Abstract
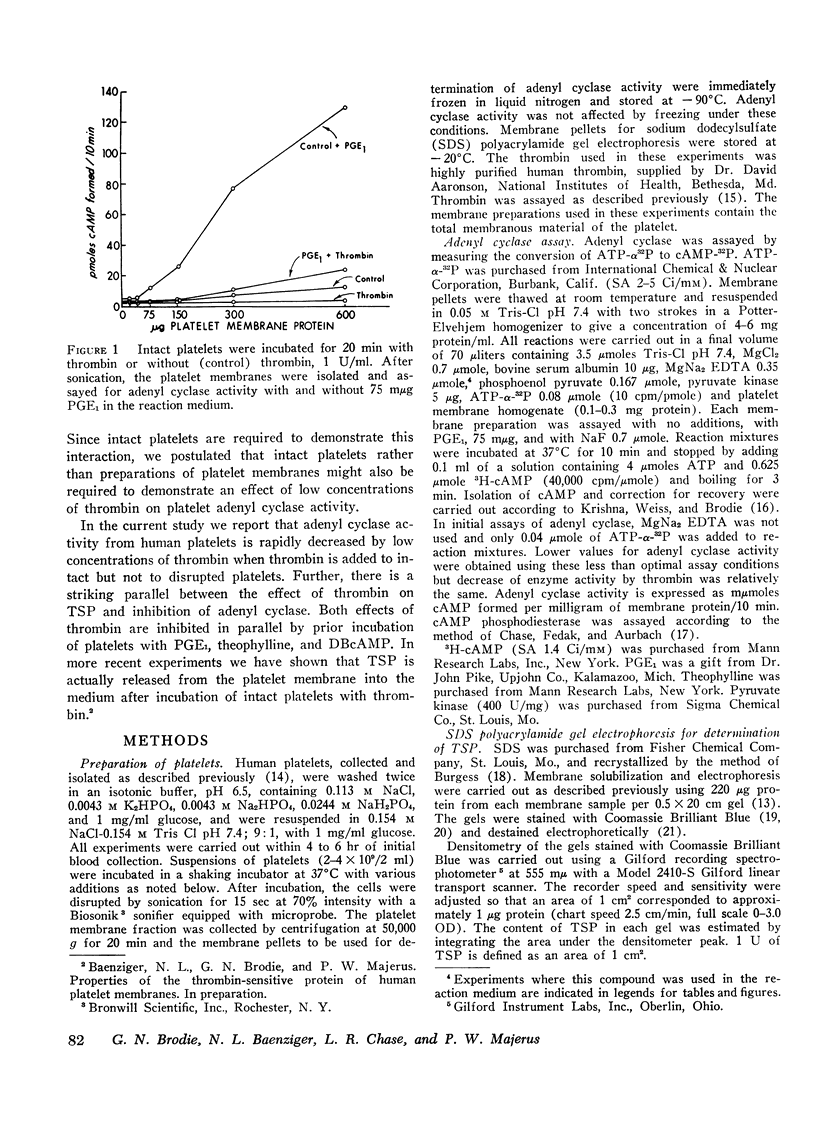
Washed human platelets were incubated with 0.1-1.0 U/ml human thrombin and the effects on adenyl cyclase activity and on a platelet membrane protein (designated thrombin-sensitive protein) were studied. Adenyl cyclase activity was decreased 70-90% when intact platelets were incubated with thrombin. The T½ for loss of adenyl cyclase activity was less than 15 sec at 1 U/ml thrombin. There was no decrease of adenyl cyclase activity when sonicated platelets or isolated membranes were incubated with these concentrations of thrombin. Loss of adenyl cyclase activity was relatively specific since the activities of other platelet membrane enzymes were unaffected by thrombin. Prior incubation of platelets with dibutyryl cyclic adenosine monophosphate (AMP), prostaglandin E1, or theophylline protected adenyl cyclase from inhibition by thrombin.
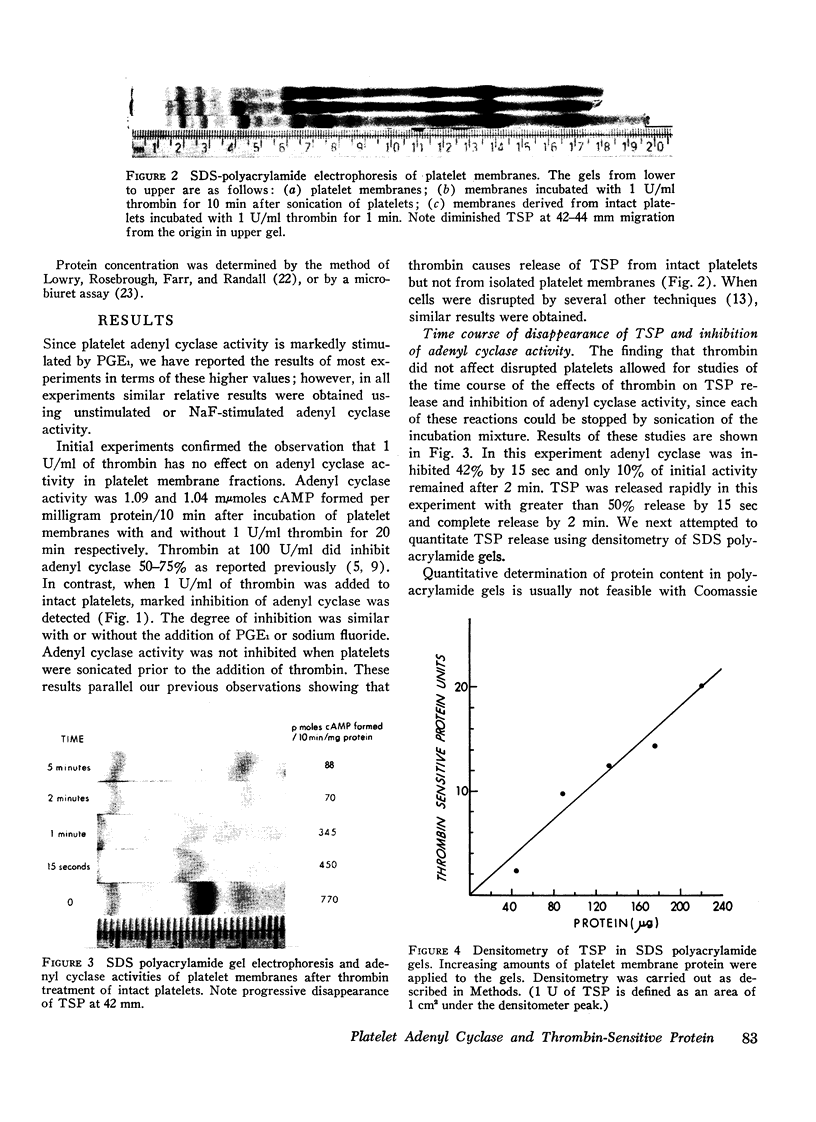
Incubation of intact but not disrupted platelets with thrombin resulted in the release of thrombin-sensitive protein from the platelet membrane. The rapid release of this protein (T½ < 15 sec) at low concentrations of thrombin suggested that removal of thrombin-sensitive protein from the platelet membrane is an integral part of the platelet release reaction. This hypothesis is supported by the parallel effects of thrombin on adenyl cyclase activity and thrombin-sensitive protein release in the presence of dibutyryl cyclic AMP, prostaglandin E1, and theophylline at varying concentrations of thrombin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulla Y. H. Beta-adrenergic receptors in human platelets. J Atheroscler Res. 1969 Mar-Apr;9(2):171–177. doi: 10.1016/s0368-1319(69)80052-9. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Brodie G. N., Majerus P. W. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971 Jan;68(1):240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Chase L. R., Fedak S. A., Aurbach G. D. Activation of skeletal adenyl cyclase by parathyroid hormone in vitro. Endocrinology. 1969 Apr;84(4):761–768. doi: 10.1210/endo-84-4-761. [DOI] [PubMed] [Google Scholar]
- Cohen I., Bohak Z., De VriesA, Katchalski E. Thrombosthenin M. Purification and interaction with thrombin. Eur J Biochem. 1969 Sep;10(2):388–394. doi: 10.1111/j.1432-1033.1969.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Emmons P. R., Hampton J. R., Harrison M. J., Honour A. J., Mitchell J. R. Effect of prostaglandin E1 on platelet behaviour in vitro and in vivo. Br Med J. 1967 May 20;2(5550):468–472. doi: 10.1136/bmj.2.5550.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- KOERNER J. F., SINSHEIMER R. L. A deoxyribonuclease from calf spleen. II. Mode of action. J Biol Chem. 1957 Oct;228(2):1049–1062. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis N., Majerus P. W. Lipid metabolism in human platelets. II. De novo phospholipid synthesis and the effect of thrombin on the pattern of synthesis. J Clin Invest. 1969 Nov;48(11):2114–2123. doi: 10.1172/JCI106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Smith M. B., Clamon G. H. Lipid metabolism in human platelets. I. Evidence for a complete fatty acid synthesizing system. J Clin Invest. 1969 Jan;48(1):156–164. doi: 10.1172/JCI105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis N. R., Vigdahl R. L., Tavormina P. A. Platelet aggregation. I. Regulation by cyclic AMP and prostaglandin E1. Biochem Biophys Res Commun. 1969 Sep 10;36(6):965–972. doi: 10.1016/0006-291x(69)90298-8. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Levine L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. II. Effect of N6-2'-o-dibutyryl cyclic 3',5'-adenosine monophosphate on platelet function. J Clin Invest. 1971 Jan;50(1):131–141. doi: 10.1172/JCI106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman E. W., Neri L. L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. Nature. 1969 Nov 8;224(5219):609–610. doi: 10.1038/224609a0. [DOI] [PubMed] [Google Scholar]
- Scott R. E. Effects of prostaglandins, epinephrine and NaF on human leukocyte, platelet and liver adenyl cyclase. Blood. 1970 Apr;35(4):514–516. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Ward S. An improved transverse destaining apparatus for acrylamide gels. Anal Biochem. 1970 Feb;33(2):259–262. doi: 10.1016/0003-2697(70)90295-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Inhibition of platelet energy production and release reaction by PGE1, theophylline and cAMP. Biochem Biophys Res Commun. 1970 Oct 9;41(1):128–134. doi: 10.1016/0006-291x(70)90478-x. [DOI] [PubMed] [Google Scholar]
- Zieve P. D., Greenough W. B., 3rd Adenyl cyclase in human platelets: activity and responsiveness. Biochem Biophys Res Commun. 1969 May 22;35(4):462–466. doi: 10.1016/0006-291x(69)90368-4. [DOI] [PubMed] [Google Scholar]




