Abstract
ApoE plays an important role in lipoprotein metabolism. This study investigated the effects of adenovirus-mediated human apoE overexpression (AdhApoE3) on sterol metabolism and in vivo reverse cholesterol transport (RCT). In wild-type mice, AdhApoE3 resulted in decreased HDL cholesterol levels and a shift toward larger HDL in plasma, whereas hepatic cholesterol content increased (P < 0.05). These effects were dependent on scavenger receptor class B type I (SR-BI) as confirmed using SR-BI-deficient mice. Kinetic studies demonstrated increased plasma HDL cholesteryl ester catabolic rates (P < 0.05) and higher hepatic selective uptake of HDL cholesteryl esters in AdhApoE3-injected wild-type mice (P < 0.01). However, biliary and fecal sterol output as well as in vivo macrophage-to-feces RCT studied with 3H-cholesterol-loaded mouse macrophage foam cells remained unchanged upon human apoE overexpression. Similar results were obtained using hApoE3 overexpression in human CETP transgenic mice. However, blocking ABCA1-mediated cholesterol efflux from hepatocytes in AdhApoE3-injected mice using probucol increased biliary cholesterol secretion (P < 0.05), fecal neutral sterol excretion (P < 0.05), and in vivo RCT (P < 0.01), specifically within neutral sterols. These combined data demonstrate that systemic apoE overexpression increases i) SR-BI-mediated selective uptake into the liver and ii) ABCA1-mediated efflux of RCT-relevant cholesterol from hepatocytes back to the plasma compartment, thereby resulting in unchanged fecal mass sterol excretion and overall in vivo RCT.
Keywords: apolipoprotein E, reverse cholesterol transport, ATP-binding cassette transporter A1, atherosclerosis, bile, cholesteryl ester transfer protein, feces, high density lipoprotein, liver, macrophage, metabolism, mice, probucol
Apolipoprotein E (apoE) plays an important role in lipoprotein metabolism and atherosclerosis. ApoE is produced and secreted predominantly by the liver (1), but it is also expressed in a variety of other tissues, including macrophages (2, 3). While loss of function of apoE in mice and in humans is associated with a proatherogenic lipoprotein profile and increased atherogenesis (4, 5), overexpression of apoE in various models has been shown to protect against atherosclerotic lesion formation (6–11). Among other metabolic effects that are potentially antiatherogenic, apoE has been reported to promote cholesterol efflux (12–14), and recent studies have suggested that lack of macrophage apoE might decrease overall reverse cholesterol transport (RCT) (15). However, the pool of macrophage-derived apoE represents a small fraction of total circulating apoE.
The classic RCT pathway is a multistep process that involves i) HDL-mediated efflux of excess cholesterol from extrahepatic cells and most relevant for atherosclerosis lipid-laden macrophages in the arterial wall, ii) uptake of HDL cholesterol into the liver, and iii) excretion of HDL cholesterol into bile and ultimately feces either directly or after metabolic conversion into bile acids (16–18). Although the liver has a key function in the RCT pathway and the majority of circulating apoE is generated by hepatocytes, the contribution of hepatic apoE to in vivo RCT has not been addressed.
Therefore, the aim of the current study was to investigate the effects of hepatic overexpression of human apoE on liver lipid metabolism, biliary sterol secretion, and in vivo macrophage-to-feces RCT. Our data demonstrate that increasing plasma levels of liver-derived apoE enhances selective uptake of HDL cholesteryl esters into the liver and induces hepatic cholesterol accumulation in a scavenger receptor class B type I (SR-BI)-dependent manner. However, this does not affect fecal mass sterol excretion and macrophage-specific RCT due to an apoE-induced enhancement of ATP-binding cassette transporter A1 (ABCA1)-mediated efflux of RCT-relevant cholesterol from hepatocytes back to the plasma compartment. These findings suggest that systemic apoE overexpression protects against atherosclerosis by mechanisms other than modulation of RCT.
MATERIALS AND METHODS
Animals
C57BL/6J mice were obtained from Charles River (Wilmington, MA). SR-BI knockout mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and backcrossed to the C57BL/6J background for a total of eight generations. Probucol (Sigma, St. Louis, MO) was mixed into powdered chow (0.5% wt/wt). For the RCT experiment, the diet was provided for 12 days before and then throughout the 48-h period of the experiment. In all other experiments, the diet was provided for 14 days. Animals were caged in animal rooms with alternating 12-h periods of light (from 7:00 AM to 7:00 PM) and dark (from 7:00 PM to 7:00 AM), with ad libitum access to water and mouse chow diet (Arie Blok, Woerden, The Netherlands). Animal experiments were performed in conformity with PHS policy and in accordance with the national laws. All protocols were approved by the responsible ethics committee of the University of Groningen and the University of Pennsylvania.
Generation of recombinant adenoviruses
The empty control adenovirus AdNull (19) and the recombinant adenovirus encoding human apoE3 (AdhApoE3) (19) were amplified and purified as reported previously (20). For in vivo experiments, mice were injected with 1 × 1011 particles/mouse of AdhApoE3 or AdNull. In vivo reverse cholesterol transport studies were carried out between day 2 and day 4 after injection of recombinant adenoviruses, a time frame when high and stable expression from an adenovirus is achieved. All other experiments described were performed on day 4 after injection of the recombinant adenoviruses.
Plasma lipid and lipoprotein analysis
Mice were bled by heart puncture after a 4-h fast at the time of death. Aliquots of plasma were stored at −80°C until analysis. Commercially available reagents were used to measure plasma total cholesterol, triglycerides (Roche Diagnostics, Basel, Switzerland), free cholesterol, and phospholipids (Diasys, Holzheim, Germany). Pooled plasma samples were subjected to fast protein liquid chromatography (FPLC) gel filtration using a Superose 6 column (GE Healthcare, Uppsala, Sweden) as described (21). Individual fractions were assayed for cholesterol concentrations as described above.
To determine plasma HDL- and nonHDL-cholesterol levels, apoB-containing lipoproteins were precipitated using polyethylene glycol in 10 mM HEPES (pH 8.0) as described (22). After centrifugation of the samples at 2,000 g and 4°C for 30 min, the HDL-containing supernatant was transferred to clean tubes. The nonHDL-containing pellet was dissolved in 0.5 M NaCO3. Cholesterol concentrations in the HDL and nonHDL fractions were determined as described above.
Analysis of liver lipid composition
Liver tissue was homogenized as described (23). Commercially available kits were used to measure contents of total cholesterol, triglycerides (Roche Diagnostics), and free cholesterol (Diasys) after extraction of lipids according to the general procedure of Bligh Dyer and redissolving lipids in water containing 2% Triton X-100 (23). Phospholipid content of the liver was determined after lipid extraction essentially as described (23).
Analysis of gene expression by real-time quantitative PCR
Total RNA from mouse livers was extracted with TriReagent (Sigma) and quantified using a Nanodrop ND-100 U-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA synthesis was performed from 1 μg of total RNA using reagents from Invitrogen (Carlsbad, CA). Real-time quantitative PCR was carried out on an ABI-Prism 7700 (Applied Biosystems, Darmstadt, Germany) sequence detector with the default settings (23). Primers and fluorogenic probes were designed with the Primer Express Software (Applied Biosystems) and synthesized by Eurogentec (Seraing, Belgium). mRNA expression levels presented were calculated relative to the average of the housekeeping gene cyclophilin and further normalized to the relative expression levels of the respective controls.
Bile collection and assessment of biliary excretion of bile acids, phospholipids, and cholesterol
Bile was collected by cannulation of the gallbladder in mice anesthetized by intraperitoneal injection of hypnorm (fentanyl/fluanisone, 1 mg/kg) and diazepam (10 mg/kg). During the bile collection, body temperature was maintained using a humidified incubator. Bile collection was performed for 30 min, and secretion rates were determined gravimetrically. Biliary bile salt, cholesterol, and phospholipid concentrations were determined and the respective biliary excretion rates calculated as described previously (23, 24).
Fecal sterol analysis
Mice were individually housed, and feces were collected over a period of 24 h and separated from the bedding. Fecal samples were dried, weighed, and thoroughly ground. Aliquots thereof were used for determination of neutral sterol and bile acid content by gas-liquid chromatography as described (23, 24).
HDL kinetics studies
HDL kinetics studies were performed essentially as published previously (21). Autologous HDL was prepared from pooled mouse plasma by sequential ultracentrifugation (density 1.063 < d < 1.21). After extensive dialysis against sterile PBS containing 0.01% EDTA, HDL was labeled with 125I-tyramine-cellobiose (TC) and cholesteryl hexadecyl ether (cholesteryl-1,2,-3H; Perkin Elmer Life Sciences) as previously described (25). For kinetic studies, 0.4 µCi of 125I and 0.7 million dpm of the 3H tracer were injected into the tail veins of fasted wild-type mice treated with AdNull or AdhApoE3. Blood samples were drawn by retroorbital bleeding at 5 min and at 1, 3, 6, 11, and 24 h after injection. Plasma decay curves for both tracers were generated by dividing the plasma radioactivity at each time point by the radioactivity at the initial 5-min time point after tracer injection. Fractional catabolic rates (FCRs) were determined from the area under the plasma disappearance curves fitted to a bicompartmental model using the SAAM II program (26). The use of 3H-cholesteryl ether does not affect turnover rates in vivo compared with 3H-cholesteryl ester-labeled HDL (21). Organ uptake of HDL apolipoproteins (125I) and HDL-CEs (3H-cholesteryl ether) was determined by measuring the counts recovered in each organ expressed as a percentage of the injected dose, which was calculated by multiplying the initial plasma counts (5-min time point) with the estimated plasma volume (3.5% of total body weight). Selective uptake into organs was determined by subtracting the percentage of the injected dose of 125I-HDL recovered in each organ from the percentage of the injected dose of 3H-HDL-CE.
In vivo RCT studies
In vivo RCT studies were performed essentially as published previously (27). Wild-type C57BL/6J donor mice were injected with 1.0 ml of 4% Brewer thioglycollate medium (Becton Dickinson, Le Point de Claix, France). Peritoneal macrophages were harvested 4 days after thioglycollate injection as described (28). Macrophages were plated in RPMI 1640 medium (Invitrogen) supplemented with 1% FBS (HyClone, Logan, UT) and penicillin (100 U/ml)/streptomycin (100 μg/ml) (Invitrogen) and were allowed to adhere for 5 h at 37°C under 5% CO2 humidified air. Nonadherent cells were removed by washing twice with PBS followed by loading of the macrophages with 50 μg/ml acetylated LDL and 3 μCi/ml 3H-cholesterol (Perkin Elmer Life Sciences, Boston, MA) for 24 h. Thereafter, cells were washed again and equilibrated in RPMI 1640 medium supplemented with 2% BSA (Sigma) for 18 h. Immediately before injection, cells were harvested and resuspended in RPMI 1640 medium. For in vivo macrophage-to-feces RCT studies, 2 million 3H-cholesterol-loaded macrophage foam cells were injected intraperitoneally into individually housed recipient mice. Plasma was collected 6 h and 24 h after macrophage injection by retroorbital puncture and for the final blood draw (48 h) by heart puncture. At the end of the experimental period, livers were harvested, snap-frozen in liquid nitrogen, and stored at −80°C until further analysis. Feces were collected continuously for 48 h.
Counts in plasma were assessed directly by liquid scintillation counting (Packard 1600CA Tri-Card, Packard, Meriden, CT). Counts within liver were determined after solubilization of the tissue using Solvable (Packard) exactly as previously reported (26). Counts recovered from the respective liver piece were back-calculated to total liver mass. Feces were separated from the bedding, dried, weighed, and thoroughly ground. Aliquots were separated into neutral sterol and bile acid fractions as previously reported (27). Briefly, samples were heated for 2 h at 80°C in alkaline methanol and then extracted three times with petroleum ether. In the top layer, radioactivity within the neutral sterol fraction was determined by liquid scintillation counting, whereas radioactivity incorporated into bile acids was assessed from the bottom layer. Counts recovered from the respective aliquots were related to the total amount of feces produced over the whole experimental period. All obtained counts were expressed relative to the administered dose.
In vitro cholesterol efflux assay
Thioglycollate-elicited peritoneal mouse macrophages were harvested as described above. Macrophages were plated in RPMI 1640 medium (Invitrogen) supplemented with 1% FBS (HyClone, Logan, UT) and penicillin (100 U/ml)/streptomycin (100 μg/ml) (Invitrogen) and were allowed to adhere for 5 h at 37°C under 5% CO2 humidified air. Nonadherent cells were removed by washing twice with PBS followed by loading of the macrophages with 50 μg/ml LDL and 1 μCi/ml 3H-cholesterol (Perkin Elmer Life Sciences, Boston, MA) for 24 h. The cells were washed again and equilibrated in RPMI 1640 medium supplemented with 2% BSA (Sigma) for 18 h. The cells were washed with PBS, and 2% mouse plasma was added. After 4 h and 8 h, radioactivity within the medium was determined by liquid scintillation counting. The cell layer was washed twice with PBS, and 0.1 M NaOH was added. Plates were incubated 30 min at room temperature, and the radioactivity remaining within the cells was assessed by liquid scintillation counting. Wells incubated with RPMI without added plasma were used as blanks to determine plasma-independent efflux, and these values were subtracted from the respective experimental values. Efflux is given as the percentage of counts recovered from the medium in relation to the total counts present on the plate (sum of medium and cells).
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences version 16.0 (SPSS Inc., Chicago, IL). Data are presented as means ± SEM. The Mann-Whitney U-test was used to compare different groups. Statistical significance for all comparisons was assigned at P < 0.05.
RESULTS
Hepatic apoE overexpression affects HDL size distribution but not plasma lipid levels
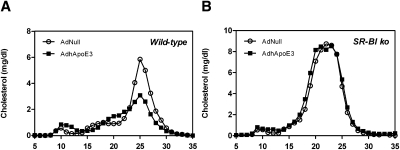
To assess the effects of hepatic overexpression of human apoE3 on plasma lipid levels, wild-type mice were injected with an empty control adenovirus AdNull or with an adenovirus expressing human apoE3. Plasma levels of total cholesterol, free cholesterol, esterified cholesterol, phospholipids, and triglycerides remained essentially unchanged in response to hepatic apoE overexpression (Table 1). However, FPLC analysis revealed a lower HDL cholesterol peak and a shift toward larger particles in the AdhApoE3-injected mice compared with controls (Fig. 1A). In parallel, plasma levels of apoA-I (P = 0.055; Supplementary Figure IA) and apoB100 (P < 0.01; Supplementary Figure IB) were lower in the mice overexpressing human apoE, whereas plasma apoB48 was not altered (n.s.; Supplementary Figure IC). To explore the distribution of human apoE across the different lipoprotein classes, Western blot analysis for apoA-I and human apoE was performed on the individual FPLC fractions. In the mice administered AdhApoE3, human apoE was present in the apoA-I-containing HDL fractions and in the nonHDL lipoprotein fractions lacking apoA-I expression (Supplementary Figure II). Because cholesteryl ester transfer protein (CETP) plays an important role in human lipoprotein metabolism but is absent in wild-type mice (29), apoE overexpression experiments were carried out in transgenic mice expressing human CETP under the control of its endogenous promoter (hCETP tg). Comparable to the results in wild-type mice, in hCETP tg mice no major changes in plasma lipids occurred in response to hepatic apoE overexpression (Supplementary Table I), and the HDL cholesterol peak was similarly decreased and was shifted toward larger HDL particles (Supplementary Figure III).
Fig. 1.
Apolipoprotein E overexpression affects plasma cholesterol distribution in an SR-BI-dependent fashion. FPLC profiles in response to apolipoprotein E overexpression in (A) wild-type mice and (B) SR-BI knockout (ko) mice. Pooled plasma samples collected on day 4 after injection with the control adenovirus AdNull or with the human apolipoprotein E3 expressing adenovirus AdhApoE3 were subjected to gel filtration chromatography analysis using a Superose 6 column as described in Materials and Methods. n = 6–8 mice for each condition. Open circles, AdNull-injected controls; closed squares, AdhApoE3-injected mice.
Hepatic apoE overexpression increases hepatic cholesterol content by stimulating selective uptake into the liver
Next, we determined whether hepatic overexpression of human apoE would affect hepatic lipid composition. Hepatic total cholesterol content was significantly increased by 24% in mice administered AdhApoE3 (P < 0.05; Table 1), largely due to a higher hepatic esterified cholesterol content (+150%; P < 0.01; Table 1). Whereas hepatic phospholipids were identical between AdNull-injected and AdhApoE3-injected mice (Table 1), apoE overexpression resulted in an elevated hepatic triglyceride content (+355%; P < 0.01; Table 1). Similar changes in hepatic cholesterol and triglyceride content were observed in response to AdhApoE3 in hCETP tg mice (Supplementary Table II).
TABLE 1.
Plasma lipids, liver lipid composition, and biliary excretion of sterols in wild-type and SR-BI knockout mice in response to hepatic apolipoprotein E overexpression
| Wild-type | SR-BI knockout | |||
| AdNull | AdhApoE3 | AdNull | AdhApoE3 | |
| Plasma | ||||
| Total cholesterol (mg/dl) | 69.5 ± 1.9 | 65.5 ± 2.6 | 147.6 ± 9.5 | 140.5 ± 12.1 |
| Free cholesterol (mg/dl) | 29.0 ± 0.7 | 31.3 ± 0.9 | 86.4 ± 4.9 | 81.3 ± 7.8 |
| Esterified cholesterol (mg/dl) | 40.5 ± 1.6 | 34.3 ± 3.1 | 61.1 ± 6.4 | 59.2 ± 5.4 |
| Phospholipids (mg/dl) | 178.6 ± 12.7 | 148.6 ± 8.0 | 191.8 ± 10.8 | 214.7 ± 16.5 |
| Triglycerides (mg/dl) | 82.9 ± 9.2 | 80.0 ± 8.9 | 59.8 ± 6.9 | 79.9 ± 10.8 |
| Liver | ||||
| Total cholesterol (nmol/mg liver) | 6.3 ± 0.5 | 7.8 ± 0.4a | 8.5 ± 0.3 | 8.3 ± 0.4 |
| Free cholesterol (nmol/mg liver) | 5.4 ± 0.4 | 5.9 ± 0.3 | 6.7 ± 0.1 | 6.4 ± 0.1 |
| Esterified cholesterol (nmol/mg liver) | 0.8 ± 0.2 | 2.0 ± 0.1a | 1.7 ± 0.3 | 2.0 ± 0.3 |
| Phospholipids (nmol/mg liver) | 28.2 ± 1.5 | 25.8 ± 0.9 | 31.9 ± 0.7 | 29.5 ± 0.6a |
| Triglycerides (nmol/mg liver) | 12.8 ± 3.1 | 58.3 ± 5.0a | 22.2 ± 1.6 | 32.8 ± 4.5 |
| Bile | ||||
| Bile flow (μl/min/100 g bw) | 10.3 ± 0.6 | 10.2 ± 0.2 | ND | ND |
| Biliary bile acid secretion (nmol/min/100 g bw) | 866 ± 86 | 762 ± 45 | ND | ND |
| Biliary phospholipid secretion (nmol/min/100 g bw) | 61.6 ± 5.0 | 82.6 ± 6.8a | ND | ND |
| Biliary cholesterol secretion (nmol/min/100 g bw) | 3.9 ± 0.2 | 4.0 ± 0.3 | ND | ND |
On day 4 after adenovirus injection, bile was collected continuously for 30 min, plasma samples were taken, and livers were harvested and snap-frozen in liquid nitrogen. Plasma lipids, liver lipids, and biliary output rates of bile acids, phospholipids, and cholesterol were determined as described in Materials and Methods. Values are means ± SEM; n = 5–8 mice for each condition. AdhApoE3, recombinant adenovirus expressing human apoE3; AdNull, empty control adenovirus; SR-BI, scavenger receptor class B type I; bw, body weight; ND, not determined.
Significantly different from the respective AdNull-injected controls as assessed by Mann-Whitney U-test (at least P < 0.05).
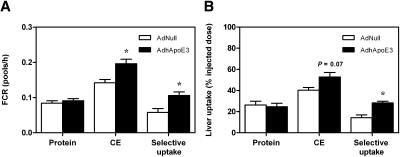
To test the hypothesis that the decrease in plasma HDL cholesterol and the concomitant increase in hepatic cholesterol content in response to apoE overexpression were due to an enhanced selective uptake of cholesteryl esters from HDL, HDL kinetic studies were carried out in wild-type mice using autologous HDL. Hepatic apoE overexpression caused an increase in the HDL cholesteryl ester FCR (0.142 ± 0.009 vs. 0.196 ± 0.013 pools/h; P < 0.05; Fig. 2A) without having significant effects on the HDL protein FCR (0.084 ± 0.007 vs. 0.091 ± 0.013 pools/h; n.s.; Fig. 2A). Therefore, the apparent whole body selective uptake as calculated by the difference between the HDL cholesteryl ester and HDL protein FCRs was significantly higher in apoE-overexpressing mice compared with controls (0.058 ± 0.011 vs. 0.106 ± 0.010 pools/h; P < 0.05; Fig. 2A). In agreement with the above results, the uptake of HDL protein into the liver remained unchanged after hepatic apoE overexpression (26.2 ± 3.7 vs. 24.6 ± 3.3%; n.s.; Fig. 2B), whereas uptake of HDL cholesteryl ester into the liver tended to be higher (40.4 ± 2.4 vs. 52.8 ± 4.2%; P = 0.07; Fig. 2B). Overall, this translated into an almost 2-fold increase in hepatic selective uptake in the AdhApoE3-injected group (14.2 ± 2.7 vs. 28.2 ± 1.6; P < 0.01; Fig. 2B). Although selective uptake of HDL cholesteryl esters in the liver was enhanced, Sr-b1 mRNA expression was lower in wild-type mice (P < 0.01; Table 2) and in hCETP tg mice overexpressing apoE (Supplementary Table III). However, neither total nor membrane-associated hepatic SR-BI protein levels were changed in the two mouse models (Supplementary Figure IV). Combined, these data demonstrate that hepatic apoE overexpression increases hepatic cholesterol content by stimulating selective uptake of HDL cholesteryl esters into the liver.
Fig. 2.
Apolipoprotein E overexpression increases selective uptake of HDL cholesteryl esters into the liver. On day 4 after injection with the control adenovirus AdNull or with the human apolipoprotein E3-expressing adenovirus, AdhApoE3 kinetic experiments were performed using autologous HDL double labeled with 125I-tyramine-cellobiose and 3H-cholesteryl ether (CE) as described in Materials and Methods. A: FCRs calculated from the respective plasma disappearance curves. B: Uptake of 125I-tyramine-cellobiose and 3H-CE by the liver. Data are presented as means ± SEM. n = 6 mice for each condition. White bars, AdNull-injected mice; black bars, AdhApoE3-injected mice. * Significantly different from the respective AdNull-injected controls as assessed by Mann-Whitney U-test (at least P < 0.05).
TABLE 2.
Hepatic mRNA expression in wild-type mice in response to hepatic apolipoprotein E overexpression
| Wild-type | ||
| AdNull | AdhApoE3 | |
| Sr-b1 | 1.00 ± 0.03 | 0.70 ± 0.03a |
| Abcb11 | 1.00 ± 0.06 | 0.60 ± 0.04a |
| Abcb4 | 1.00 ± 0.07 | 0.91 ± 0.05 |
| Abcg5 | 1.00 ± 0.08 | 0.62 ± 0.04a |
| Abcg8 | 1.00 ± 0.06 | 0.69 ± 0.06a |
| Cyp7a1 | 1.00 ± 0.23 | 0.91 ± 0.13 |
| Cyp27a1 | 1.00 ± 0.09 | 0.46 ± 0.04a |
| Cyp8b1 | 1.00 ± 0.07 | 0.74 ± 0.02a |
| Srebp2 | 1.00 ± 0.10 | 0.70 ± 0.02a |
| Ldlr | 1.00 ± 0.09 | 0.71 ± 0.04 |
| Hmgcr | 1.00 ± 0.16 | 0.74 ± 0.09 |
| Abca1 | 1.00 ± 0.02 | 0.92 ± 0.06 |
Livers of mice administered the respective adenoviruses were harvested on day 4 after adenovirus injection and snap-frozen in liquid nitrogen. mRNA expression levels were determined by real-time quantitative PCR as described in Materials and Methods. Values are means ± SEM; n = 6 mice for each condition. Within each set of experiments, gene expression levels are related to the respective AdNull-injected controls. AdhApoE3, recombinant adenovirus expressing human apoE3; AdNull, empty control adenovirus.
Significantly different from the respective AdNull-injected controls as assessed by Mann-Whitney U-test (at least P < 0.05).
Increased hepatic cholesterol content in response to hepatic apoE overexpression is dependent on SR-BI
SR-BI is the major receptor responsible for the selective uptake of HDL cholesterol into the liver (21, 30). To confirm a critical role of SR-BI mediating altered plasma lipoprotein distribution and hepatic cholesterol content as a consequence of apoE overexpression, the effects of AdNull or AdhApoE3 were investigated in SR-BI-deficient mice. In agreement with results in wild-type mice, injection of a human apoE expressing adenovirus did not alter plasma levels of total cholesterol, free cholesterol, esterified cholesterol, phospholipids, and triglycerides in SR-BI knockout mice (Table 1). However, the marked alterations observed in the lipoprotein distribution in response to apoE overexpression in wild-type mice were not present in SR-BI knockouts, as reflected by virtually identical FPLC profiles in the AdNull-injected compared with the AdhApoE3-injected group (Fig. 1B). In line with these results, the hepatic content of total cholesterol (Table 1), free cholesterol (Table 1), and esterified cholesterol (Table 1) was not affected by apoE overexpression in SR-BI knockout mice. Nevertheless, AdhApoE3 injection in the SR-BI knockouts caused a slight but significant decrease in hepatic phospholipid content (−8%; P < 0.05; Table 1), whereas the hepatic triglyceride content tended to be higher (+48%; P = 0.06; Table 1). These data indicate that the apoE-mediated changes in lipoprotein distribution and hepatic cholesterol content are dependent on SR-BI.
Hepatic apoE overexpression does not affect biliary and fecal sterol excretion
To explore whether higher SR-BI-mediated hepatic cholesterol uptake after apoE overexpression in wild-type mice would translate into changes in biliary sterol secretion, a continuous bile cannulation experiment was performed in wild-type mice receiving AdNull or AdhApoE3. Neither bile flow (Table 1) nor biliary secretion rates of bile acids (Table 1) were affected by hepatic overexpression of human apoE3. Although the biliary secretion rate of phospholipids was 1.3-fold higher (P < 0.05; Table 1), the secretion rate of cholesterol into bile remained unchanged in wild-type mice (Table 1). However, in hCETP tg mice, lower biliary output of cholesterol was noted in the group injected with AdhApoE3, whereas there was no effect on the biliary secretion rates of bile acids and phospholipids (Supplementary Figure V).
Hepatic mRNA expression of the hepatobiliary phospholipid transporter Abcb4 (also known as multidrug resistance protein 2, Mdr2) was not changed by apoE overexpression in wild-type mice, whereas expression levels of the bile salt export pump Abcb11 (also known as Bsep; P < 0.01) and the cholesterol half-transporters Abcg5 and Abcg8 were decreased (P < 0.01 for both) (Table 2). ApoE overexpression reduced expression of the key enzyme for the alternative bile acid synthesis pathway in the liver, Cyp27a1 (P < 0.01) and the enzyme responsible for cholate synthesis, Cyp8b1 (P < 0.05) but did not affect expression of the rate-limiting enzyme for classic bile acid synthesis, Cyp7a1 (Table 2). Hepatic gene expression of the sterol regulatory binding protein 2 (Srebp2) was suppressed by apoE overexpression (P < 0.05), whereas mRNA expression of its two target genes LDL receptor (Ldlr) and the rate-limiting enzyme for cholesterol synthesis, HMG-CoA reductase (Hmgcr), was not significantly affected (Table 2). There was also no difference in Abca1 mRNA levels in the liver between AdhApoE3 injected wild-type mice and controls. In hCETP tg mice, virtually identical changes in liver gene expression were found in response to apoE overexpression (Supplementary Table III).
Consistent with unaltered biliary secretion of cholesterol and bile acids in wild-type mice, the fecal mass output of neutral sterols (3.11 ± 0.22 vs. 2.49 ± 0.34 μmol/day; Supplementary Table IV) and bile acids (2.33 ± 0.19 vs. 2.65 ± 0.11 μmol/day; Supplementary Table V) was not influenced by overexpression of apoE. Likewise, hCETP tg mice injected with AdhApoE3 also excreted similar amounts of neutral sterols (Supplementary Table IV) and bile acids (Supplementary Table V) into the feces compared with AdNull administered controls. Taken together, these results indicate that apoE overexpression promotes hepatic cholesterol uptake without increasing biliary and fecal sterol excretion.
Hepatic apoE overexpression does not affect macrophage-to-feces RCT
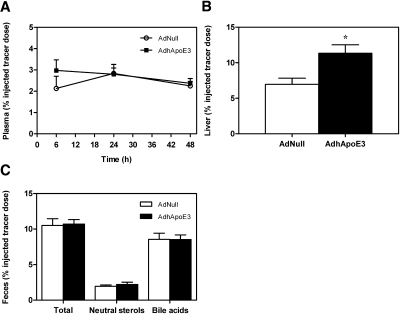
Because apoE overexpression increased hepatic selective uptake via SR-BI, an important step in the RCT pathway, but did not influence mass biliary and fecal sterol excretion, we next investigated whether apoE overexpression might affect overall RCT. In vivo RCT was traced after intraperitoneal injection of primary mouse macrophages loaded with 3H-cholesterol in control and apoE-overexpressing wild-type mice. The appearance of tracer in plasma was not significantly different between controls and mice injected with AdhApoE3 at 6 h (2.1 ± 0.6 vs. 3.0 ± 0.5% injected tracer dose; n.s.), 24 h (2.8 ± 0.4 vs. 2.8 ± 0.3% injected tracer dose; n.s.), and 48 h (2.3 ± 0.2 vs. 2.4 ± 0.2% injected tracer dose; n.s.) after macrophage injection (Fig. 3A). Although apoE overexpression led to a 63% increase in macrophage-derived 3H-cholesterol within the liver (7.0 ± 0.9 vs. 11.3 ± 1.2% injected tracer dose; P < 0.05; Fig. 3B), overall, in vivo RCT remained essentially unchanged as reflected by no effect on the total excretion of 3H-tracer into the feces (10.5 ± 1.0 vs. 10.7 ± 0.6% injected tracer dose; n.s.; Fig. 3C). In addition, no significant changes were observed in the fecal loss of 3H-tracer within neutral sterols (1.9 ± 0.2 vs. 2.2 ± 0.3% injected tracer dose; n.s.) or within the bile acid fraction (8.6 ± 0.9 vs. 8.5 ± 0.7% injected tracer dose; n.s.) in response to apoE overexpression (Fig. 3C). Moreover, in the CETP transgenic mouse model, RCT was not influenced by AdhApoE3 (Supplementary Figure VI). These observations indicate that hepatic apoE overexpression has no apparent effect on macrophage RCT.
Fig. 3.
Apolipoprotein E overexpression does not affect in vivo macrophage-to-feces reverse cholesterol transport in wild-type mice. On day 2 after injection with the control adenovirus AdNull or with the human apolipoprotein E3-expressing adenovirus AdhApoE3, mice received intraperitoneal injections with 3H-cholesterol-loaded primary mouse macrophage foam cells as described in Materials and Methods. A: Time course of 3H-cholesterol recovery in plasma. B: 3H-cholesterol within liver 48 h after macrophage administration. C: 3H-cholesterol appearance in feces collected continuously from 0 to 48 h after macrophage administration and separated into bile acid and neutral sterol fractions as indicated. Data are expressed as percentage of the injected tracer dose and presented as means ± SEM. n = 8 mice for each condition. White bars, AdNull-injected mice; black bars, AdhApoE3-injected mice. * Significantly different from the respective AdNull-injected controls as assessed by Mann-Whitney U-test (at least P < 0.05).
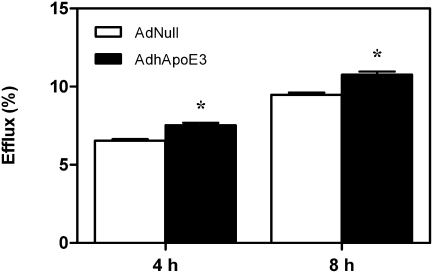
Probucol treatment decreases plasma lipid and lipoprotein levels
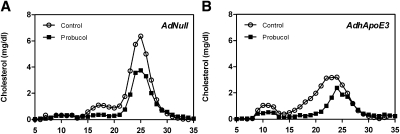
It has been suggested recently that inhibition of hepatic ABCA1 activity by probucol reduces ABCA1-mediated cholesterol efflux from hepatocytes, thereby increasing cholesterol excretion into the bile and feces (31). Cholesterol efflux from lipid-laden macrophages in vitro was significantly higher toward plasma from ApoE-overexpressing wild-type mice compared with controls after 4 h (6.5 ± 0.1 vs. 7.5 ± 0.2%; P < 0.01) and 8 h (9.5 ± 0.1 vs. 10.8 ± 0.2%; P < 0.01) of incubation (Fig. 4). We therefore hypothesized that potentially increased ABCA1-mediated cholesterol efflux from hepatocytes might explain the unchanged biliary and fecal sterol output in response to apoE overexpression. To explore this hypothesis, wild-type mice were fed a control chow diet or a chow diet supplemented with 0.5% (wt/wt) probucol and were injected on day 10 of diet feeding with AdNull or AdhApoE3. Treatment with probucol decreased plasma total cholesterol concentrations in AdNull-injected (−51%; P < 0.01) and AdhApoE3-injected mice (−66%; P < 0.01; Table 3). This was due to a significant lowering of plasma free cholesterol (−52% and −71% for AdNull and AdhApoE3, respectively; P < 0.01 for both) as well as esterified cholesterol (−51% and −61% for AdNull and AdhApoE3, respectively; P < 0.01 for both) in the mice administered probucol (Table 3). As expected from the role of liver ABCA1 in HDL formation, HDL-cholesterol levels in plasma were markedly reduced by dietary probucol (−41% and −42% for AdNull and AdhApoE3, respectively; P < 0.01 for both). However, probucol also resulted in decreased plasma nonHDL-cholesterol levels (−66% and −79% for AdNull and AdhApoE3, respectively; P < 0.01 for both). In line with these results, FPLC analysis demonstrated a clear reduction in all lipoprotein classes in AdNull-injected mice fed the probucol diet compared with mice fed the control diet (Fig. 5A). Similar changes in the plasma lipoprotein distribution were observed in human apoE-overexpressing mice in response to probucol (Fig. 5B). Finally, administration of probucol resulted in a significant decrease in plasma phospholipids and triglycerides in mice injected with the control adenovirus AdNull (−37% and −38% for phospholipids and triglycerides, respectively; P < 0.01 and P < 0.05, respectively) or AdhApoE3 (−57% and −66% for phospholipids and triglycerides, respectively; P < 0.01 for both) (Table 3).
Fig. 4.
Apolipoprotein E overexpression increases cholesterol efflux from macrophage foam cells toward plasma. Thioglycollate-elicited peritoneal mouse macrophages were loaded with 50 μg/ml acetylated LDL and 1 μCi/ml [3H]cholesterol as described in Materials and Methods. Subsequently, 2% plasma was added to the cells. After 4 h and 8 h, radioactivity within the medium and radioactivity remaining within the cells was determined by liquid scintillation counting. Efflux is given as the percentage of counts recovered from the medium in relation to the total counts present on the plate (sum of medium and cells). Data are presented as means ± SEM. n = 8 mice for each condition. White bars, AdNull-injected mice; black bars, AdhApoE3-injected mice. * Significantly different from the respective AdNull-injected controls as assessed by Mann-Whitney U-test (at least P < 0.05).
TABLE 3.
Plasma lipids, liver lipid composition, and biliary excretion of sterols in response to probucol treatment
| AdNull | AdhApoE3 | |||
| Control | Probucol | Control | Probucol | |
| Plasma | ||||
| Total cholesterol (mg/dl) | 72.3 ± 1.7 | 35.3 ± 1.3a | 63.7 ± 2.8 | 21.9 ± 1.3a |
| Free cholesterol (mg/dl) | 22.2 ± 0.4 | 10.7 ± 0.6a | 29.8 ± 2.0 | 8.5 ± 1.1a |
| Esterified cholesterol (mg/dl) | 50.1 ± 1.8 | 24.6 ± 1.1a | 34.0 ± 2.0 | 13.4 ± 0.6a |
| Phospholipids (mg/dl) | 154.1 ± 7.4 | 97.7 ± 5.8a | 137.8 ± 9.5 | 59.8 ± 1.3a |
| Triglycerides (mg/dl) | 58.2 ± 7.1 | 36.0 ± 3.5a | 95.3 ± 7.9 | 32.4 ± 7.0a |
| Liver | ||||
| Total cholesterol (nmol/mg liver) | 7.2 ± 0.2 | 7.5 ± 0.2 | 9.1 ± 0.4 | 9.2 ± 0.5 |
| Free cholesterol (nmol/mg liver) | 5.9 ± 0.1 | 6.0 ± 0.2 | 7.4 ± 0.5 | 6.9 ± 0.3 |
| Esterified cholesterol (nmol/mg liver) | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.7 ± 0.2 | 2.3 ± 0.2 |
| Phospholipids (nmol/mg liver) | 29.2 ± 0.7 | 26.5 ± 0.6a | 26.7 ± 0.6 | 27.8 ± 0.6 |
| Triglycerides (nmol/mg liver) | 21.4 ± 1.7 | 22.3 ± 3.3 | 63.7 ± 7.1 | 59.4 ± 8.6 |
| Bile | ||||
| Bile flow (μl/min/100 g bw) | 9.3 ± 0.6 | 8.2 ± 0.7 | 7.3 ± 1.1 | 10.6 ± 0.9 |
| Biliary bile acid secretion (nmol/min/100 g bw) | 469 ± 90 | 357 ± 73 | 427 ± 81 | 466 ± 51 |
| Biliary phospholipid secretion (nmol/min/100 g bw) | 41.6 ± 3.1 | 37.7 ± 5.6 | 43.0 ± 5.4 | 64.3 ± 5.1a |
| Biliary cholesterol secretion (nmol/min/100 g bw) | 3.5 ± 0.4 | 3.6 ± 0.5 | 2.9 ± 0.4 | 5.2 ± 0.7a |
Mice were fed a control chow diet or a chow diet containing 0.5% probucol for 2 weeks. On day 4 after adenovirus injection, bile was collected continuously for 30 min, plasma samples were taken, and livers were harvested and snap-frozen in liquid nitrogen. Plasma lipids, liver lipids, and biliary output rates of bile acids, phospholipids, and cholesterol were determined as described in Materials and Methods. Values are means ± SEM; n = 6 mice for each condition. AdhApoE3, recombinant adenovirus expressing human apoE3; AdNull, empty control adenovirus; bw, body weight.
Significantly different from the respective controls as assessed by Mann-Whitney U-test (at least P < 0.05).
Fig. 5.
Probucol treatment decreases plasma cholesterol levels. FPLC profiles in response to probucol treatment in (A) AdNull-injected and (B) AdhApoE3-injected mice. Mice were fed a control chow diet or a chow diet containing 0.5% probucol for 2 weeks. Pooled plasma samples collected on day 4 after injection with the control adenovirus AdNull or with the human apolipoprotein E3-expressing adenovirus AdhApoE3 were subjected to gel filtration chromatography analysis using a Superose 6 column as described in Materials and Methods. n = 6 mice for each condition. Open circles, chow-fed controls; closed squares; probucol-treated mice.
Probucol treatment does not change the hepatic cholesterol content in response to hepatic apoE overexpression
In mice that received the control adenovirus AdNull, the hepatic content of total cholesterol was not different between groups on control and probucol-containing diet (Table 3). ApoE overexpression consistently increased total cholesterol levels in the liver; however, there was no additional effect of probucol (Table 3). The amount of free cholesterol and esterified cholesterol in the liver was not changed in response to probucol in both the mice administered AdNull and AdhApoE3 (Table 3). Probucol treatment resulted in a lower hepatic phospholipid content in the AdNull (−9%; P < 0.05) but not the AdhApoE3 group (Table 3). There was no effect of probucol on the hepatic triglyceride content (Table 3). Thus, probucol does not change the hepatic cholesterol mass content in response to hepatic apoE overexpression.
Probucol treatment increases biliary and fecal sterol secretion in apoE-overexpressing mice
Bile cannulation experiments revealed that dietary probucol had no effect on bile flow or on the biliary secretion of bile acids, phospholipids, and cholesterol in mice injected with the control adenovirus (Table 3). However, in AdhApoE3-administered mice, bile flow tended to increase in response to probucol (P = 0.055; Table 3). Whereas the biliary output rate of bile acids remained unchanged, biliary secretion rates of phospholipids were 1.5-fold higher in apoE-overexpressing mice in response to probucol (P < 0.05), and the biliary secretion rate of cholesterol increased significantly by 1.8-fold (P < 0.05) (Table 3).
Hepatic mRNA expression of the bile acid transporter Abcb11, the phospholipid transporter Abcb4, and the cholesterol half-transporters Abcg5 and Abcg8 was not modified by probucol treatment in control mice or in mice that overexpress apoE in the liver (Table 4). No significant changes in the hepatic gene expression level of the bile acid synthesizing enzymes Cyp7a1, Cyp27a1, and Cyp8b1 were observed, except for higher relative mRNA levels of Cyp7a1 in apoE-overexpressing mice upon probucol administration (P < 0.05; Table 4). Furthermore, expression of Srebp2 and its target genes ldlr and hmgcr in the liver was not different between mice fed a chow diet and mice fed a probucol-enriched diet (Table 4). Finally, as has been reported previously (31), no change in the hepatic mRNA expression of Abca1 was detected in the probucol-treated mice (Table 4).
TABLE 4.
Hepatic mRNA expression in response to probucol treatment
| AdNull | AdhApoE3 | |||
| Control | Probucol | Control | Probucol | |
| Sr-b1 | 1.00 ± 0.05 | 0.88 ± 0.03a | 1.00 ± 0.03 | 1.08 ± 0.03a |
| Abcb11 | 1.00 ± 0.06 | 1.16 ± 0.04 | 1.00 ± 0.05 | 1.14 ± 0.08 |
| Abcb4 | 1.00 ± 0.07 | 1.05 ± 0.09 | 1.00 ± 0.06 | 1.12 ± 0.05 |
| Abcg5 | 1.00 ± 0.10 | 0.91 ± 0.09 | 1.00 ± 0.06 | 1.20 ± 0.09 |
| Abcg8 | 1.00 ± 0.07 | 0.93 ± 0.08 | 1.00 ± 0.06 | 1.13 ± 0.13 |
| Cyp7a1 | 1.00 ± 0.15 | 0.60 ± 0.09 | 1.00 ± 0.13 | 1.58 ± 0.12a |
| Cyp27a1 | 1.00 ± 0.05 | 0.90 ± 0.03 | 1.00 ± 0.07 | 1.11 ± 0.07 |
| Cyp8b1 | 1.00 ± 0.08 | 0.96 ± 0.09 | 1.00 ± 0.06 | 1.04 ± 0.09 |
| Srebp2 | 1.00 ± 0.04 | 0.96 ± 0.05 | 1.00 ± 0.05 | 0.81 ± 0.06 |
| Ldlr | 1.00 ± 0.08 | 1.14 ± 0.08 | 1.00 ± 0.06 | 0.94 ± 0.06 |
| Hmgcr | 1.00 ± 0.07 | 0.98 ± 0.09 | 1.00 ± 0.05 | 0.83 ± 0.09 |
| Abca1 | 1.00 ± 0.04 | 1.07 ± 0.02 | 1.00 ± 0.04 | 1.04 ± 0.04 |
Mice were fed a control chow diet or a chow diet containing 0.5% probucol for 2 weeks. Livers of mice administered the respective adenoviruses were harvested on day 4 after adenovirus injection and snap-frozen in liquid nitrogen. mRNA expression levels were determined by real-time quantitative PCR as described in Materials and Methods. Values are means ± SEM; n = 6 mice for each condition. Within each set of experiments, gene expression levels are related to the respective chow-fed controls. AdhApoE3, recombinant adenovirus expressing human apoE3; AdNull, empty control adenovirus.
Significantly different from the respective controls as assessed by Mann-Whitney U-test (at least P < 0.05).
Analysis of fecal contents showed that treatment with probucol did not influence the fecal excretion of neutral sterols (4.74 ± 0.28 vs. 5.07 ± 0.18 μmol/day) and bile acids (2.52 ± 0.22 vs. 3.02 ± 0.28 μmol/day) in mice that received the control adenovirus AdNull. In contrast and in good agreement with the elevated biliary cholesterol secretion, fecal excretion of neutral sterols was significantly enhanced by probucol in mice overexpressing apoE (3.69 ± 0.21 vs. 4.97 ± 0.37 μmol/day; P < 0.05). Nonetheless, no effect of probucol on the fecal bile acid output was found in these mice (2.20 ± 0.14 vs. 2.66 ± 0.23 μmol/day). Combined, these data demonstrate that biliary and fecal sterol secretion are increased upon probucol treatment in apoE-overexpressing mice.
Probucol treatment increases macrophage-to-feces RCT in apoE-overexpressing mice
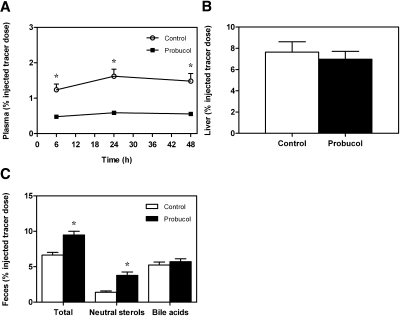
Because probucol enhanced biliary and fecal sterol secretion in mice overexpressing human apoE, we investigated whether this would also translate into an improvement in overall RCT from macrophages to feces. After intraperitoneal injection of 3H-cholesterol-loaded macrophages, counts within plasma were profoundly lower at the 6 h (1.24 ± 0.16 vs. 0.48 ± 0.04% injected tracer dose; P < 0.01; Fig. 6A), 24 h (1.62 ± 0.20 vs. 0.59 ± 0.04% injected tracer dose; P < 0.01; Fig. 6A), and 48 h time point (1.48 ± 0.22 vs. 0.56 ± 0.06% injected tracer dose; P < 0.01; Fig. 6A) in the probucol-treated apoE-overexpressing mice compared with apoE-overexpressing controls. However, the amount of macrophage-derived tracer recovered within the liver was not affected by probucol in mice with hepatic apoE overexpression (7.6 ± 1.0 vs. 7.0 ± 0.7% injected tracer dose; n.s.; Fig. 6B). Consistent with the higher biliary and fecal mass excretion of sterols, probucol significantly enhanced the total excretion of 3H-cholesterol originating from macrophages into the feces of AdhApoE3-injected mice (6.6 ± 0.4 vs. 9.5 ± 0.5% injected tracer dose; P < 0.01; Fig. 6C). Because tracer recovery in the fecal bile acid fraction remained unaltered (5.3 ± 0.4 vs. 5.7 ± 0.4% injected tracer dose; n.s.; Fig. 6C), this was attributable to a 2.7-fold higher excretion of 3H-cholesterol in the fecal neutral sterol fraction (1.4 ± 0.2% vs. 3.8 ± 0.5% injected tracer dose; P < 0.01; Fig. 6C). These findings demonstrate that probucol results in an increased movement of cholesterol from macrophages to the feces in mice with hepatic apoE overexpression.
Fig. 6.
Probucol treatment increases in vivo macrophage-to-feces reverse cholesterol transport in apolipoprotein E-overexpressing mice. Mice were fed a control chow diet or a chow diet containing 0.5% probucol for 12 days before and then throughout the 48-h period of the experiment. On day 2 after injection with the human apolipoprotein E- expressing adenovirus, AdhApoE3 mice received intraperitoneal injections with 3H-cholesterol-loaded primary mouse macrophage foam cells as described in Materials and Methods. A: Time course of 3H-cholesterol recovery in plasma. B: 3H-cholesterol within liver 48 h after macrophage administration. C: 3H-cholesterol appearance in feces collected continuously from 0 to 48 h after macrophage administration and separated into bile acid and neutral sterol fractions as indicated. Data are expressed as percentage of the injected tracer dose and presented as means ± SEM. n = 8 mice for each condition. White bars, chow-fed controls; black bars, probucol-treated mice. * Significantly different from the respective controls as assessed by Mann-Whitney U-test (at least P < 0.05).
DISCUSSION
This study demonstrates that hepatic overexpression of human apoE not only promotes SR-BI-mediated selective uptake of HDL cholesterol into the liver but also enhances the resecretion of RCT-relevant cholesterol via hepatocyte ABCA1 back to the plasma compartment. As a result, biliary and fecal mass sterol excretion and RCT remain unchanged in apoE-overexpressing mice with active ABCA1, whereas all of these parameters increase significantly when ABCA1 activity is blocked with probucol. Collectively, these results point to a metabolic shunt potentially connecting the SR-BI and the ABCA1 pathway with a high relevance for the regulation of RCT.
The RCT pathway represents an important atheroprotective functionality of HDL (16, 17). RCT comprises cholesterol efflux from macrophage foam cells within the vessel wall, the transport of this cholesterol within HDL through the plasma compartment, the subsequent hepatic uptake via SR-BI or as a holoparticle, and excretion into the bile and feces (16, 17). In vitro, apoE expression by macrophages has been shown to stimulate cholesterol efflux (12–14). In addition, in vivo macrophage-to-feces RCT was lower when apoE-deficient macrophages were injected into wild-type mice compared with wild-type macrophages injected into wild-type mice (15). Combined with our present results, these data suggest that apoE expression by macrophages might determine the general availability of macrophage-derived cholesterol for the RCT pathway directly at the point of entry, whereas in subsequent steps of RCT hepatic ABCA1 counteracts these effects.
Experiments in SR-BI knockout mice as well as HDL kinetic studies in the current report demonstrated that apoE overexpression specifically increased selective uptake of HDL cholesterol into the liver. Importantly, the expression levels of hepatic SR-BI did not change in response to apoE overexpression. Therefore, our present findings are complementary to previous work demonstrating impaired hepatic selective HDL cholesterol uptake in mice lacking apoE (32), although the apoE knockout mouse model exhibits a considerably altered plasma lipid profile with substantially increased levels of apoB-containing lipoprotein remnants (32). In addition, selective uptake of HDL cholesterol into HepG2 cells after treatment with a blocking antibody directed against apoE was reduced (33). It is possible that apoE stimulates SR-BI-mediated selective uptake due to an improved interaction of the HDL particle with SR-BI (32), although the exact mechanisms will require further investigation.
Although hepatic human apoE overexpression increased the hepatic cholesterol content, biliary cholesterol excretion and the overall elimination of cholesterol from the body did not increase in wild-type mice or hCETP tg mice. The current observation that increased SR-BI-mediated selective uptake does not necessarily translate into enhanced biliary and fecal sterol excretion in the face of unaltered hepatic SR-BI expression is in agreement with earlier observations made by us in mice overexpressing group IIA secretory phospholipase A2 (23) or endothelial lipase (EL) (21, 34). Transgenic overexpression of secretory phospholipase A2 or adenovirus-mediated overexpression of EL also resulted in an increased flux of cholesterol into the liver, whereas biliary cholesterol secretion and mass fecal excretion of sterols remained unaffected (21, 23, 34). On the other hand, altering the hepatic expression level of SR-BI has clear effects on biliary cholesterol secretion as well as RCT with overexpression resulting in an increase and knockdown in a decrease of both of these parameters (24, 34, 35). How can this discrepancy be explained? Recent data generated in probucol-fed mice demonstrated that a decrease in hepatic ABCA1 activity results in increased in vivo RCT (31). We therefore speculate that increased cholesterol uptake into the liver via SR-BI results in transport to a specific intrahepatic compartment also accessible for ABCA1-mediated resecretion back into the plasma compartment to generate new HDL particles. Increasing hepatic SR-BI expression levels (24, 34) or decreasing the activity of ABCA1 might shift the balance toward biliary secretion (present report and Reference 31). However, the nature of these intrahepatic cholesterol pools is unknown. Future in vitro studies should therefore focus on the intracellular trafficking of cholesterol and, using a pulse-chase set-up, on the incorporation of HDL-derived cholesterol tracers into newly formed HDL in models with altered expression of hepatocyte ABCA1. Ideally, such studies should be carried out in polarized liver cells. To formally relate the cholesterol mass changes observed in our current study to each other, quantifying cholesterol fluxes would be required, which has not been done in our present study and thus represents a potential limitation in the interpretation of our current work.
Systemic apoE (7–11) and macrophage-derived apoE (36–39) have been shown to protect against atherosclerotic lesion formation due to effects not immediately involving RCT. ApoE promotes the clearance of atherogenic lipoproteins (6, 7, 10), and also a number of pleiotropic effects of apoE might be important. For example, apoE has antioxidative properties. ApoE prevented oxidative cell death in cultured neuronal cells and inhibited copper-mediated oxidation of LDL, a key event in the initiation and progression of atherosclerotic lesions (40). Moreover, apoE knockout mice have significantly increased in vivo oxidative stress, as determined by plasma, urinary, and vascular isoprostane levels (41), whereas hepatic overexpression of apoE in LDL receptor knockout mice resulted in a reduced oxidative stress burden and decreased atherosclerosis independent of plasma lipid and lipoprotein levels (9). Anti-inflammatory activities of apoE also add to its atheroprotective properties. In vitro studies have shown that apoE can suppress proliferation of cultured peripheral blood T lymphocytes (42) and shifts polarization of mouse macrophages to the anti-inflammatory M2 phenotype (43). In vivo, apoE-deficient mice exhibit an exaggerated proinflammatory cytokine response after LPS injection compared with wild-type mice (44, 45), which could be partially normalized by hepatic overexpression of human apoE3 (45). In addition, apoE can prevent LPS-induced mortality in wild-type mice (44). More recently, hepatic expression of human apoE has been shown to limit monocyte entry into the vessel wall and thereby to contribute significantly to regression of preexisting plaques in apoE knockout mice (46). Finally, apoE might be antiatherogenic by inhibiting platelet aggregation (47) as well as proliferation of vascular smooth muscle cells (48).
In summary, this study demonstrates that hepatic overexpression of human apoE3 not only facilitates SR-BI-mediated selective uptake of HDL cholesterol into the liver but also increases ABCA1-mediated resecretion of RCT-relevant cholesterol back into the plasma compartment. Decreasing hepatocyte ABCA1 activity might therefore represent a strategy to enhance the anti-atherosclerotic efficacy of apoE. However, before applying such strategies, the underlying mechanisms need to be delineated in more detail.
Supplementary Material
Acknowledgments
The authors thank the Vector Core of the University of Pennsylvania for producing the vectors.
Footnotes
Abbreviations:
- CE
- cholesteryl ether
- CETP
- cholesteryl ester transfer protein
- FCR
- fractional catabolic rate
- FPLC
- fast protein liquid chromatography
- RCT
- reverse cholesterol transport
- SR-BI
- scavenger receptor class B type I
This work was supported by the Netherlands Organization for Scientific Research VIDI grant 917-56-358 (U.J.F.T.), by the Top Institute (TI) Food and Nutrition (U.J.F.T.), and by grants HL022633 and HL059407 from the NHLBI (D.J.R.). P.C.N.R. is an Established Investigator of the Netherlands Heart Foundation (2009T038).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five tables, six figures, and supplemental Methods.
REFERENCES
- 1.Mahley R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 240: 622–630 [DOI] [PubMed] [Google Scholar]
- 2.Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. 1985. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J. Biol. Chem. 260: 2452–2457 [PubMed] [Google Scholar]
- 3.Basu S. K., Goldstein J. L., Brown M. S. 1983. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 219: 871–873 [DOI] [PubMed] [Google Scholar]
- 4.Plump A. S., Smith J. D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. 1992. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71: 343–353 [DOI] [PubMed] [Google Scholar]
- 5.Schaefer E. J., Gregg R. E., Ghiselli G., Forte T. M., Ordovas J. M., Zech L. A., Brewer H. B., Jr 1986. Familial apolipoprotein E deficiency. J. Clin. Invest. 78: 1206–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashyap V. S., Santamarina-Fojo S., Brown D. R., Parrott C. L., Applebaum-Bowden D., Meyn S., Talley G., Paigen B., Maeda N., Brewer H. B., Jr 1995. Apolipoprotein E deficiency in mice: gene replacement and prevention of atherosclerosis using adenovirus vectors. J. Clin. Invest. 96: 1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukamoto K., Tangirala R., Chun S. H., Pure E., Rader D. J. 1999. Rapid regression of atherosclerosis induced by liver-directed gene transfer of ApoE in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19: 2162–2170 [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto K., Tangirala R. K., Chun S., Usher D., Pure E., Rader D. J. 2000. Hepatic expression of apolipoprotein E inhibits progression of atherosclerosis without reducing cholesterol levels in LDL receptor-deficient mice. Mol. Ther. 1: 189–194 [DOI] [PubMed] [Google Scholar]
- 9.Tangirala R. K., Pratico D., FitzGerald G. A., Chun S., Tsukamoto K., Maugeais C., Usher D. C., Pure E., Rader D. J. 2001. Reduction of isoprostanes and regression of advanced atherosclerosis by apolipoprotein E. J. Biol. Chem. 276: 261–266 [DOI] [PubMed] [Google Scholar]
- 10.Kitajima K., Marchadier D. H., Miller G. C., Gao G. P., Wilson J. M., Rader D. J. 2006. Complete prevention of atherosclerosis in apoE-deficient mice by hepatic human apoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler. Thromb. Vasc. Biol. 26: 1852–1857 [DOI] [PubMed] [Google Scholar]
- 11.Kawashiri M., Zhang Y., Usher D., Reilly M., Pure E., Rader D. J. 2001. Effects of coexpression of the LDL receptor and apoE on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 42: 943–950 [PubMed] [Google Scholar]
- 12.Mazzone T., Reardon C. 1994. Expression of heterologous human apolipoprotein E by J774 macrophages enhances cholesterol efflux to HDL3. J. Lipid Res. 35: 1345–1353 [PubMed] [Google Scholar]
- 13.Lin C. Y., Duan H., Mazzone T. 1999. Apolipoprotein E-dependent cholesterol efflux from macrophages: kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J. Lipid Res. 40: 1618–1627 [PubMed] [Google Scholar]
- 14.Langer C., Huang Y., Cullen P., Wiesenhutter B., Mahley R. W., Assmann G., von Eckardstein A. 2000. Endogenous apolipoprotein E modulates cholesterol efflux and cholesteryl ester hydrolysis mediated by high-density lipoprotein-3 and lipid-free apolipoproteins in mouse peritoneal macrophages. J Mol Med (Berl). 78: 217–227 [DOI] [PubMed] [Google Scholar]
- 15.Zanotti I., Pedrelli M., Poti F., Stomeo G., Gomaraschi M., Calabresi L., Bernini F. 2011. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 31: 74–80 [DOI] [PubMed] [Google Scholar]
- 16.Lewis G. F., Rader D. J. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96: 1221–1232 [DOI] [PubMed] [Google Scholar]
- 17.Cuchel M., Rader D. J. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113: 2548–2555 [DOI] [PubMed] [Google Scholar]
- 18.Nijstad N., Gautier T., Briand F., Rader D. J., Tietge U. J. 2011. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology. 140: 1043–1051 [DOI] [PubMed] [Google Scholar]
- 19.Maugeais C., Tietge U. J., Tsukamoto K., Glick J. M., Rader D. J. 2000. Hepatic apolipoprotein E expression promotes very low density lipoprotein-apolipoprotein B production in vivo in mice. J. Lipid Res. 41: 1673–1679 [PubMed] [Google Scholar]
- 20.Tietge U. J., Kozarsky K. F., Donahee M. H., Rader D. J. 2003. A tetracycline-regulated adenoviral expression system for in vivo delivery of transgenes to lung and liver. J. Gene Med. 5: 567–575 [DOI] [PubMed] [Google Scholar]
- 21.Nijstad N., Wiersma H., Gautier T., van der Giet M., Maugeais C., Tietge U. J. 2009. Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotein by endothelial lipase. J. Biol. Chem. 284: 6093–6100 [DOI] [PubMed] [Google Scholar]
- 22.Kappelle P. J., de Boer J. F., Perton F. G., Annema W., de Vries R., Dullaart R. P., Tietge U. J. 2011. Increased LCAT activity and hyperglycaemia decrease the antioxidative functionality of HDL. Eur. J. Clin. Invest. doi: 10.1111/j.1365-2362.2011.02604.x [DOI] [PubMed] [Google Scholar]
- 23.Tietge U. J., Nijstad N., Havinga R., Baller J. F., van der Sluijs F. H., Bloks V. W., Gautier T., Kuipers F. 2008. Secretory phospholipase A2 increases SR-BI-mediated selective uptake from HDL but not biliary cholesterol secretion. J. Lipid Res. 49: 563–571 [DOI] [PubMed] [Google Scholar]
- 24.Wiersma H., Gatti A., Nijstad N., Oude Elferink R. P., Kuipers F., Tietge U. J. 2009. Scavenger receptor class B type I mediates biliary cholesterol secretion independent of ATP-binding cassette transporter g5/g8 in mice. Hepatology. 50: 1263–1272 [DOI] [PubMed] [Google Scholar]
- 25.Tietge U. J., Maugeais C., Cain W., Rader D. J. 2003. Acute inflammation increases selective uptake of HDL cholesteryl esters into adrenals of mice overexpressing human sPLA2. Am. J. Physiol. Endocrinol. Metab. 285: E403–E411 [DOI] [PubMed] [Google Scholar]
- 26.Tietge U. J., Maugeais C., Cain W., Grass D., Glick J. M., de Beer F. C., Rader D. J. 2000. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J. Biol. Chem. 275: 10077–10084 [DOI] [PubMed] [Google Scholar]
- 27.Annema W., Nijstad N., Tolle M., de Boer J. F., Buijs R. V., Heeringa P., van der Giet M., Tietge U. J. 2010. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J. Lipid Res. 51: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tietge U. J., Pratico D., Ding T., Funk C. D., Hildebrand R. B., Van Berkel T., Van Eck M. 2005. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J. Lipid Res. 46: 1604–1614 [DOI] [PubMed] [Google Scholar]
- 29.Guyard-Dangremont V., Desrumaux C., Gambert P., Lallemant C., Lagrost L. 1998. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120: 517–525 [DOI] [PubMed] [Google Scholar]
- 30.Out R., Hoekstra M., Spijkers J. A., Kruijt J. K., van Eck M., Bos I. S., Twisk J., Van Berkel T. J. 2004. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J. Lipid Res. 45: 2088–2095 [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S., Tanigawa H., Li X., Komaru Y., Billheimer J. T., Rader D. J. 2011. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 124: 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai T., Rinninger F., Varban L., Fairchild-Huntress V., Liang C. P., Chen W., Seo T., Deckelbaum R., Huszar D., Tall A. R. 1999. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc. Natl. Acad. Sci. USA. 96: 12050–12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leblond L., Marcel Y. L. 1993. Uptake of high density lipoprotein cholesterol ester by HepG2 cells involves apolipoprotein E localized on the cell surface. J. Biol. Chem. 268: 1670–1676 [PubMed] [Google Scholar]
- 34.Wiersma H., Gatti A., Nijstad N., Kuipers F., Tietge U. J. 2009. Hepatic SR-BI, not endothelial lipase, expression determines biliary cholesterol secretion in mice. J. Lipid Res. 50: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Da Silva J. R., Reilly M., Billheimer J. T., Rothblat G. H., Rader D. J. 2005. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 115: 2870–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linton M. F., Atkinson J. B., Fazio S. 1995. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267: 1034–1037 [DOI] [PubMed] [Google Scholar]
- 37.Bellosta S., Mahley R. W., Sanan D. A., Murata J., Newland D. L., Taylor J. M., Pitas R. E. 1995. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J. Clin. Invest. 96: 2170–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasty A. H., Linton M. F., Brandt S. J., Babaev V. R., Gleaves L. A., Fazio S. 1999. Retroviral gene therapy in ApoE-deficient mice: ApoE expression in the artery wall reduces early foam cell lesion formation. Circulation. 99: 2571–2576 [DOI] [PubMed] [Google Scholar]
- 39.Tennert C., Teupser D., Mueller M. A., Wilfert W., Renner-Muller I., Stein O., Stein Y., Sippel A. E., Wolf E., Thiery J. 2007. Effect of macrophage ApoE on atherosclerosis in LDL-receptor deficient mice. Biochem. Biophys. Res. Commun. 361: 574–579 [DOI] [PubMed] [Google Scholar]
- 40.Miyata M., Smith J. D. 1996. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 14: 55–61 [DOI] [PubMed] [Google Scholar]
- 41.Pratico D., Tangirala R. K., Rader D. J., Rokach J., FitzGerald G. A. 1998. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 4: 1189–1192 [DOI] [PubMed] [Google Scholar]
- 42.Kelly M. E., Clay M. A., Mistry M. J., Hsieh-Li H. M., Harmony J. A. 1994. Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cell. Immunol. 159: 124–139 [DOI] [PubMed] [Google Scholar]
- 43.Baitsch D., Bock H. H., Engel T., Telgmann R., Muller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., et al. 2011. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31: 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Oosten M., Rensen P. C., Van Amersfoort E. S., Van Eck M., Van Dam A. M., Breve J. J., Vogel T., Panet A., Van Berkel T. J., Kuiper J. 2001. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J. Biol. Chem. 276: 8820–8824 [DOI] [PubMed] [Google Scholar]
- 45.Ali K., Middleton M., Pure E., Rader D. J. 2005. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ. Res. 97: 922–927 [DOI] [PubMed] [Google Scholar]
- 46.Potteaux S., Gautier E. L., Hutchison S. B., van Rooijen N., Rader D. J., Thomas M. J., Sorci-Thomas M. G., Randolph G. J. 2011. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest. 121: 2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riddell D. R., Graham A., Owen J. S. 1997. Apolipoprotein E inhibits platelet aggregation through the L-arginine:nitric oxide pathway. Implications for vascular disease. J. Biol. Chem. 272: 89–95 [DOI] [PubMed] [Google Scholar]
- 48.Kothapalli D., Fuki I., Ali K., Stewart S. A., Zhao L., Yahil R., Kwiatkowski D., Hawthorne E. A., FitzGerald G. A., Phillips M. C., et al. 2004. Antimitogenic effects of HDL and APOE mediated by Cox-2-dependent IP activation. J. Clin. Invest. 113: 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.