Abstract
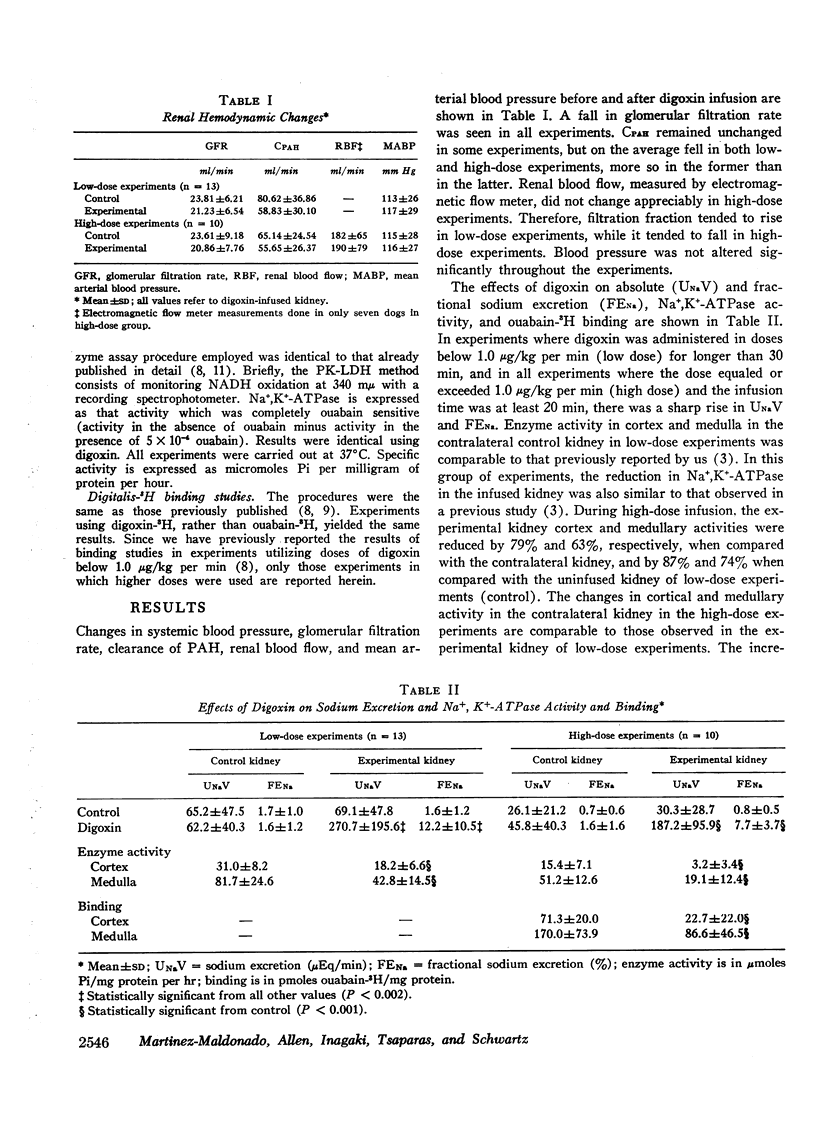
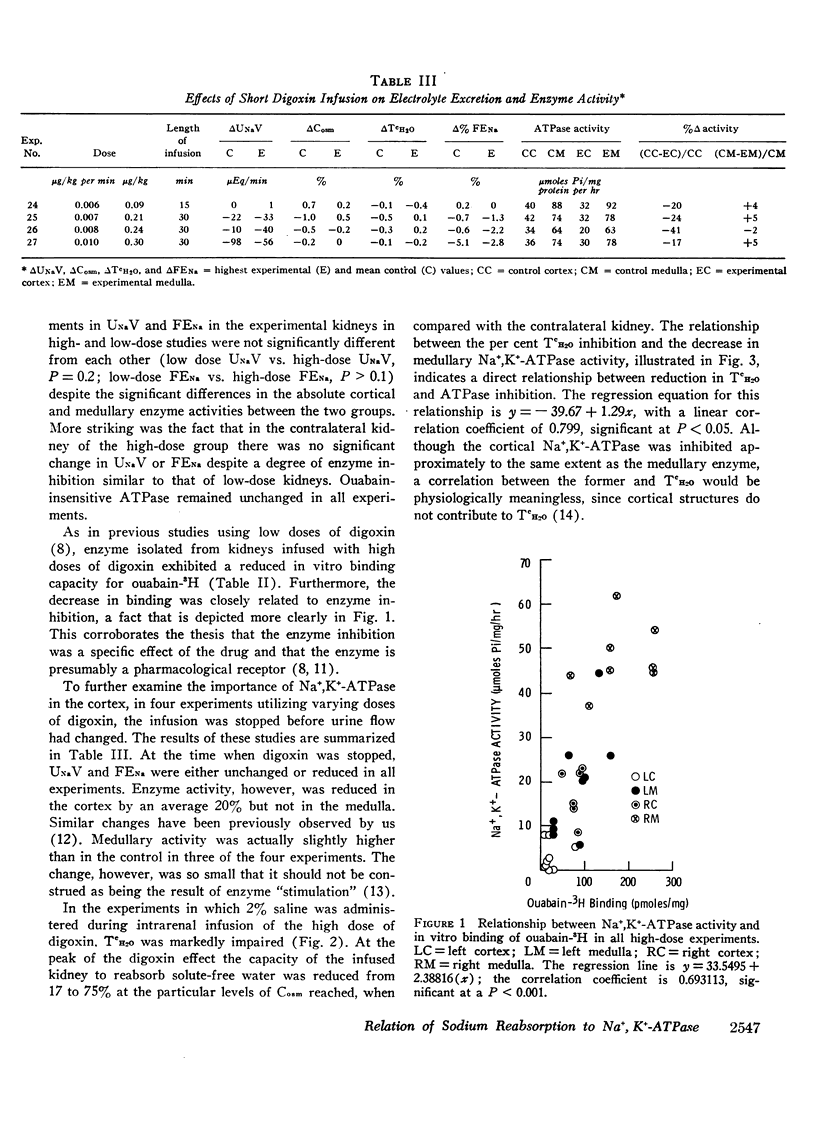
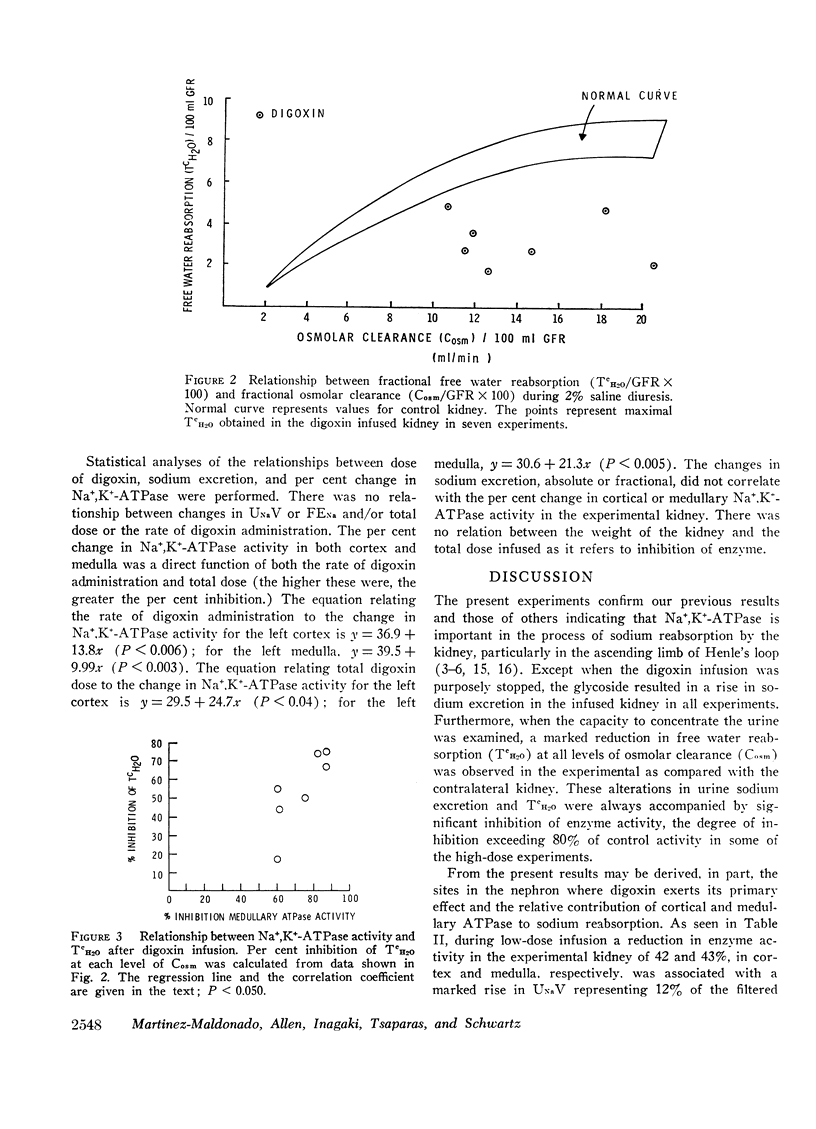
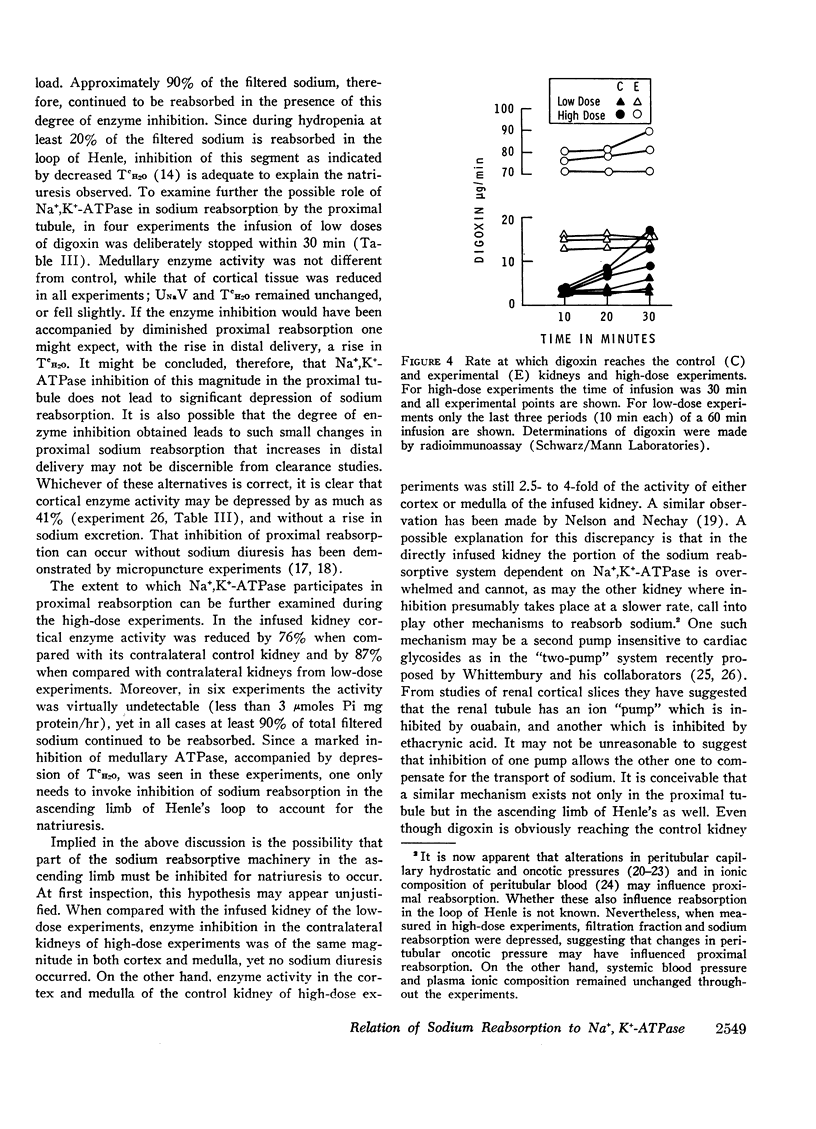
The role of renal Na+,K+-ATPase in sodium reabsorption was further examined in dogs in which digoxin, a specific inhibitor of the enzyme system, was infused into one renal artery in doses ranging from 0.4 to 0.9 μg/kg/min (low dose) and from 1.0 to 4.0 μg/kg/min (high dose). A significant natriuresis occurred with both dose ranges which was accompanied by inhibition of Na+,K+-ATPase of cortex and medulla in the infused kidney. Despite over 90% enzyme inhibition in many experiments, at least 80% of the filtered sodium continued to be reabsorbed. The per cent change in enzyme activity correlated with the rate of digoxin administration and the total dose administered but not with changes in sodium excretion. Changes in medullary Na+,K+-ATPase activity, however, bore a direct relationship to alterations in fractional solute free water reabsorption (TcH2O). Inhibition of cortical enzyme activity alone was not associated with natriuresis, suggesting that medullary enzyme activity must be depressed for increased sodium excretion to occur during digoxin infusion. In high-dose experiments, significant inhibition of cortical and medullary enzyme in the contralateral control kidney was also observed, but natriuresis did not occur. In these experiments the rate at which digoxin reached the control kidney rose progressively but never equaled the rates in the directly infused kidney with either dose. Nevertheless, it is clear that under certain circumstances enzyme inhibition of either cortex or medulla need not be accompanied by natriuresis. We conclude that the major role of renal Na+,K+-ATPase is in sodium reabsorption in the medulla (ascending limb of Henle's loop) and that it has a relatively small role in proximal sodium reabsorption. The kidney can rely on other sodium reabsorptive mechanisms depending on the rate of enzyme inhibition, so that natriuresis may not occur at all if depression in activity occurs “slowly.” The nature of these mechanisms is not clear.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C., Martinez-Maldonado M., Eknoyan G., Suki W. N., Schwartz A. Relation between digitalis binding in vivo and inhibition of sodium, potassium-adenosine triphosphatase in canine kidney. Biochem Pharmacol. 1971 Jan;20(1):73–80. doi: 10.1016/0006-2952(71)90473-4. [DOI] [PubMed] [Google Scholar]
- BONTING S. L., SIMON K. A., HAWKINS N. M. Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys. 1961 Dec;95:416–423. doi: 10.1016/0003-9861(61)90170-9. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Electrical potential difference across proximal convoluted tubules. Am J Physiol. 1970 Dec;219(6):1714–1716. doi: 10.1152/ajplegacy.1970.219.6.1714. [DOI] [PubMed] [Google Scholar]
- Earley L. E., Friedler R. M. The effects of combined renal vasodilatation and pressor agents on renal hemodynamics and the tubular reabsorption of sodium. J Clin Invest. 1966 Apr;45(4):542–551. doi: 10.1172/JCI105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknoyan G., Suki W. N., Rector F. C., Jr, Seldin D. W. Functional characteristics of the diluting segment of the dog nephron and the effect of extracellular volume expansion on its reabsorptive capacity. J Clin Invest. 1967 Jul;46(7):1178–1188. doi: 10.1172/JCI105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler E. D., Torretti J., Epstein F. H. The distribution of sodium-potassium--activated adenosine triphosphatase in medulla and cortex of the kidney. J Clin Invest. 1971 Jun;50(6):1329–1337. doi: 10.1172/JCI106612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L., Skou J. C. Preparation of highly active (Na+ + K+)-ATPase from the outer medulla of rabbit kidney. Biochem Biophys Res Commun. 1969 Sep 24;37(1):39–46. doi: 10.1016/0006-291x(69)90877-8. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Howards S. S., Wright F. S., Davis B. B., Berliner R. W. Effect of dilution and expansion of blood volume on proximal sodium reabsorption. Am J Physiol. 1968 Nov;215(5):1041–1048. doi: 10.1152/ajplegacy.1968.215.5.1041. [DOI] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M., Allen J. C., Eknoyan G., Suki W., Schwartz A. Renal concentrating mechanism: possible role for sodium-potassium activated adenosine triphosphatase. Science. 1969 Aug 22;165(3895):807–808. doi: 10.1126/science.165.3895.807. [DOI] [PubMed] [Google Scholar]
- Martinez-Maldonado M., Eknoyan G., Allen J. C., Suki W. N., Schwartz A. Urine dilution and concentration after digoxin infusion into the renal artery of dogs. Proc Soc Exp Biol Med. 1970 Jul;134(3):855–860. doi: 10.3181/00379727-134-34898. [DOI] [PubMed] [Google Scholar]
- Matsui H., Schwartz A. Purification and properties of a highly active ouabain-sensitive Na+, K+-dependent adenosinetriphosphatase from cardiac tissue. Biochim Biophys Acta. 1966 Nov 15;128(2):380–390. doi: 10.1016/0926-6593(66)90185-8. [DOI] [PubMed] [Google Scholar]
- Nechay B. R., Nelson J. A. Renal ouabain-sensitive adenosine triphosphatase activity and Na+ reabsorption. J Pharmacol Exp Ther. 1970 Dec;175(3):717–726. [PubMed] [Google Scholar]
- Nelson J. A., Nechay B. R. Effects of cardiac glycosides of renal adenosine triphosphatase activity and Na+ reabsorption in dogs. J Pharmacol Exp Ther. 1970 Dec;175(3):727–740. [PubMed] [Google Scholar]
- PALMER R. F., NECHAY B. R. BIPHASIC RENAL EFFECTS OF OUABAIN IN THE CHICKEN: CORRELATION WITH A MICROSOMAL NA+-K+ STIMULATED ATP-ASE. J Pharmacol Exp Ther. 1964 Oct;146:92–98. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Dubach U. C. Activity of (Na+K+)-stimulated adenosintriphosphatase in the rat nephron. Pflugers Arch. 1969;306(3):219–226. doi: 10.1007/BF00592433. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Sejersted O. M., Lie M., Kiil F. Effect of ouabain on metabolic rate in renal cortex and medulla. Am J Physiol. 1971 May;220(5):1488–1493. doi: 10.1152/ajplegacy.1971.220.5.1488. [DOI] [PubMed] [Google Scholar]
- Seldin D. W., Eknoyan G., Suki W. N., Rector F. C., Jr Localization of diuretic action from the pattern of water and electrolyte excretion. Ann N Y Acad Sci. 1966 Nov 22;139(2):328–343. doi: 10.1111/j.1749-6632.1966.tb41207.x. [DOI] [PubMed] [Google Scholar]
- WILDE W. S., HOWARD P. J. Renal tubular action of ouabain on Na and K transport during stop-flow and slow-flow technique. J Pharmacol Exp Ther. 1960 Oct;130:232–238. [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]


