Abstract
Cell polarity is a fundamental aspect of eukaryotic cells. A central question for cell biologists is how the polarity of a cell is established and maintained. Root hairs are exceptionally polarized structures formed from specific root epidermal cells. The morphogenesis of root hairs is characterized by the localized cell growth in a small dome at the tip of the hair, a process called tip growth. Root hairs are thus an attractive model system to study the establishment and maintenance of cell polarity in eukaryotes. Research on Arabidopsis root hairs has identified a plethora of molecular and cellular components that are important for root hair tip growth. Recently, studies on RHD3 and Atlastin have revealed a surprising similarity with respect to the role of the tubular ER network in tip growth of root hairs in plants and the axonal outgrowth of corticospinal neurons in neurological disorders known as hereditary spastic paraplegia (HSP). In this mini-review, we highlight recent progress in understanding of the function and regulation of RHD3 in the generation of the tubular ER network and discussed ways in which RHD3 could be involved in the establishment and maintenance of root hair tip growth.
Keywords: Golgi distribution, polarized trafficking, RHD3/Atlastin, Root hairs, tubular ER
Root hairs are single, tubular-shaped cells formed from specific root epidermal cells. They are exceptionally polarized structures whose morphogenesis is characterized by the localized growth in a small dome at the tip of the hair, a process called tip growth. The tip growth of root hairs resembles the neuronal outgrowth of neurons at both morphological and subcellular levels.1,2 Therefore root hairs are an attractive model system for studying the establishment and maintenance of localized cell growth in eukaryotes.
Studies on Arabidopsis root hairs have led to the identification of a plethora of cellular components and machineries important for root hair tip growth. Those include dynamic actin and microtubule cytoskeleton,3-6 coordinated exocytic and endocytic vesicle trafficking in the apical dome,7,8 properly modified cellular membranes and extracellular cell wall matrix.9-13 Furthermore, an elaborated network of signaling molecules, such as reactive oxygen species (ROS),14 calcium,15,16 and phosphoinositides17,18 is also implicated in root hair tip growth. It is clear that polarized cell growth in root hairs is tightly regulated.
RHD3 Regulates Homotypic Fusion of ER Tubules
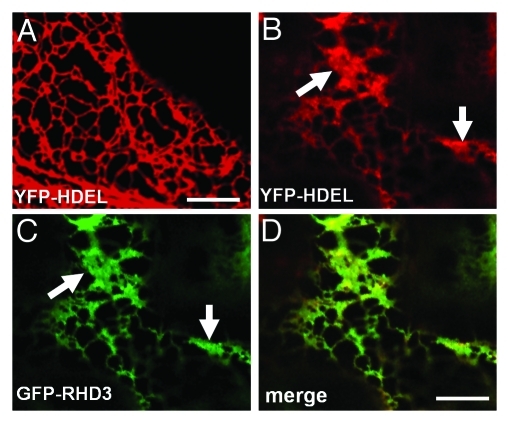
RHD3 is a protein isolated in a genetic screen for mutants defective in root hair development.19 In rhd3 mutants, the root hairs are short and wavy (19). RHD3 is a plant member of dynamin-like Atlastin GTPases.20 In humans, improper alterations in Atlastin-1 frequently cause hereditary spastic paraplegia (HSP),21 a group of neurological disorders in which the development of the long axons of corticospinal neurons is affected.22 The morphology of the long axons of corticospinal neurons in HSPs is reminiscent of short and wavy root hairs in rhd3 mutants.19,22 Three recent studies revealed that both RHD3 and Atlastin-1 play a similar role in the generation of the interconnected ER tubules20,23,24 in plants and animals, respectively. In Drosophila, overexpression of Atlastin induces the formation of aberrant ER sheets.24 It was proposed that Atlastin proteins regulate homotypic fusion of ER tubules.24 Similarly, when transiently expressed with a high OD600 = 0.3, RHD3 was also able to induce aberrant ER sheets (Fig. 1). It seems that, similar to animal members of the Atlastin GTPase class, RHD3 also mediates homotypic fusion of ER tubules in plant cells.
Figure 1.
Transient expression of GFP-RHD3 with a high optical density of agrobacterium (OD600 = 0.3) induces aberrant ER sheets. (A) A confocal microscopy of a tobacco epidermal cell expressing YFP-HDEL alone (OD600 = 0.01). Note a fine polygonal network of the ER is highlighted. Scale bar = 10 μm. (B-D) A confocal microscopy of a tobacco epidermal cell co-expressing YFP-HDEL (OD600 = 0.01) (B) and GFP-RHD3 (OD = 0.3) (C). Note aberrant ER sheets are highlighted by YFP-HDEL and GFP-RHD3 (arrows). (D) is a merged image of (B) and (C). Scale bar in (D) for (B-D) = 10 μm. Transient expression was conducted according to Chen et al. (2011).23
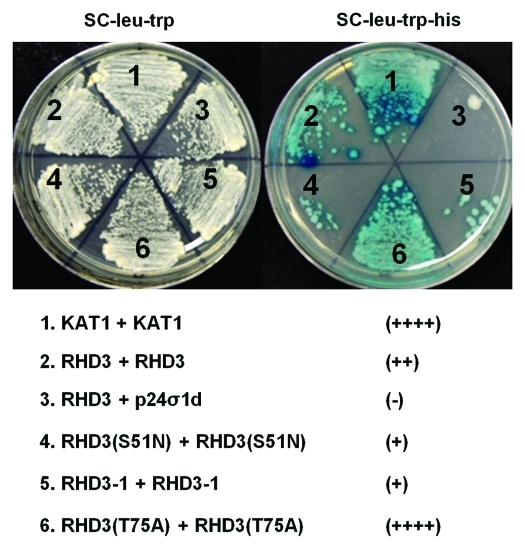
All Atlastin GTPases contain classic GTP signature motifs characteristic of dynamin GTPases.20 They also possess a conserved coiled-coil middle domain and two transmembrane domains at the C-terminus.20 Recombinant Atlastin-1 molecules undergo a guanine-nucleotide-dependent oligomerization and dissociation25,26 and the oligomerization of Atlastin-1 requires the coiled-coil middle domain.27 The mode of the atlastin action in cells is, however, not known. In plant cells, RHD3 molecules undergo a homotypic interaction at discrete ER points on ER tubules.23 Although both GTP- and GDP-locked RHD3 has dominant negative effect,23 it is interesting to note that GTP-locked RHD3 has an enhanced formation of RHD3 punctae while GDP-locked RHD3 displays a reduced formation of RHD3 punctae.23 We therefore propose a dynamin-like working model for RHD3 in the fusion of ER tubules28 that, on the ER tubules, RHD3 can undergo a GTP-induced oligomerization to form RHD compartments to squeeze ER tubules. Upon GTP hydrolysis, RHD3 undergoes a conformational change and dissociation at discrete ER points to stretch ER tubules so that fusion of ER tubules becomes possible. GTP-dependent oligomerization and GTP hydrolysis induced dissociation in the generation of interconnected ER tubules should be tightly coupled. In support of this view, we recently found that the homotypic interaction of RHD3 can be enhanced by RHD3(T75A) but reduced by RHD3(S51N) (Fig. 2). In addition, RHD3–1 is an allele with an A575V mutation in the conserved coiled-coil middle domain of RHD3,19 the homotypic interaction of RHD3 is also reduced in RHD3–1 (Fig. 2).
Figure 2.
Homotypic interaction of RHD3 is altered by mutant forms of RHD3. Split ubiquitin-based interaction assay of wild type RHD3 (#2) and mutant forms of RHD3 (#4, #5 and #6) on hisditine minus medium SC-Leu-Trp-His (right plate). The interaction was confirmed by a β-galactosidase activity assay (right plate). The left plate was cells growing on synthetic complete medium SC-Leu-Trp as mating controls. KAT1-KAT1 (#1)47 and RHD3-P24σ1d (#3) are used as controls. Note the altered RHD3 interaction by S51N (#4), RHD3–1(A575V) (#5) and T75A (#6) mutations. The number of “+” indicates the intensity of interactions evaluated by colony growth on SC-Leu-Trp-His vs. SC-Leu-Trp. The positive control KAT1-KAT1 was scored as ++++. The assay was conducted according to Chen et al. (2011).23
As an extended network of interconnected tubules stretching throughout the cytoplasm, the shape of the ER often undergoes drastic changes in response to both developmental cues and outside influences.29-31 Are there any factors regulating the function of RHD3? In this regard, it is interesting to note that rhd3–1 is epistatic to rhd2–1.32 RHD2 is an NADPH oxidase responsible for localized production of ROS.14 RHD2-derived ROS is known to stimulate Ca2+ influx into the cytoplasm.14 It is not known if ER organization in plants is under the regulation of Ca2+, but application of Ca2+ in mammalian cells induces ER restructuring.31
How could a Defect in Tubular ER Network Impair Polarized Cell Growth?
Clearly, short and wavy rhd3 root hairs of Arabidopsis and Atlastin-defective HSPs are remarkable examples of the importance of ER organization in polarized cell growth. How could a defect in the ER impair polarized cell growth? The ER is the port of entry for all membrane proteins and secretory proteins, however, in cells expressing RHD3(S51N), general protein secretion is not prevented.23 Interestingly however, Golgi stacks tend to aggregate; many of them undergo slow wiggling motion along the unbranched ER tubules.23 In plant cells, individual Golgi stacks are singly distributed and closely associated with ER tubules.33,34 Similarly in neurons, in addition to a centralized Golgi compartment in the cell body, isolated Golgi outposts, which play an important role in polarized neuronal trafficking, are also distributed along the ER throughout the dendritic arbor.35
It is known that agglomerated Golgi stacks affect the linear pattern of CESA6 (a subunit of the plasma membrane localized cellulose synthase) in the plasma membrane, but the trafficking of CESA6 to the plasma membrane is not prevented.36,37 The accumulation of celluloses in rhd3 is reduced,38 thus it would be interesting to examine the patterning of the CESA complex in the plasma membrane of rhd3. In plants, polarized tip growth requires localized modification of hemicelluloses and pectins.10,12 Targeting of other cell wall modified enzymes to the growing dome10,12,13 of rhd3 root hairs could also be tested.
Root hair tip growth requires a localized production of ROS mediated by RHD2,14 whose localization requires a coordinated post-Golgi vesicle trafficking at the tip region.17,39 Perhaps it is also worth examining the cellular distribution of RHD2 in rhd3. In the apical dome of root hairs, tip focused cytoplasmic calcium is oscillating in response to tip growth15 and there is a local positive feedback between RHD2 and Ca2+.39 The ER plays an important role in regulating cytoplasmic Ca2+ distribution,40 thus it is possible that the Ca2+ gradient at the tip of the rhd3 root hairs is perturbed. Considering the possibility that RHD3 is under the regulation of RHD2,32 perhaps RHD3 is an important component in the RHD2-Ca2+ regulation loop.
Atlastin in animal cells is known to inhibit BMP signaling by affecting either endocytic trafficking of the BMP receptor and/or secretion of BMP antagonists.41,42 Although receptor-mediated endocytosis in root hairs has not been demonstrated, endocytosis is active in root hairs.8 In rhd3–1, internalization of FM4–64 is less active.43 It is not known if in plants there are analogous BMP receptors in operation, but glutamate receptors, an important type of neuronal receptors are present in plants44 and neuronal-like activity of glutamate signaling is involved in cell development45 and ligand-gated calcium fluxes.46 Thus it may be interesting to examine the subcellular distribution of some glutamate receptors in rhd3 root hairs.
Concluding Remarks
With the role of RHD3 in ER network formation and organization revealed, the stage is set for us to understand how RHD3 works inside root hairs and to investigate how the ER participates in polarized cell growth. Arabidopsis can be manipulated genetically and the development of root hairs can be easily monitored. Therefore a powerful approach will be the identification and characterization of suppressors and enhancers of rhd3–1. Considering the remarkable functional similarity between RHD3 and Atlsatin-1,20,23 further research on RHD3 in root hairs will also provide valuable therapeutic insights into HSP.
Acknowledgments
We think Xingyun Qi (McGill University, Montreal, Canada) for critical reading of this review. This work was supported by a discovery grant from The National Science and Engineering Research Council of Canada and a startup grant from McGill University (Montreal, Canada) to H. Z.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/17477
References
- 1.Baluška F. Recent surprising similarities between plant cells and neurons. Plant Signal Behav. 2010;5:87–9. doi: 10.4161/psb.5.2.11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluška F, Volkmann D, Barlow PW. Eukaryotic cells and their cell bodies: Cell Theory revised. Ann Bot (Lond) 2004;94:9–32. doi: 10.1093/aob/mch109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baluška F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, et al. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000;227:618–32. doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- 4.Bao Y, Kost B, Chua NH. Reduced expression of alpha-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 2001;28:145–57. doi: 10.1046/j.1365-313X.2001.01142.x. [DOI] [PubMed] [Google Scholar]
- 5.Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–65. doi: 10.1046/j.1365-313X.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- 6.Ketelaar T, de Ruijter NC, Emons AM. Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell. 2003;15:285–92. doi: 10.1105/tpc.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol. 2008;181:1155–68. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samaj J, Muller J, Beck M, Bohm N, Menzel D. Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 2006;11:594–600. doi: 10.1016/j.tplants.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Böhme K, Li Y, Charlot F, Grierson C, Marrocco K, Okada K, et al. The Arabidopsis COW1 gene encodes a phosphatidylinositol transfer protein essential for root hair tip growth. Plant J. 2004;40:686–98. doi: 10.1111/j.1365-313X.2004.02245.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell. 2005;17:584–96. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, et al. Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol. 2005;168:801–12. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vissenberg K, Fry SC, Verbelen JP. Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycosylase action in Arabidopsis roots. Plant Physiol. 2001;127:1125–35. doi: 10.1104/pp.010295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson RE, Burn JE, Birch R, Baskin TI, Arioli T, Betzner AS, et al. Morphology of rsw1, a cellulose-deficient mutant of Arabidopsis thaliana. Protoplasma. 2001;215:116–27. doi: 10.1007/BF01280308. [DOI] [PubMed] [Google Scholar]
- 14.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 15.Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–8. doi: 10.1104/pp.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Véry AA, Davies JM. Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2000;97:9801–6. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Bak G, Choi Y, Chuang WI, Cho HT. Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol. 2008;147:624–35. doi: 10.1104/pp.108.117341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thole JM, Vermeer JE, Zhang Y, Gadella TW, Jr., Nielsen E. Root hair defective4 encodes a phosphatidylinositol-4-phosphate phosphatase required for proper root hair development in Arabidopsis thaliana. Plant Cell. 2008;20:381–95. doi: 10.1105/tpc.107.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Lockwood SK, Hoeltzel MF, Schiefelbein JW. The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 1997;11:799–811. doi: 10.1101/gad.11.6.799. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, et al. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–61. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soderblom C, Blackstone C. Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol Ther. 2006;109:42–56. doi: 10.1016/j.pharmthera.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Reid E. Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J Med Genet. 2003;40:81–6. doi: 10.1136/jmg.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Stefano G, Brandizzi F, Zheng H. Arabidopsis RHD3 mediates the generation of the tubular ER network and is required for Golgi distribution and motility in plant cells. J Cell Sci. 2011;124:2241–52. doi: 10.1242/jcs.084624. [DOI] [PubMed] [Google Scholar]
- 24.Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–83. doi: 10.1038/nature08280. [DOI] [PubMed] [Google Scholar]
- 25.Bian X, Klemm RW, Liu TY, Zhang M, Sun S, Sui X, et al. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proc Natl Acad Sci USA. 2011;108:3976–81. doi: 10.1073/pnas.1101643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrnes LJ, Sondermann H. Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A. Proc Natl Acad Sci USA. 2011;108:2216–21. doi: 10.1073/pnas.1012792108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss TJ, Andreazza C, Verma A, Daga A, McNew JA. Membrane fusion by the GTPase atlastin requires a conserved C-terminal cytoplasmic tail and dimerization through the middle domain. Proc Natl Acad Sci USA. 2011;108:11133–8. doi: 10.1073/pnas.1105056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–86. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucharz K, Krogh M, Ng AN, Toresson H. NMDA receptor stimulation induces reversible fission of the neuronal endoplasmic reticulum. PLoS ONE. 2009;4:e5250. doi: 10.1371/journal.pone.0005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179:895–909. doi: 10.1083/jcb.200705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian K, Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–71. doi: 10.1016/S0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 32.Schiefelbein JW, Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–43. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–7. doi: 10.1046/j.1365-313X.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 34.Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, et al. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 1999;121:1127–42. doi: 10.1104/pp.121.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–71. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, et al. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 2009;21:1141–54. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol. 2009;11:797–806. doi: 10.1038/ncb1886. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, Zhong R, Morrison WH, 3rd, Ye ZH. The Arabidopsis RHD3 gene is required for cell wall biosynthesis and actin organization. Planta. 2003;217:912–21. doi: 10.1007/s00425-003-1067-7. [DOI] [PubMed] [Google Scholar]
- 39.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–4. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 40.Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fassier C, Hutt JA, Scholpp S, Lumsden A, Giros B, Nothias F, et al. Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat Neurosci. 2010;13:1380–7. doi: 10.1038/nn.2662. [DOI] [PubMed] [Google Scholar]
- 42.Kang KH, Bier E. dHIP14-dependent palmitoylation promotes secretion of the BMP antagonist Sog. Dev Biol. 2010;346:1–10. doi: 10.1016/j.ydbio.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H, Kunst L, Hawes C, Moore I. A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J. 2004;37:398–414. doi: 10.1046/j.1365-313X.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 44.Lam HM, Chiu J, Hsieh MH, Meisel L, Oliveira IC, Shin M, et al. Glutamate-receptor genes in plants. Nature. 1998;396:125–6. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Zhu S, Song X, Shen Y, Chen H, Yu J, et al. A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell. 2006;18:340–9. doi: 10.1105/tpc.105.037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennison KL, Spalding EP. Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 2000;124:1511–4. doi: 10.1104/pp.124.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, et al. + channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA. 2004;101:12242–7. doi: 10.1073/pnas.0404467101. [DOI] [PMC free article] [PubMed] [Google Scholar]