Abstract
Thoracic lymph duct cannulations were performed shortly after a meal in rabbits trained to ingest a moderate fat, low cholesterol diet. A tracer dose of cholesterol-3H was administered to label exogenous (dietary) cholesterol during absorption. Sequential lymph samples were collected up to 24 hr postprandially, after which ultracentrifugal fractionation of lymph lipoproteins was carried out. The d < 1.006 lipoproteins were separated into two classes, chylomicra and very low density lipoproteins (VLDL).
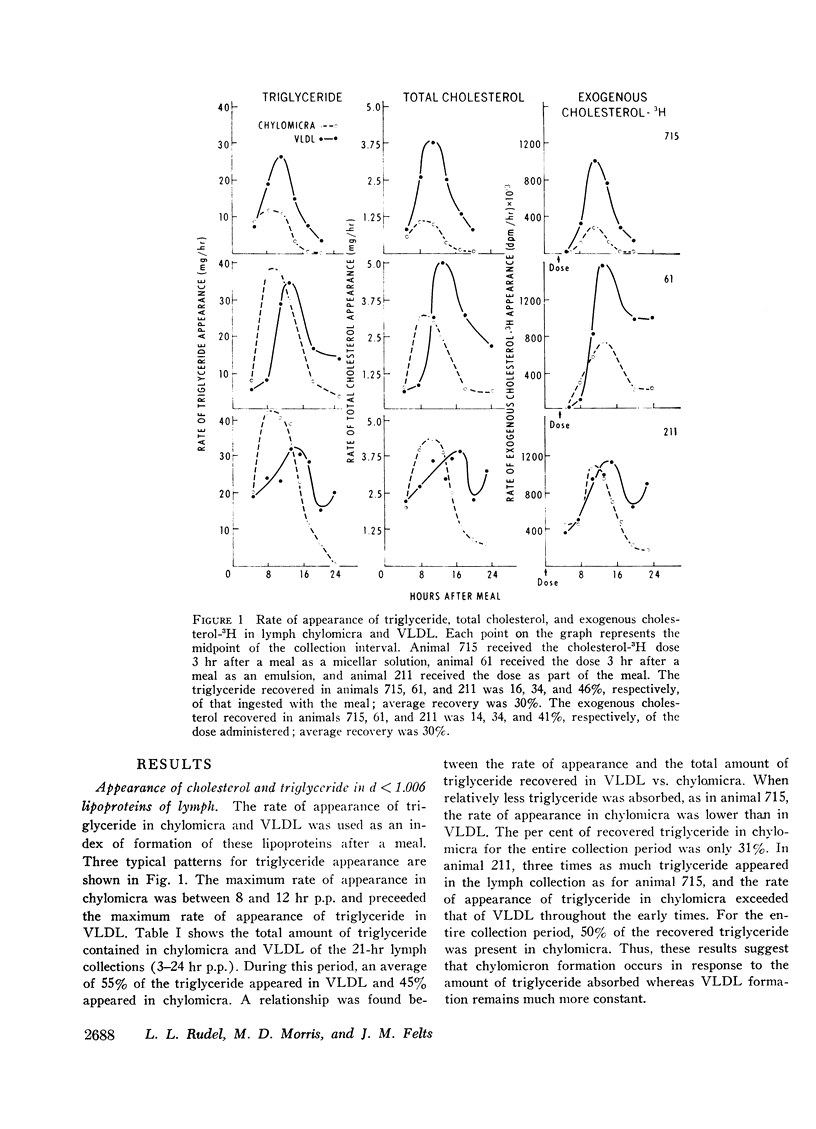
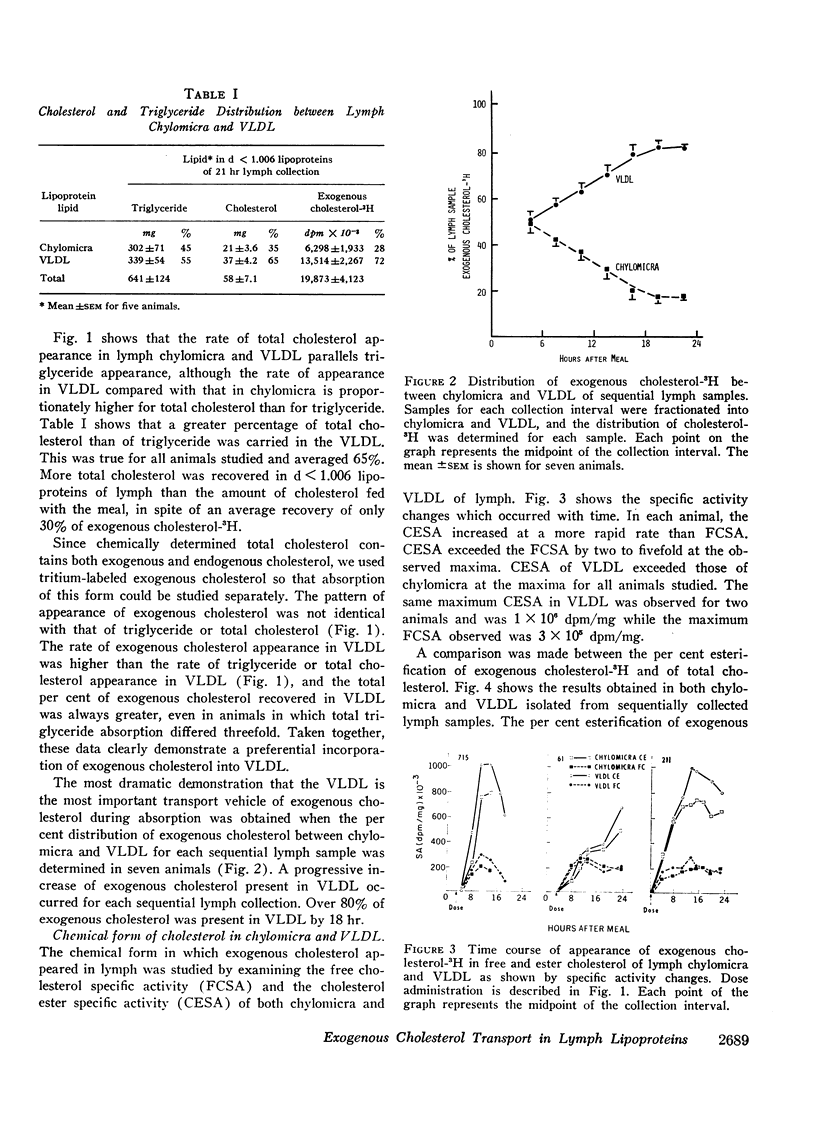
A comparison was made between chylomicra and VLDL of lymph in the transport of exogenous cholesterol after ingestion of a single meal. The per cent of exogenous cholesterol present in VLDL of sequential lymph collections progressively increased with time after a meal and by 18 hr had reached a value of 80% or greater. In chylomicra the per cent of exogenous cholesterol of sequential lymph collections progressively decreased. Therefore, exogenous cholesterol was preferentially transported in VLDL compared with chylomicra.
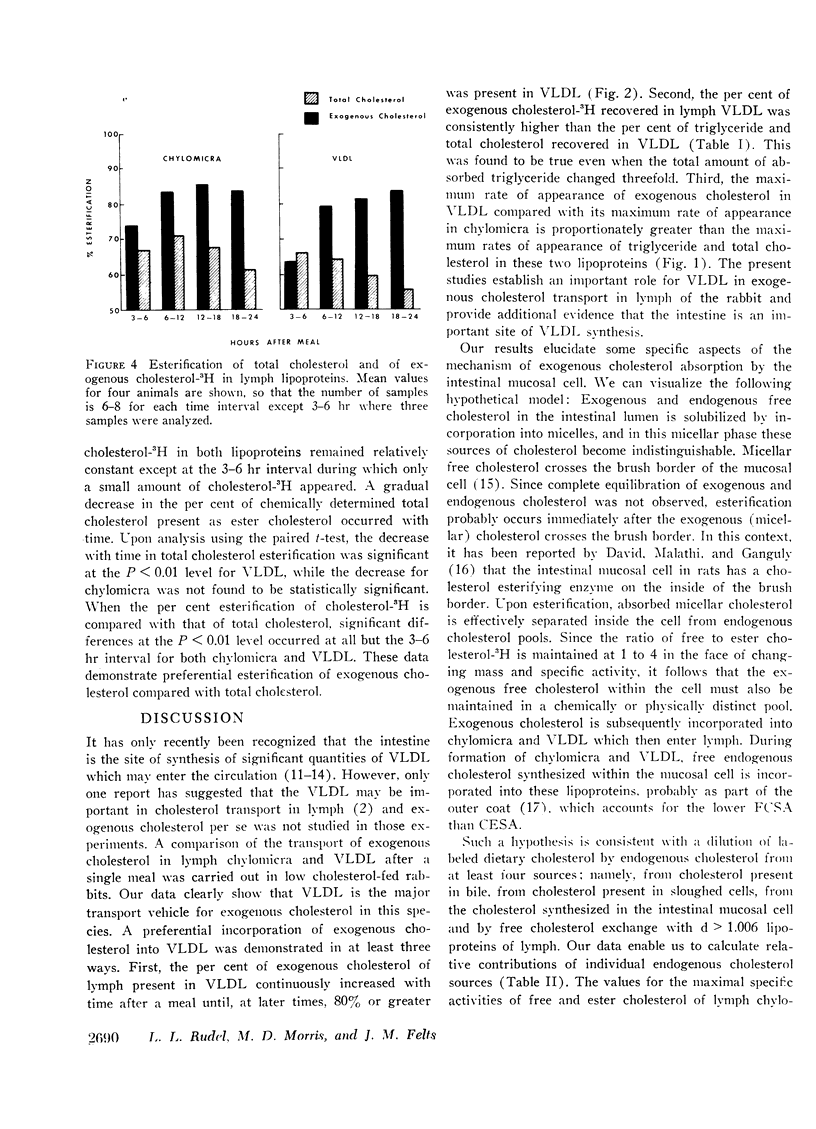
Cholesterol ester specific activity (CESA) of lymph chylomicra and VLDL increased at a more rapid rate than free cholesterol specific activity (FCSA). CESA of VLDL was three times higher than FCSA at the maximum. Exogenous cholesterol which appeared in both chylomicra and VLDL was consistently 80% esterified. while the per cent of total cholesterol esterified decreased with time and was significantly lower than that for exogenous cholesterol from 6 to 24 hr postprandially. These results demonstrate preferential esterification of exogenous cholesterol during absorption and indicate that a mechanism exists within the intestinal mucosal cell to maintain both free and esterified exogenous cholesterol in a chemically distinct pool from endogenous cholesterol during incorporation into both chylomicra and VLDL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. Origin and characteristics of endogenous lipid in thoracic duct lymph in rat. J Lipid Res. 1966 Jan;7(1):158–166. [PubMed] [Google Scholar]
- CLARKSON T. B. ATHEROSCLEROSIS--SPONTANEOUS AND INDUCED. Adv Lipid Res. 1963;1:211–252. [PubMed] [Google Scholar]
- David J. S., Malathi P., Ganguly J. Role of the intestinal brush border in the absorption of cholesterol in rats. Biochem J. 1966 Mar;98(3):662–668. doi: 10.1042/bj0980662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S. Cholesterol ester metabolism. Physiol Rev. 1965 Oct;45(4):747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P. S., Gidez L. I., Eder H. A. Extrahepatic synthesis of lipoproteins of plasma and chyle: role of the intestine. J Clin Invest. 1966 Mar;45(3):297–300. doi: 10.1172/JCI105343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]
- Wilson J. D., Reinke R. T. Transfer of locally synthesized cholesterol from intestinal wall to intestinal lymph. J Lipid Res. 1968 Jan;9(1):85–92. [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- Zilversmit D. B., Courtice F. C., Fraser R. Cholesterol transport in thoracic duct lymph of the rabbit. J Atheroscler Res. 1967 May-Jun;7(3):319–329. doi: 10.1016/s0368-1319(67)80059-0. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. The surface coat of chylomicrons: lipid chemistry. J Lipid Res. 1968 Mar;9(2):180–186. [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]


