Abstract
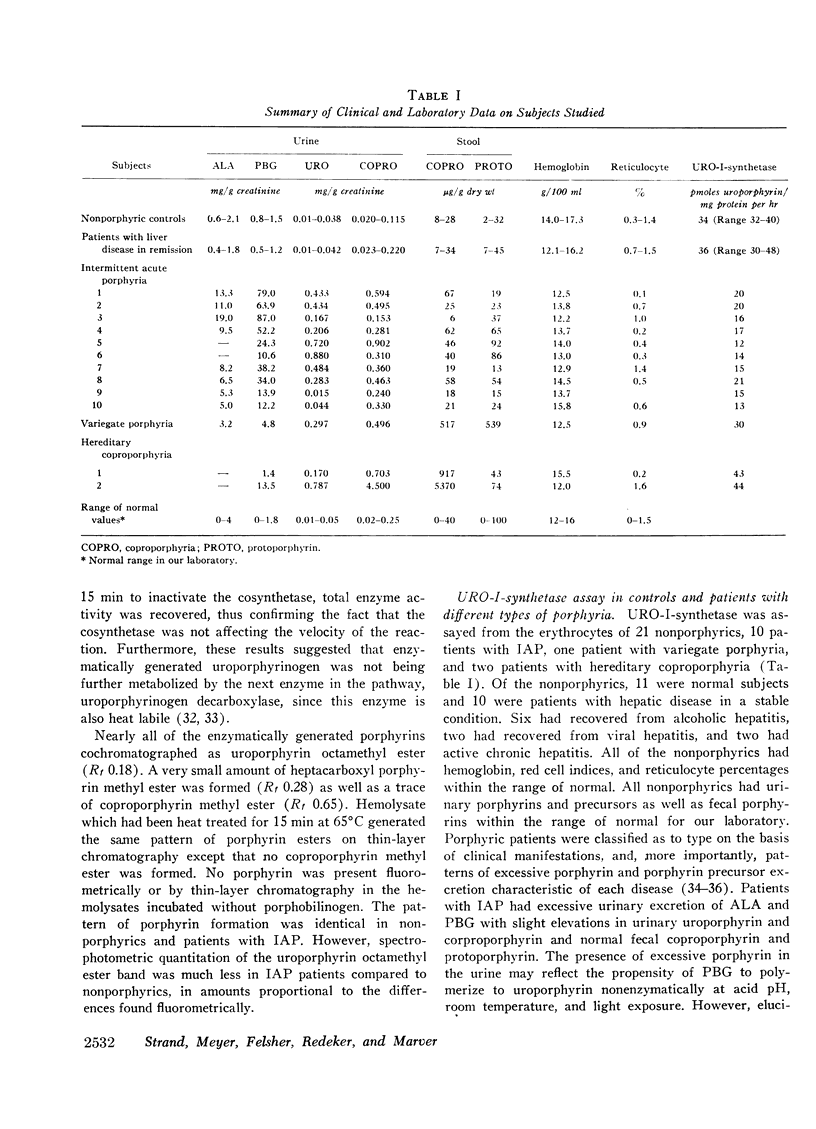
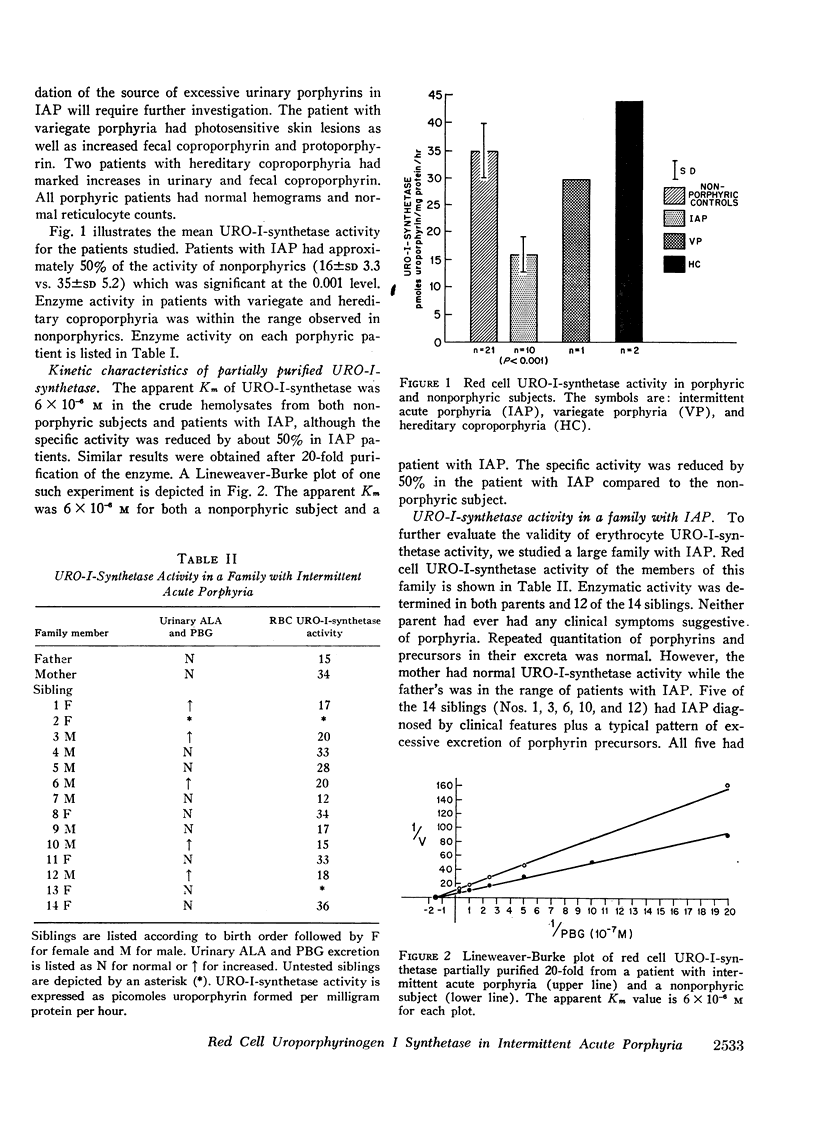
Intermittent acute porphyria has recently been distinguished biochemically from other genetic hepatic porphyrias by the observation of diminished hepatic uroporphyrinogen I synthetase activity and increased δ-aminolevulinic acid synthetase activity. Since deficient uroporphyrinogen I synthetase may be reflected in nonhepatic tissues, we have assayed this enzyme in red cell hemolysates from nonporphyric subjects and from patients with genetic hepatic porphyria. Only patients with intermittent acute porphyria had decreased erythrocyte uroporphyrinogen I synthetase activity which was approximately 50% of normal. The apparent Km of partially purified uroporphyrinogen I synthetase was 6 × 10−6m in both nonporphyrics and patients with intermittent acute porphyria. These data provide further evidence for a primary mutation affecting uroporphyrinogen I synthetase in intermittent acute porphyria. Further-more, results of assay of red cell uroporphyrinogen I synthetase activity in a large family with intermittent acute porphyria suggest that this test may be a reliable indicator of the heterozygous state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- Bloomer J. R., Berk P. D., Bonkowsky H. L., Stein J. A., Berlin N. I., Tschudy D. P. Blood volume and bilirubin production in acute intermittent porphyria. N Engl J Med. 1971 Jan 7;284(1):17–20. doi: 10.1056/NEJM197101072840104. [DOI] [PubMed] [Google Scholar]
- COOKSON G. H., RIMINGTON C. Porphobilinogen. Biochem J. 1954 Jul;57(3):476–484. doi: 10.1042/bj0570476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M. Analytical and preparative thin-layer chromatography of porphyrin methyl esters. Z Klin Chem Klin Biochem. 1970 May;8(3):197–207. doi: 10.1515/cclm.1970.8.3.197. [DOI] [PubMed] [Google Scholar]
- Doss M., Bode U. Dünnschichtchromatographische Trennung von Porphyrinen, Hämin und Lipoiden auf Kieselgel-H-Platten zur Bestimmung der Erythrozytenporphyrine als Methylester. J Chromatogr. 1968 Jun 4;35(2):248–256. doi: 10.1016/s0021-9673(01)82381-5. [DOI] [PubMed] [Google Scholar]
- Dowdle E. B., Mustard P., Eales L. Delta-aminolaevulinic acid synthetase activity in normal and porphyric human livers. S Afr Med J. 1967 Nov 4;41(42):1093–1096. [PubMed] [Google Scholar]
- Doyle D., Schimke R. T. The genetic and developmental regulation of hepatic delta-aminolevulinate dehydratase in mice. J Biol Chem. 1969 Oct 25;244(20):5449–5459. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Goldberg A., Moore M. R., Beattie A. D., Hall P. E., McCallum J., Grant J. K. Excessive urinary excretion of certain porphyrinogenic steroids in human acute intermittent porphyria. Lancet. 1969 Jan 18;1(7586):115–118. doi: 10.1016/s0140-6736(69)91134-9. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- HENNESSEY M. A., WALTERSDORPH A. M., HUENNEKENS F. M., GABRIO B. W. Erythrocyte metabolism. VI. Separation of erythrocyte enzymes from hemoglobin. J Clin Invest. 1962 Jun;41:1257–1262. doi: 10.1172/JCI104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. II. Effects of hemin and bilirubin on enzyme induction. J Biochem. 1968 Apr;63(4):446–452. doi: 10.1093/oxfordjournals.jbchem.a128796. [DOI] [PubMed] [Google Scholar]
- Heilmeyer L., Clotten R. Zur biochemischen Pathogenese der Pophyria acuta intermittens. Klin Wochenschr. 1969 Jan 15;47(2):71–74. doi: 10.1007/BF01745768. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Gross S. R. Chemical induction of hepatic porphyria in inbred strains of mice. Arch Biochem Biophys. 1970 Nov;141(1):284–292. doi: 10.1016/0003-9861(70)90134-7. [DOI] [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Abnormal steroid hormone metabolism in the genetic liver disease acute intermittent porphyria. Ann N Y Acad Sci. 1971 Jul 6;179:611–624. doi: 10.1111/j.1749-6632.1971.tb46937.x. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Marver H. S. Biochemical defects in two types of human hepatic porphyria. N Engl J Med. 1970 Oct 29;283(18):954–958. doi: 10.1056/NEJM197010292831803. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin E. Y. Uroporphyrinogen 3 cosynthetase from mouse spleen. Biochemistry. 1968 Nov;7(11):3781–3788. doi: 10.1021/bi00851a001. [DOI] [PubMed] [Google Scholar]
- Llambías E. B., Batlle A. M. Porphyrin biosynthesis. 8. Avian erythrocyte porphobilinogen deaminase-uroporphyrinogen 3 cosynthetase, its purification, properties and the separation of its components. Biochim Biophys Acta. 1971 Jan 13;227(1):180–191. doi: 10.1016/0005-2744(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- McIntyre N., Pearson A. J., Allan D. J., Craske S., West G. M., Moore M. R., Beattie A. D., Paxton J., Goldberg A. Hepatic delta-aminolaevulinic acid synthetase in an attack of hereditary coproporphyria and during remission. Lancet. 1971 Mar 20;1(7699):560–564. doi: 10.1016/s0140-6736(71)91161-5. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Strand L. J., Doss M., Rees A. C., Marver H. S. Intermittent acute porphyria--demonstration of a genetic defect in porphobilinogen metabolism. N Engl J Med. 1972 Jun 15;286(24):1277–1282. doi: 10.1056/NEJM197206152862401. [DOI] [PubMed] [Google Scholar]
- Miyagi K., Cardinal R., Bossenmaier I., Watson C. J. The serum porphobilinogen and hepatic porphobilinogen deaminase in normal and porphyric individuals. J Lab Clin Med. 1971 Nov;78(5):683–695. [PubMed] [Google Scholar]
- Nakao K., Wada O., Kitamura T., Uono K., Urata G. Activity of amino-laevulinic acid synthetase in normal and porphyric human livers. Nature. 1966 May 21;210(5038):838–839. doi: 10.1038/210838b0. [DOI] [PubMed] [Google Scholar]
- Romeo G., Levin E. Y. Uroporphyrinogen decarboxylase from mouse spleen. Biochim Biophys Acta. 1971 Feb 23;230(2):330–341. doi: 10.1016/0304-4165(71)90220-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A., Hsia Y. E. Methylmalonic aciduria: metabolic block localization and vitamin B 12 dependency. Science. 1968 Nov 15;162(3855):805–807. doi: 10.1126/science.162.3855.805. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Manning J., Marver H. S. The induction of -aminolevulinic acid synthetase in cultured liver cells. The effects of end product and inhibitors of heme synthesis. J Biol Chem. 1972 May 10;247(9):2820–2827. [PubMed] [Google Scholar]
- Sweeney V. P., Pathak M. A., Asbury A. K. Acute intermittent porphyria. Increased ALA-synthetase activity during an acute attack. Brain. 1970;93(2):369–380. doi: 10.1093/brain/93.2.369. [DOI] [PubMed] [Google Scholar]
- TSCHUDY D. P., PERLROTH M. G., MARVER H. S., COLLINS A., HUNTER G., Jr, RECHCIGL M., Jr ACUTE INTERMITTENT PORPHYRIA: THE FIRST "OVERPRODUCTION DISEASE" LOCALIZED TO A SPECIFIC ENZYME. Proc Natl Acad Sci U S A. 1965 Apr;53:841–847. doi: 10.1073/pnas.53.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeini L., Watson C. J. The clinical porphyrias. Semin Hematol. 1968 Oct;5(4):335–369. [PubMed] [Google Scholar]
- Tomio J. M., García R. C., San Martín de Viale L. C., Grinstein M. Porphyrin biosynthesis. VII. Porphyrinogen carboxy-lyase from avian erythrocytes. Purification and properties. Biochim Biophys Acta. 1970 Feb 11;198(2):353–363. doi: 10.1016/0005-2744(70)90068-9. [DOI] [PubMed] [Google Scholar]
- Waldenström J., Haeger-Aronsen B. The porphyrias: a genetic problem. Prog Med Genet. 1967;5:58–101. doi: 10.1016/b978-1-4831-6757-2.50006-3. [DOI] [PubMed] [Google Scholar]


