-sepharose and used to immunize the rabbits by means of an immunization protocol similar to that described above for the PATE protein. Analysis of the obtained polyclonal anti-PATE-B antisera showed recognition of the PATE-B protein down to a dilution of at least 1:3000. For the generation of anti-PATE-M polyclonal Abs, mice were immunized (as above) with recombinant bacterial human PATE-M protein. This PATE-M protein comprises sequences coded by exon 1 and exon 3 and is designated PATE-M small (Levitin et al., 2008). Analysis of the obtained Abs showed recognition and specificity for both the complete human PATE-M protein (exons 1, 2 and 3) and PATE-M small, down to a 32 000-fold dilution of the anti-sera. Affinity-purified anti-PATE-M Abs were used for some experiments, which were obtained by the passage of the polyclonal anti-PATE-M antisera through an Affigel-10 (BioRad) column to which human PATE-M-hFc fusion protein had been covalently linked.
Immunofluorescent staining of ejaculated sperm cells and testicular biopsies
Ejaculated fresh or frozen human sperm cells were isolated by the Isolate kit (Irvine Scientific) followed by centrifugation for 15 min at 400g. The sperm cells were diluted in PBS and centrifuged for 10 min at 400g to discard seminal plasma, and resuspended in PBS. This step was performed three times. For the investigation of sperm cells from ejaculates, 15-µl smears containing ∼200 000 cells were made on a clean slide and left to dry for 10 min. Examination of cells from testicular biopsies involved a wet cytological smear of the biopsy sample which was carried out as follows. Testicular tissue was shredded and the released cells were washed and suspended in sucrose (0.1-M BDH, Dorest, UK), after which they were spread on microscope slides layered with paraformaldehyde solution at pH 9.2 (Fluka, Bosch, Switzerland) and Triton X-100 (Sigma, St. Louis, MO, USA). The slides were washed twice with PBS, the cells were fixed in 4% formaldehyde for 60 min, washed twice with PBS and blocked for 30 min in 3% bovine serum albumin. The slides were washed twice with PBS and incubated with 50-µl primary Ab overnight at 4°C in a humid chamber. After incubation, the slides were washed four times with PBS and rhodamine-conjugated secondary Ab goat anti-rabbit immunoglobulin G Alexa Fluor 568 (Sigma; for PATE and PATE-B primary Ab) or goat-anti-mouse (Jackson; for PATE-M primary Ab) were applied in 50 µl (1:100 dilution) and incubated for 1 h at 25°C in a humid chamber in the dark. They were then washed three times with PBS, the sperm acrosome was stained with 6% Pisum sativum agglutinin fluorescein isothiocyanate (PSA-FITC) and counterstained for 1 h at 25°C in a humid chamber in the dark. The slides were then washed three times with PBS and once with distilled water and left to air dry. 4′,6-Diamidino-2-phenylindole (DAPI; 16 µl) supplemented with anti-fading reagent (Vysis, Inc., IL, USA) was applied for nucleus staining and a coverslip was placed on the sperm smear and left overnight at 4°C, after which the samples were observed with a fluorescent microscope. At least 600 sperm cells were counted in each specimen.
A dual staining of sperm cells was performed as described above to determine the presence and localization of PATE-like proteins in capacitated, acrosome-intact and acrosome-reacted sperm cells. Fresh ejaculate was divided into three portions. The first tube contained untreated ejaculated sperm cells 30 min after ejaculation (0 h), the second tube contained sperm cells incubated for 4 h in HTF containing 3% HSA (capacitating treatment labeled ‘4 h no ionophore’) and the third tube contained sperm cells incubated for 3 h in HTF medium with 3% HSA followed by 1 h in 5 μM A23187 calcium ionophore (Sigma) in HTF at 37°C (labeled ‘4 h ionophore-treated’). Each test was performed independently in duplicate in the samples from three different donors, and at least 600 sperm cells were counted on each slide.
When testicular biopsies were used, 5 μm sections of paraffin-embedded testicular tissue were mounted on slides, deparaffinized and heated to induce antigen retrieval at a controlled temperature in a microwave processor in 10-mM citrate buffer, pH 6, for 5 min at 97°C. The slides were immunostained using the above-mentioned sperm immunostaining protocol.
Immunohistochemistry
Immunohistochemical staining of PATE-like proteins was performed on 24 biopsies using a three-step indirect process. Sections of paraffin-embedded testicular biopsies fixed in Bouin's solution were processed by the labeled-(strept) avidin–biotin peroxidase complex method. The pAbs against PATE and PATE-M were used as primary Abs. Immunohistochemistry was performed using the Histostain Plus Broad Spectrum kit (Invitrogen, CA, USA). This kit uses biotinylated secondary Abs to locate the bound primary Ab, followed by the binding of streptavidin horse-radish peroxidase conjugate. The complex is visualized with hydrogen peroxidase substrate and 3,30-diaminobenzidine (DAB) tetrahydrochloride chromogen, which produces a dark brown precipitate that is readily detected by light microscopy. The sections were counterstained with Mayer's hematoxylin, dehydrated and mounted for microscopic examination.
Human hemizona assay
Unfertilized oocytes from failed IVF procedures were rinsed three times in the control medium, and then cut using Leitz micromanipulators. The matching hemizonae were separately co-incubated (4 h, 37°C) in 50-µl droplets containing spermatozoa derived from samples preincubated in 1:50 or 1:200 dilutions of Abs against PATE. One control included the sibling hemizona which was incubated with untreated sperm cells, and this represented a score of 100% to which all the other treatments were compared. The other control included sperm cells that were incubated with 1:50 and 1:200 dilutions of pre-immune rabbit serum. Four zona pellucidas were bisected and incubated with the pre-immune rabbit serum as well as with the untreated sperm cells. After the co-incubation period, the hemizonae were vigorously pipetted to dislodge loosely attached sperm cells. The number of the tightly bound sperm cells was counted twice using a phase contrast microscope (Gamzu et al., 1994). The results are given as a hemizona index (HZI) of treated sperm attached to the hemizona divided by the number of untreated sperm bound to the sibling hemizona (expressed as percentage ± SD).
Zona-free HEPT
The sperm penetration assay, or zona-free hamster oocyte penetration assay, measures the ability of sperm to undergo capacitation, acrosome reaction, fusion–penetration through the oolemma, and decondensation within the cytoplasm of an oocyte (Samplaski et al., 2010). The effect of Abs against PATE on sperm/oocyte fusion was evaluated by HEPT as previously described (Samuel et al., 1987). Briefly, fresh human semen obtained from known fertile donors was mixed with an equal volume of TEST yolk buffer (Irvine Scientific), incubated for 10 min at 37°C, cooled at room temperature and transferred overnight to 4°C. Sperm sedimentation took place during 22 h in vertical tubes and the clear supernatant was then discarded. Sedimented sperm cells were washed and resuspended in Biggers Whitten Whittingham (BWW) medium. Abs against PATE were added to tubes containing sperm at 1:20 and 1:100 dilutions. Another tube containing sperm without Ab was used as a control. The BWW medium (100 µl) was carefully added to each tube, and all tubes were incubated at 37°C at an angle of ∼30° for ∼1.5 h to allow the sperm to swim-up. The upper 100 µl were collected from each tube, washed with BWW and resuspended, giving a final suspension of 5 million motile spermatozoa per ml (according to the WHO criteria, 2010). Standard procedures were utilized for the recruitment of hamster oocytes. Between 20 and 25 freshly prepared zona-free eggs were added to each drop of 100 µl sperm cells. The drops were covered with paraffin oil and incubated under identical conditions for an additional 2 h. The eggs were collected, washed three times in drops of BWW and mounted on slides. The eggs were flattened and fixed with 2–5% glutaraldehyde for 1 min, ethyl alcohol 95% for 1 min and stained with acetolacmoid stain [0.5% Lacmoid stain (Sigma) in 45% acetic acid] for 1 min. They were then examined under a phase contrast microscope. A swelling sperm head was the criteria for fertilization. The number of attached sperm cells and the number of swollen sperm heads in each egg were counted. The percentage of ova penetrated by spermatozoa (penetration rate, PR) and the number of sperm penetrations per oocyte (penetration index, PI) were recorded.
Statistical analysis
Data are expressed as mean ± SD. The different groups of HZA were compared by the paired sample t-test. In the HEPT, the PR was compared by the Pearson χ2 test followed by the Fisher's exact test. The comparison between different groups of PI and attachment was evaluated by the non-parametric Kruskal–Wallis test followed by the Mann–Whitney test. A P value <0.05 was considered as significant. All statistics were performed at the Statistical Department of Tel Aviv University using SPSS version 15.0 software package.
Results
RNA expression of PATE-like genes in testicular biopsies
The histological classification of the testicular biopsies was reinforced by the assessment of expressed germ cell-specific markers (RBM1, DAZ, CDY2, BOULE and CDY). Biopsies with Sertoli cells only did not express any of these markers. PATE and PATE-M were expressed almost exclusively in biopsies with germ cells, as mRNA was present in only a few biopsies containing Sertoli-only cells. Weak expression of PATE-B (a pale band in RT–PCR) was detected in a few biopsies with normal spermatogenesis (two of six samples) even after testing with three different sets of primers and increasing the number of cycles to 40 (Table I).
Table I.
Detection of mRNA for the prostate and testis expression (PATE)-like genes by RT–PCR in 95 human testicular biopsies, categorized according to histological pattern.
| Gene | Pathology |
|||
|---|---|---|---|---|
| Obstructive (normal) (n = 17, %) | Focal spermatogenesis (n = 36, %) | Maturation arrest (n = 8, %) | Sertoli cell only (n = 34, %) | |
| PATE | 100 | 78 | 100 | 3 |
| PATE-M | 100 | 72 | 100 | 9 |
| aPATE-B | 33a | 0a | Not tested | 0a |
The percentage of biopsies expressing the PATE-like genes was calculated for each category.
n, number of specimens.
aTested in 6 obstructive, 23 focal spermatogenesis and 2 Sertoli cell-only specimens.
Expression and localization of PATE-like proteins in testicular tissue
Twenty-four biopsies underwent immunohistochemistry and immunofluorescence staining tests with available Abs for PATE, PATE-M and PATE-B proteins. Ten of them were defined as obstructive azoospermia, three as maturation arrest, seven as focal spermatogenesis and four as Sertoli cell-only azoospermia. Similar to the RNA results, PATE and PATE-M proteins were detected in germ cells during spermatogenesis but not in somatic cells (Sertoli cells). These proteins were immunohistochemically detected in the cytoplasm of spermatogonia and spermatocytes, as well as in all stages of spermatid cells (Fig. 1). Immunofluorescence staining of PATE and PATE-M proteins revealed cytoplasmic staining in circles around the nucleus in most cells and staining in the spermatid cells (data not shown). Low background staining was demonstrated when the biopsies were analyzed for autofluorescence when only a secondary Ab was used.
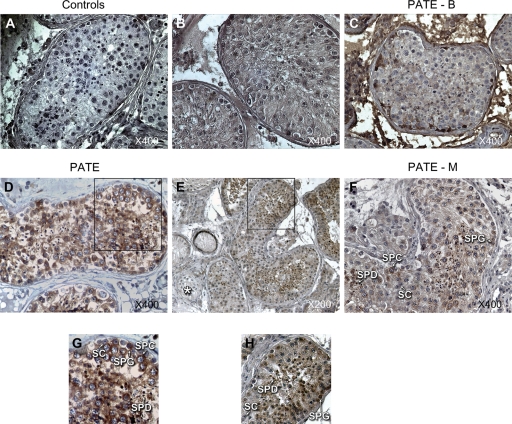
Figure 1.
Immunohistochemistry staining of prostate and testis expression (PATE) and PATE-M proteins in paraffin-embedded human testicular biopsy. DAB staining generated a brown color at the position of the Ab. (A–D) and (F) Testicular section of obstructive azoospermia with complete spermatogenesis, magnification of ×400. (A) Control for immunostaining without any primary rabbit Ab. (B) Control with pre-immune rabbit serum as primary Ab. Similar results were observed with pre-immune mouse serum (not shown). (C) Anti-PATE-B staining of a testicular section showing the absence of expression of the PATE-B protein. (D) Anti-PATE detected PATE protein in all stages of spermatogenesis. Note that Sertoli cells (SC) are uniformly negative for the PATE protein. (E) Staining of a testicular section of a focal spermatogenesis sample with anti-PATE antibodies showing the expression of PATE protein in the cytoplasm of spermatogonia (SPG) and spermatocytes (SPC) and in all stages of spermatids (SPD) but not in tubules containing only SC (asterisk at bottom left of E), magnification of ×200. (F) Anti-PATE-M staining showing the expression of PATE-M protein in the cytoplasm of SPG and SPC and in SPD but not in SC. (G) and (H) indicate the square insets of (D and E).
PATE-B protein could not be detected with the rabbit anti-PATE-B antisera in the paraffin-embedded biopsies by immunohistochemistry (Fig. 1) or by immunofluorescence staining methods (data not shown). This suggests that the PATE-B protein is either not present in testis or that it is present at very low levels in testicular cells. This finding correlates well with our findings of PATE-B expression as analyzed by RT–PCR in 23 biopsies derived from testicular samples that showed focal spermatogenesis. None of these samples demonstrated the presence of PATE-B mRNA by RT–PCR, whereas PATE and PATE-M mRNA was detected in ∼75% of such cases (Table I). Furthermore, the anti-PATE-B rabbit antisera was used at a concentration (sera dilution of 1:100) 25-fold higher than that used for anti-PATE sera to detect the PATE protein (sera dilution 1:2500). The absence of any staining at this high concentration of the anti-PATE-B antisera additionally serves as a convenient control for the specificity of the anti-PATE antisera that readily detects the PATE protein in all the germ cells.
Expression and localization of PATE-like proteins in testicular cytology
Immunofluorescence staining was performed on isolated cells from three testicular biopsies (taken during TESE procedures in obstructive azoospermic men). The staining pattern (Fig. 2) was similar in the three patients analyzed. This test confirmed previous results that localized the PATE and PATE-M proteins to the cytoplasm of spermatocytes and verified the specific localization of these proteins in different stages of spermatid cell development. When considering all cells (a total of 600–1000 cells) present in the examined fields, the percentage of stained cells was 85, 50 and 2% for PATE, PATE-M and PATE-B proteins, respectively. After the preparation, the majority of cells observed in these fields are germ cells and some are Sertoli cells. Nevertheless, it is difficult to identify all the different kinds of germ cells that are present. The PATE and PATE-M proteins were present in all steps of spermatid cell development, i.e. in the cytoplasm and around the region later destined to be the acrosome, in the early stages, and in a band-like pattern around the acrosome in the later stages. This localization of the PATE-M protein to the cytoplasm correlated with the immunohistochemical analyses (Fig. 1), that also showed the protein to be present solely in the cytoplasm of the cell. As whole cell mounts were used for these analyses (Fig. 2), the cytoplasmic contents of the cell encompass the nucleus of the cell, giving the impression of nuclear-localized PATE-M protein. However, our extensive investigations using many different focal planes supported cytoplasmic, and not nuclear, localization of all the PATE-like proteins. Entire Sertoli cells could not be detected using this technique: Sertoli cell nuclei were observed only in slides stained for PATE-M protein and, as expected, no expression of this protein was detected. Only a few testicular cells (2% stained cells) of the specimens expressed PATE-B protein. In such cells PATE-B protein was observed in the cytoplasm of spermatocytes and around the acrosome of the spermatids.
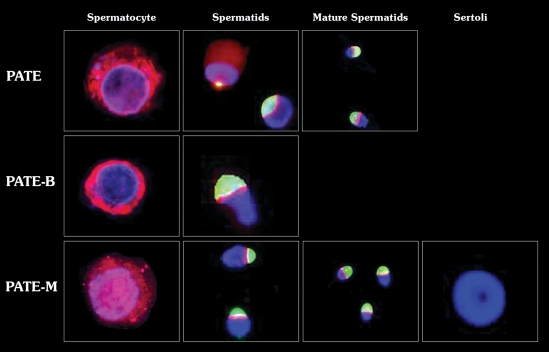
Figure 2.
PATE, PATE-B and PATE-M proteins localization in germ cells isolated from human testicular tissue. PATE proteins were stained with rhodamine-conjugated secondary Ab (red), the acrosome with Pisum sativum agglutinin fluorescein isothiocyanate (PSA–FITC, green) and the nucleus with 4′,6-diamidino-2-phenylindole DAPI (blue). The percentage of stain cells was 85, 50 and 2% for PATE, PATE-M and PATE-B proteins, respectively. Staining of PATE and PATE M proteins was detected in the cytoplasm of spermatocytes and spermatids and around the acrosome in different stages of spermatids. PATE-B protein was observed in the cytoplasm of spermatocytes and around the acrosome of the spermatids. Magnification ×1000.
Localization of PATE-like proteins on ejaculated sperm cells
PATE-like proteins were immunostained to confirm that the PATE protein is associated with human sperm as well as to determine its location on sperm. PATE and PATE-M proteins were localized in a band-like pattern lying in the equatorial segment of the sperm head, and PATE-B proteins appeared in a band-like pattern in the post-acrosomal region of the sperm head (Fig. 3). Only second Ab was used as a negative control. PATE, PATE-B and PATE-M proteins were detected in 90, 80 and 50% of the counted sperm cells, respectively. A dual staining of acrosomal cap (PSA-FITC) and PATE-like proteins (rhodamine-conjugated secondary Ab) enabled us to further identify the specific localization of the PATE-like proteins in the acrosome intact (AI) and acrosome-reacted (AR) sperm cells (Fig. 3). It should be noted that induction of the acrosome reaction does not result in the complete simultaneous removal of the acrosome from all such treated sperm cells, and that some cells display intermediate PSA-FITC levels. Sperm cells showing more than two-thirds of the acrosome without fluorescence or with a fluorescing band at the equatorial segment were considered to be AR (Risopatrón et al., 2001). A band-like pattern was detected at the equatorial zone for the PATE and PATE-M proteins in both the AR and the AI sperm cells, whereas this pattern was seen at the post-acrosomal region for the PATE-B protein. The number of sperm cells stained at the equatorial zone (PATE and PATE-M) and at the post-acrosomal region (PATE-B) was counted in AR and in AI sperm cells. The same staining pattern was detected in both AI and AR sperm cells, for all three PATE-like proteins tested. The expression and localization patterns of PATE-like genes and proteins in testis and ejaculate are summarized in Table II.
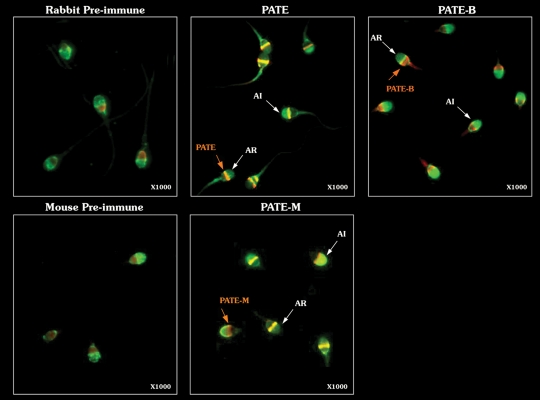
Figure 3.
Expression and localization of PATE, PATE-B, PATE-M on acrosome intact (AI) and acrosome-reacted (AR)-ejaculated human sperm cells. Sperm cells were incubated with polyclonal antibodies directed against each PATE-like protein and with PSA-FITC (green color) for acrosome staining. Antibodies that bound the PATE-like proteins were detected with rhodamine-conjugated secondary Ab (red). The acrosome was considered to be intact when the anterior half of the head of a sperm cell was fluorescent bright green. Relevant negative controls (pre-immune sera) for the rabbit anti-sera and mouse anti-sera are shown. A band-like pattern was detected in the equatorial zone for PATE and PATE-M and at the post-acrosomal region for PATE-B, in AR and in the AI sperm cells. No similar staining of PATE-like proteins was seen in the control cells, and only a non-specific smear was occasionally seen in the heads of control sperm cells. Magnification ×1000.
Table II.
Summary of gene expression and protein localization of the PATE-like family in human testis.
| Gene | RNA expression in testis | Protein localization in testicular germ cells (% of stained testicular cells) | Protein localization in ejaculated sperm cells (% of stained sperm cells) |
|---|---|---|---|
| PATE | In germ cells | Cytoplasm of spermatogonia and spermatocyte. Equatorial segment of spermatids (85%) | Equatorial segment (90%) |
| PATE-M | In germ cells | Cytoplasm of spermatogonia and spermatocyte. Equatorial segment of spermatids (50%) | Equatorial segment (50%) |
| PATE-B | Present in only a few biopsies | Cytoplasm of spermatocytes. Equatorial segment of spermatids (2%) | Post-acrosomal segment (80%) |
PATE was chosen for further study in sperm–zona binding and sperm–oolemma binding tests because it was the most widely expressed among the PATE-like genes in germ cells and spermatozoa and because sufficient amount of Ab was available.
Polyclonal Ab against the PATE protein and sperm–zona binding in the HZA
The role of the PATE protein during fertilization was assessed by an HZA. The test was repeated independently three times with three different donors and was performed in duplicate or triplicate (18 oocytes). The average HZI was 89.6 ± 10% in sperm treated with a 1:50 dilution of pAb against PATE, and 87 ± 36% in sperm treated with a 1:200 dilution of the same Ab. Sperm cells incubated with the pre-immune rabbit serum showed an average HZI of 128 ± 53% of zona pellucida binding ability compared with the untreated sperm cells. As such, there were no significant differences between the treated sperm cells and either the control untreated sperm cells or the pre-immune rabbit serum-treated sperm cells, and between the Ab diluted 1:50- and 1:200-treated sperm cells. Furthermore, these experiments were repeated with an additional 20 oocytes using dilutions of anti-PATE pAb ranging from 1:10 to 1:800. These experiments showed no effect on the binding of sperm cells to the hemizona, even at the very highest concentration of pAb (1:10). This clearly demonstrates that there is no effect of the Ab on the sperm-cell binding ability.
Polyclonal Ab against the PATE protein and sperm–oolemma binding in the zona-free HEPT
The sperm were incubated with various dilutions of pAb against PATE. The percentage of hamster oolemma which were penetrated by spermatozoa (PR) and the average number of sperm penetrations per oolemma (PI) were measured. While the sperm PR was 95.6% without pAb, the rates decreased significantly (Pearson χ2, P< 0.001) and in a dose-dependent fashion after prior incubation of sperm with pAb against PATE (1:100 = 87.5%; 1:20, 32.1%). The PI also decreased significantly (Kruskal–Wallis test, P< 0.001) following incubation of sperm with pAb against PATE (without Ab 7.3 ± 3.9; Ab dilution of 1:100, 4 ± 3.5; Ab dilution of 1:20, 0.6 ± 1.2). The average number (±SD) of sperm cells attached per oolemma did not differ significantly between the control and the pAb-treated groups [without Ab 37.4 ± 36.4 (n = 23); 1:100, 46.8 ± 32.1 (n = 8]; 1:20, 45.4 ± 36.1 (n = 28)].
Discussion
Resolving the molecular basis of sperm–egg interaction is of immense strategic importance to the development of novel methods for fertility regulation and for the diagnosis of human infertility. As part of the global joint effort to understand this interaction, we focused our attention on the novel PATE-like family (Levitin et al., 2008). One member of this family, the PATE protein, is localized in sperm cells and, as such, may be involved in sperm–oocyte interaction. In the present study, we report that the PATE and PATE-M genes were expressed in testicular germ cells and PATE-B gene was expressed rarely and at much lower levels. The respective proteins were present in the head of ejaculated spermatozoa. PATE and PATE-M were localized to the equatorial segment and PATE-B to the post-acrosomal region of ejaculated spermatozoa and this localization did not change following the acrosome reaction. Moreover, the PATE protein appeared to be involved in sperm–oolemma fusion but not in earlier steps of fertilization, such as sperm–zona pellucida binding, acrosome reaction and sperm–oolemma attachment.
PATE mRNA was reportedly expressed in tissues of the male genital tract, including the prostate, testis, epididymis and seminal vesicle (Soler-Garcıá et al., 2005). In the present study, the expression of the PATE and PATE-M genes was detected in only a few biopsies that were deprived of germ cells, and contained only somatic cells (Sertoli cell only; Table I). The exceptions (e.g. 1 of the 34 samples categorized as Sertoli cell only for PATE, and 3 samples for PATE-M) in gene expression could have resulted from the non-homogenous distribution of germ cells in human testis, such that one small biopsy could be depleted of germ cells while another biopsy from the same testis may contain a few germ cells (Silber et al., 1997). The absence of PATE mRNAs in some specimens with focal spermatogenesis may be attributed to the same reason. The absence of germ cell-specific markers (RBM1, DAZ, CDY2, BOULE and CDY) in Sertoli-cell-only biopsies and their presence in focal spermatogenesis biopsies reinforce the histological classification and minimize any possible misclassification. Such seldom occurring atypical expression had been observed previously with some testicular genes (Kleiman et al., 2007, 2011). Assessment of the level of expression by quantitative RT–PCR may have clarified if the expression of PATE-M observed in the three Sertoli cell-only specimens was caused by very low levels of transcript. Low levels, close to the basal level, of expression are seldom detected by regular RT–PCR (Kleiman et al., 2011).
In agreement with the very low expression of PATE-B previously observed in testis by northern blot and RT–PCR (Levitin et al., 2008), PATE-B gene was barely detected by RT–PCR in 2 of the 6 biopsies with normal spermatogenesis and in none of the 23 with hypospermatogenesis. In accordance with this, PATE-B protein was detected only in very few testicular germ cells (Table II).
In situ hybridization of PATE mRNA in testis indicated its predominant expression in the spermatogonia, leading to the hypothesis that the PATE protein may be involved in spermatogenesis, in addition to its association with sperm cells (Soler-Garcıá et al., 2005). Our current histological and cytological studies demonstrated that PATE and PATE-M proteins are chiefly expressed in germ cells and localized the proteins in the cytoplasm of spermatogonia, spermatocytes and spermatids, as well as in a band-like pattern around the forming acrosome in all stages of spermatids and sperm cells (Figs. 1 and 2). The expression of these proteins in all stages of germ cell development is suggestive of their possible general function during spermatogenesis. However, PATE-B protein was detected only in sporadic germ cells by cytological immunofluorescence studies on testes but not in paraffin-embedded sections, probably owing to its low level of expression in testes (almost undetectable by RT–PCR) and the lower sensitivity of immunohistochemistry method in comparison to immunofluorescence.
In ejaculated sperm cells, PATE and PATE-M proteins are present in the equatorial segment, while PATE-B is present in the post-acrosomal region of the sperm head. The discrepancy between the subcellular localization of PATE/PATE-M and PATE-B suggests that these highly homologous proteins may have different functions in sperm cells. The localization of PATE-B was originally reported as being in the acrosome (Levitin et al., 2008). Our studies repeatedly observed post-acrosomal localization in 80% of the sperm cells, and not in the acrosome, counted in three different normozoospermic men. Its low level in testes in contrast with the high percentage of ejaculated sperm cells containing PATE-B protein suggest that it might be secreted by sexual accessory tissues into the semen fluid where it becomes associated with sperm cells in a similar manner as proposed for the PATE protein (Soler-Garcıá et al., 2005). Both proteins are secreted from transfected cells into the medium (Soler-Garcıá et al., 2005; Levitin et al., 2008). Interestingly, it has been shown that PATE-B up-regulates the activity of homomeric nAChR7 (nicotinic acetylcholine receptor 7), which is also present in the posterior post-acrosomal region of sperm cells (Kumar and Meizel, 2005; Levitin et al., 2008).
For successful fertilization, the sperm cell undergoes a complex series of steps. It undergoes capacitation, penetrates the cumulus, binds to the zona pellucida, acrosome reacts, penetrates the zona, attaches to the oolemma, undergoes gamete membrane fusion, penetrates the ooplasm and participates in activation of the oocyte (Yanagimachi, 1994; Stein et al., 2004; Rubinstein et al., 2006).
The role of PATE-B protein localized in the post-acrosomal region is unknown at present. Proteins that are localized to the post-acrosomal region could have a role in oocyte activation (Sutovsky et al., 2003), therefore it would be interesting to investigate the involvement of PATE-B in fertilization.
PATE is a sperm-associated secreted protein detected by immunofluorescence in a band-like pattern to the equatorial segment of the sperm head (Soler-Garcıá et al., 2005 and Fig. 3 of the present study). Due to the fact that proteins that are localized to the sperm equatorial segment are known to have a role during fertilization (Evans et al., 1997; Hamatani et al., 2000; Wolkowicz et al., 2008), the PATE protein became a candidate for being involved in fertilization. As expected from its equatorial segment localization on the sperm cell, binding and acrosome reaction assays failed to indicate any involvement of PATE protein in sperm–zona pellucida binding. Indeed, these tests were performed based on the gene and protein similarity between PATE and SP-10, which localizes to the acrosome and equatorial segment of the sperm head, and based on the various reports regarding SP-10 involvement in zona pellucida binding and oolema fusion steps (Foster et al., 1994; Coonrod et al., 1996; Hamatani et al., 2000).
Pre-incubation of sperm cells with an Ab against the PATE protein significantly decreased the average number of sperm cell penetrations per oolemma but had no influence on the number of sperm cells that attached to the oolemma. This may imply that different sets of proteins are involved in the processes of attachment and fusion–penetration, and that the PATE protein may participate only in the fusion –penetration step of fertilization. It should be noted that our results indicating the participation of PATE protein in the process of sperm–oolemma fusion–penetration were obtained with polyclonal antisera directed against the rPATE. These intriguing initial findings obviously need to be examined in more extensive investigations with appropriate negative controls, such as pre-immune sera and the inclusion of soluble competing PATE protein in the assay, as well as the use of highly purified anti-PATE Ab generated by affinity purification procedures.
Analysis of the PATE protein amino acid sequence provided support for its involvement in the sperm–oolemma fusion and penetration steps. The predicted 127 amino acid sequence analysis showed that PATE is a cysteine-rich protein with a secretory phospholipase A2 (sPLA2) motif at the carboxyl terminus (Bera et al., 2002; Soler-Garcıá et al., 2005). The sPLA2 motif is found in a group of enzymes with a conserved catalytic site and Ca2+-binding loop that catalyzes the release of fatty acids and lysophospholipids from glycerophospholipids in the presence of Ca2+ (Masuda et al., 2004). The presence of the phospholipase motif and the low molecular weight of the PATE protein suggest that it could exhibit phospholipase activity (Bera et al., 2002). Masuda et al (2004) evaluated the localization of sPLA2 in the somatic and germ cells of the testis, in the epididymis, vas deferens, seminal vesicles and prostate of mouse male reproductive organs. Possible functions of sPLA2 in mammalian male reproductive organs include (i) production of lipid mediators (Masuda et al., 2004), (ii) regulation of spermatogenesis (Masuda et al., 2004), (iii) regulation of sperm functions, including the acrosome reaction and fertilization (Fleming and Yanagimachi, 1984; Riffo and Parraga, 1996) and (iv) defense against micro-organisms invading these tissues (Buckland et al., 2000). The phospholipase activity may be an important property of the PATE protein, given that mammalian sperm undergo extensive plasma membrane remodeling during maturation in the epididymal duct (Christova et al., 2002), and thus may be involved in both maturation and function of germ cells. Riffo and Párraga (1997) localized PLA2 on the anterior tip of the human sperm head and demonstrated through HEPT experiments that Abs against PLA2 inhibit sperm–oolemma fusion but not sperm–oolemma attachment (as was proposed in the present paper for the functional role of PATE). An additional phospholipase, predominantly localized to the equatorial region of the sperm, was suggested to be involved in oocyte activation (Young et al., 2009).
The functional role of PATE in sperm–egg interaction is further inferred from characterization of the molecular sequence. Both PATE and SP-10 amino acid sequences contain a recognizable TFP/Ly-6/uPAR family motif (Ploug and Ellis, 1994). This family is serologically and structurally related to cell surface proteins, which are anchored to the plasma membrane through a C-terminal glycosyl-phosphatidylinositol (GPI) attachment (McKenzie et al., 1977). They are also related to the protein family members that contain the TFP/Ly-6/uPAR domain but do not comprise a GPI anchor, and are thus likely to be secreted from the cell. The TFP/Ly-6/uPAR family includes complement-binding regulatory protein CD59 (Wang et al., 1995), urokinase-type plasminogen activator (Ohkura et al., 1994) and snake venom postsynaptic neurotoxin (Tsetlin and Hucho, 2004). As a member of the secreted TFP/Ly-6/uPAR protein family, the PATE-like proteins participate in cell–cell interactions.
In conclusion, the PATE protein is expressed on the equatorial segment of human sperm and seems to participate in the steps of sperm–oolemma fusion and penetration, as a member of a set of proteins which are involved in this process. Its function during spermatogenesis and maturation still needs to be assessed. The function of PATE-M and PATE-B has not yet been clarified. The present study provides further data for understanding the expression of PATE-like genes and the localization of the proteins, together with evidence for a function during spermatogenesis and fertilization. Further studies are warranted to clarify the additional functions of the PATE protein in the testis, the role of the other PATE-like proteins in spermatogenesis and fertilization, and to discover their putative ligand(s) in the oolemma.
Authors' roles
M.M. performed the experiments and contributed to the study design, compiled and interpreted the data. L.Y. advised, interpreted and reviewed. H.Y. involved in critical discussion and review of last draft. L.O. critically reviewed the manuscript. H.R. involved in collection of samples and review of the preliminary manuscript. B.A. critically reviewed the last draft of the manuscript. B.S. helped with the immunostaining and camera operation. L.F. helped and advised with the antibodies experiments. W.M. involved in critical discussion. P.I. involved in supply of PATE antibodies and reviewed the manuscript. W. involved in supply of PATE-M and PATE-B antibodies, advised and reviewed the manuscript. G.P. designed and reviewed. S.E.K. designed the study, data analysis and interpretation and drafting of the manuscript.
Funding
The research was carried out under the auspices of the Alan and Ada Selwyn Chair in Clinical Infertility Research and Molecular Medicine (Melbourne, Australia) granted to one of the authors (G.P.). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Conflict of interest
None declared.
Acknowledgements
The authors thank the laboratory technicians Marina Ilatov, of The Institute for the Study of Fertility, Tel Aviv Medical Center, and Sarita Kaufman, Male Infertility and IVF Unit, Assaf Harofeh Medical Center, Zerifin for skillful and excellent technical work and Ilana Galernter (Statistical Department, Tel Aviv University) for expert statistical analysis.
References
- Anderson DJ, Abbott AF, Jack RM. The role of complement component C3b and its receptors in sperm–oocyte interaction. Proc Natl Acad Sci USA. 1993;90:10051–10055. doi: 10.1073/pnas.90.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts EG, Wijchman JG, Jager S, Hoekstra D. Protein involvement in the fusion between the equatorial segment of acrosome-reacted human spermatozoa and liposomes. Biochem J. 1997;325:191–198. doi: 10.1042/bj3250191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera TK, Maitra R, Iavarone C, Salvatore G, Kumar V, Vincent JJ, Sathyanarayana BK, Duray P, Lee BK, Pastan I. PATE, a gene expressed in prostate cancer, normal prostate, and testis, identified by a functional genomic approach. Proc Natl Acad Sci U S A. 2002;99:3058–3063. doi: 10.1073/pnas.052713699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Heeley EL, Wilton DC. Bacterial cell membrane hydrolysis by secreted phospholipases A(2): a major physiological role of human group IIa sPLA(2) involving both bacterial cell wall penetration and interfacial catalysis. Biochimica et Biophysica Acta. 2000;1484:195–206. doi: 10.1016/s1388-1981(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Christova Y, James PS, Cooper TG, Jones R. Lipid diffusion in the plasma membrane of mouse spermatozoa: changes during epididymal maturation, effects of pH, osmotic pressure, and knockout of the c-ros gene. J Androl. 2002;23:384–392. [PubMed] [Google Scholar]
- Coonrod SA, Herr JC, Westhusin ME. Inhibition of bovine fertilization in vitro by antibodies to SP-10. J Reprod Fertil. 1996;107:287–297. doi: 10.1530/jrf.0.1070287. [DOI] [PubMed] [Google Scholar]
- Cuasnicu PS, Gonzalez Echeverria F, Piazza AD, Cameo MS, Blaquier JA. Antibodies against epididymal glycoproteins block fertilizing ability in rat. J Reprod Fertil. 1984;72:467–471. doi: 10.1530/jrf.0.0720467. [DOI] [PubMed] [Google Scholar]
- Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1) Dev Biol. 2008;320:12–18. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerman DA, Myles DG, Primakoff P. A role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell. 2006;10:831–837. doi: 10.1016/j.devcel.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Evans JP, Schultz RM, Kopf GS. Mouse sperm-egg plasma membrane interactions: analysis of roles of egg integrins and the mouse sperm homologue of PH-30 (fertilin) beta. J Cell Sci. 1995;108:3267–3278. doi: 10.1242/jcs.108.10.3267. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kopf GS, Schultz RM. A characterization of the binding of recombinant mouse sperm fertilin beta subunit to mouse eggs: evidence for adhesive activity via an egg beta1 integrin mediated interaction. Dev Biol. 1997;187:79–93. doi: 10.1006/dbio.1997.8611. [DOI] [PubMed] [Google Scholar]
- Fleming AD, Yanagimachi R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J Exp Zool. 1984;229:485–489. doi: 10.1002/jez.1402290317. [DOI] [PubMed] [Google Scholar]
- Foster JA, Klotz KL, Flickinger CJ, Thomas TS, Wright RM, Castillo JR, Herr JC. Human SP-10: acrosomal distribution, processing, and fate after the acrosome reaction. Biol Reprod. 1994;51:1222–1231. doi: 10.1095/biolreprod51.6.1222. [DOI] [PubMed] [Google Scholar]
- Gamzu R, Yogev L, Amit A, Lessing J, Homonnai ZT, Yavetz H. The hemizona assay is of good prognostic value for the ability of sperm to fertilize oocytes in vitro. Fertil Steril. 1994;62:1056–1059. doi: 10.1016/s0015-0282(16)57073-3. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Tanabe K, Kamei K, Sakai N, Yamamoto Y, Yoshimura Y. A monoclonal Ab to human SP-10 inhibits in vitro the binding of human sperm to hamster oolemma but not to human Zona pellucida. Biol Reprod. 2000;62:1201–1208. doi: 10.1095/biolreprod62.5.1201. [DOI] [PubMed] [Google Scholar]
- Herrero MB, Mandal A, Digilio LC, Coonrod SA, Maier B, Herr JC. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm–egg binding and fertilization. Dev Biol. 2005;284:126–142. doi: 10.1016/j.ydbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hoshi K, Sasaki H, Yanagida K, Sato A, Tsuiki A. Localization of fibronectin on the surface of human spermatozoa and relation to the sperm–egg interaction. Fertil Steril. 1994;61:542–547. doi: 10.1016/s0015-0282(16)56590-x. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Nakanishi T, Matsumoto M, Nomura M, Seya T, Okabe M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol Cell Biol. 2003;23:2614–2622. doi: 10.1128/MCB.23.7.2614-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Kleiman SE, Lagziel A, Yogev L, Botchan A, Paz G, Yavetz H. Expression of CDY1 may identify complete spermatogenesis. Fertil Steril. 2001;75:166–173. doi: 10.1016/s0015-0282(00)01639-3. [DOI] [PubMed] [Google Scholar]
- Kleiman SE, Yogev L, Hauser R, Botchan A, Maymon BB, Paz G, Yavetz H. Expression profile of AZF genes in testicular biopsies of azoospermic men. Hum Reprod. 2007;22:151–158. doi: 10.1093/humrep/del341. [DOI] [PubMed] [Google Scholar]
- Kleiman SE, Lehavi O, Hauser R, Botchan A, Paz G, Yavetz H, Yogev L. CDY1 and BOULE transcripts assessed in the same biopsy as predictive markers for successful testicular sperm retrieval. Fertil Steril. 2011;95:2297–2302. doi: 10.1016/j.fertnstert.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kumar P, Meizel S. Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J Biol Chem. 2005;280:25928–25935. doi: 10.1074/jbc.M502435200. [DOI] [PubMed] [Google Scholar]
- Levitin F, Weiss M, Hahn Y, Stern O, Papke RL, Matusik R, Nandana SR, Ziv R, Pichinuk E, Salame S, et al. PATE gene clusters code for multiple, secreted, TFP/Ly-6/uPAR proteins that are expressed in reproductive and neural-rich tissues and possess neuromodulatory activity. J Biol Chem. 2008;283:16928–16939. doi: 10.1074/jbc.M801454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Murakami M, Matsumoto S, Eguchi N, Urade Y, Lambeau G, Gelb MH, Ishikawa Y, Ishii T, Kudo I. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim Biophys Acta. 2004;1686:61–76. doi: 10.1016/j.bbalip.2004.08.017. [DOI] [PubMed] [Google Scholar]
- McKenzie IF, Gardiner J, Cherry M, Snell GD. Lymphocyte antigens: Ly-4, Ly-6, and Ly-7. Transplant Proc. 1977;9:667–669. [PubMed] [Google Scholar]
- Nixon B, Aitkena RJ, McLaughlin E. A. New insights into the molecular mechanisms of sperm–egg interaction. Cell Mol Life Sci. 2007;64:1805–1823. doi: 10.1007/s00018-007-6552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N, Inoue S, Ikeda K, Hayashi K. The two subunits of a phospholipase A2 inhibitor from the plasma of Thailand cobra having structural similarity to urokinase-type plasminogen activator receptor and Ly-6 related proteins. Biochem Biophys Res Commun. 1994;204:1212–1218. doi: 10.1006/bbrc.1994.2592. [DOI] [PubMed] [Google Scholar]
- Ploug M, Ellis V. Structure–function relationships in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom alpha-neurotoxins. FEBS Lett. 1994;349:163–168. doi: 10.1016/0014-5793(94)00674-1. [DOI] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Riffo MS, Parraga M. Study of the acrosome reaction and the fertilizing ability of hamster epididymal cauda spermatozoa treated with antibodies against phospholipase A2 and/or lysophosphatidylcholine. J Exp Zool. 1996;275:459–468. doi: 10.1002/(SICI)1097-010X(19960815)275:6<459::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Riffo MS, Párraga M. Role of phospholipase A2 in mammalian sperm–egg fusion: development of hamster oolemma fusibility by lysophosphatidylcholine. J Exp Zool. 1997;279:81–88. [PubMed] [Google Scholar]
- Risopatrón J, Peña P, Miska W, Sánchez R. Evaluation of the acrosome reaction in human spermatozoa: comparison of cytochemical and fluorescence techniques. Andrologia. 2001;33:63–67. doi: 10.1046/j.1439-0272.2001.00405.x. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Ziyyat A, Wolf J-P, Le Naour F, Boucheix C. The molecular players of sperm–egg fusion in mammals. Semin Cell Dev Biol. 2006;17:254–263. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol. 2010;17:839–847. doi: 10.1111/j.1442-2042.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- Samuel T, Soffer Y, Caspi E. Sperm–egg penetration of human spermatozoa treated with various rabbit antisera to human sperm antigens. Clin Exp Immunol. 1987;67:454–459. [PMC free article] [PubMed] [Google Scholar]
- Silber SJ, Nagy Z, Devroey P, Tournaye H, Van Steirteghem AC. Distribution of spermatogenesis in the testicles of azoospermic men: the presence or absence of spermatids in the testes of men with germinal failure. Hum Reprod. 1997;12:2422–2428. doi: 10.1093/humrep/12.11.2422. [DOI] [PubMed] [Google Scholar]
- Soler-Garcıá AA, Maitra R, Kumar V, Ise T, Nagata S, Beers R, Bera TK, Pastan I. The PATE gene is expressed in the accessory tissues of the human male genital tract and encodes a secreted sperm-associated protein. Reproduction. 2005;129:515–524. doi: 10.1530/rep.1.00576. [DOI] [PubMed] [Google Scholar]
- Stein KK, Primakoff P, Myles D. Sperm–egg fusion: events at the plasma membrane. J Cell Sci. 2004;117:6269–6274. doi: 10.1242/jcs.01598. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech. 2003;61:362–378. doi: 10.1002/jemt.10350. [DOI] [PubMed] [Google Scholar]
- Toshimori K. Maturation of mammalian spermatozoa: modifications of the acrosome and plasma membrane leading to fertilization. Cell Tissue Res. 1998;293:177–187. doi: 10.1007/s004410051110. [DOI] [PubMed] [Google Scholar]
- Tsetlin VI, Hucho F. Snake and snail toxins acting on nicotinic acetylcholine receptors: fundamental aspects and medical applications. FEBS Lett. 2004;557:9–13. doi: 10.1016/s0014-5793(03)01454-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dang J, Johnson LK, Selhamer JJ, Doe WF. Structure of the human urokinase receptor gene and its similarity to CD59 and the Ly-6 family. Eur J Biochem. 1995;227:116–122. doi: 10.1111/j.1432-1033.1995.tb20366.x. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nature Cell Biol. 2001;3:59–64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Wolkowicz MJ, Digilio L, Klotz K, Shetty J, Flickinger CJ, Herr JC. Equatorial segment protein (ESP) is a human alloantigen involved in sperm–egg binding and fusion. J Androl. 2008;29:272–282. doi: 10.2164/jandrol.106.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi Y. Mammalian fertilization. In: Knobil E, Neill JS, editors. The Physiology of Reproduction. New York: Raven Press, Ltd; 1994. pp. 189–317. [Google Scholar]
- Yogev L, Gamzu R, Paz G, Kleiman S, Botchan A, Hauser R, Yavetz H. Pre-freezing sperm preparation does not impair thawed spermatozoa binding to the zona pellucida. Hum Reprod. 1999;14:114–117. doi: 10.1093/humrep/14.1.114. [DOI] [PubMed] [Google Scholar]
- Yogev L, Zeharia E, Kleiman SE, Maymon BB, Hauser R, Botchan A, Yavetz H, Paz G. Use of sex chromosome bivalent pairing in spermatocytes of nonobstructive azoospermic men for the prediction of successful sperm retrieval. Fertil Steril. 2006;86:106–112. doi: 10.1016/j.fertnstert.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Young C, Grasa P, Coward K, Davis LC, Parrington J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril. 2009;91(5 Suppl.):2230–2242. doi: 10.1016/j.fertnstert.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Yuan R, Primakoff P, Myles DG. A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm–egg plasma membrane adhesion and fusion. J Cell Biol. 1997;137:105–112. doi: 10.1083/jcb.137.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]