Differences in pathophysiology may affect the diagnosis, prevention, and treatment of diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders (AANHPIs). Equally important are differences in cultural beliefs, dietary habits, and behavioral patterns among AANHPIs that require culturally effective translation of interventions into the community. These issues were discussed by clinicians and investigators at a conference held in Honolulu, Hawaii, in September 2011 with the theme “Diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders: A Call to Action” by a coalition of health care organizations, clinicians, and scientists with strong interests in the topic and in the health of AANHPI populations (1). The discourse begins with an evaluation of pathophysiologic differences, followed by a discussion of the standard diagnostic criteria and current treatment algorithms as well as dietary recommendations. The focus then shifts to various diabetes prevention studies specific to AANHPIs and their relevance to this growing ethnic minority group in America. This review concludes with two investigations that demonstrate culturally appropriate interventions and a brief description of the role played by the National Diabetes Education Program (NDEP).
PATHOPHYSIOLOGIC DIFFERENCES
Pathophysiology—type 1 diabetes
Diabetes has reached an epidemic level around the world, with most of the increase attributable to type 2 diabetes in developing Asian countries such as India and China (2). Type 1 diabetes is also increasing, although at a much less dramatic rate than type 2 diabetes and is now also increasingly associated with obesity and insulin resistance (3). The highest rates for type 1 diabetes are found in Northern European and Scandinavian countries and among the Caucasian population of the U.S. In contrast, type 1 diabetes is approximately 5 to 10 times lower in prevalence in those of Asian than those of European descent (4). In the U.S., the incidence of type 1 diabetes is lower by two- to fivefold in blacks, Asian Americans, and Hispanics compared with Caucasians (5). A study of ethnically diverse American children with diabetes showed that most children aged <9 years had typical type 1 diabetes (6). However, among children aged 10 to 19 years, merely 10% of Caucasian children had type 2 diabetes in contrast to 40% of Asian American and Pacific Islander children (7).
In Caucasians, type 1 diabetes is closely associated with certain HLA haplotypes, such as DR3/4 and DQB1, and other genes such as insulin, cytotoxic t-lymphocyte antigen 4 (INS, CTLA4), but only ∼30% of East Asians and Asian Americans have these genotypes (8). The presence of antibodies typically associated with type 1 diabetes was also low, less than 50% in various East Asian groups (9). The low occurrence of DR3 and DR4 in Asian American children with type 1 diabetes correlated with their low incidence for type 1 diabetes. However, despite the low frequency of the at-risk HLA genotype in Asians, among siblings who are positive for the genotype, in Japan for example, there is an increased rate of developing type 1 diabetes that is comparable to Caucasians (10). Other differences in genetic markers exist between Asians and Caucasians with type 1 diabetes; for example, a variant of small ubiquitin-like modifier 4 (SUMO4) was associated with diabetes in East Asians but not in Caucasians (9).
A recent study showed that type 1 diabetes can be difficult to clinically differentiate from type 2 diabetes in East Asian Americans, especially among those aged <35 years, because BMI is equally low, ∼24 kg/m2, in both types (11). This is very different from other ethnic groups, where the BMIs of type 2 diabetes patients are usually high and >27 kg/m2. However, type 1 diabetes can be distinguished from type 2 diabetes in these patients by differences in several biochemical parameters such as higher concentrations of HDL and adiponectin and lower concentrations of c-peptide in type 1 diabetes. In addition, insulin clamp studies showed significantly higher glucose disposal rates, similar to nondiabetic control subjects in type 1 diabetes than in type 2 diabetes, indicating reduced insulin sensitivity in type 2 diabetic patients even with BMIs similar to Asian Americans with type 1 diabetes. Low positivity of auto-antibodies at ∼30% in Asian American patients with type 1 diabetes also raises questions about mechanisms underlying the destruction of β-cells in this population.
Pathophysiology—type 2 diabetes
The pathogenesis of type 2 diabetes is closely associated with increased body weight, obesity, sedentary lifestyle, inflammation, and insulin resistance. However, the relationship between excess adiposity and diabetes is not so straightforward in AANHPIs. Native Hawaiians and other Pacific Islanders have a higher prevalence of type 2 diabetes and are predisposed to obesity. Surprisingly, South Asians (Indians) and East Asians (Chinese, Koreans, and Japanese) typically have a lower mean BMI and waist circumference but a higher prevalence of diabetes and other metabolic conditions compared with Caucasians at similar levels of BMI and waist circumference.
To help explain this paradox, the role of body composition in diabetes risk was assessed further in multiple publications from the Japanese American Community Diabetes Study, a prospective study of second- and third-generation Japanese Americans of 100% Japanese ancestry. Results of this study have demonstrated the overriding importance of visceral fat as a risk factor for adverse metabolic outcomes such as coronary heart disease, hypertension, impaired glucose tolerance (IGT), type 2 diabetes, metabolic syndrome, and insulin resistance (12). Visceral fat measured by computed tomography as intra-abdominal fat area, in contrast to the abdominal and thigh subcutaneous fat depots, was linearly and positively associated with the risk of developing type 2 diabetes over a 10-year period (13). Several hypotheses have been advanced to explain this relationship between visceral fat accumulation and diabetes risk, including deposition of free fatty acids mobilized from visceral fat into the liver’s portal circulation and the presence of adipocyte-associated inflammatory markers (14).
In addition, a cross-sectional comparison of Caucasians in Pittsburgh and native Japanese found a higher mean visceral fat area in each quartile of waist circumference that reached statistical significance in two quartiles (15). In another cross-sectional comparison, Filipino women in California with a normal Asian-adjusted BMI (<23 kg/m2) similarly had a greater volume of intra-abdominal fat compared with white or African American women also with a normal BMI (<25 kg/m2) (16). The available evidence therefore argues that Asian ethnicity may be predisposed to greater deposition of fat in the visceral depot.
Little is known about differences in regional fat distribution in other Asian or Pacific Islander or Native Hawaiian ethnic groups. At a BMI cut point of 30 kg/m2 in European men and women residents of New Zealand that corresponded to a mean percentage of body fat by dual X-ray absorptiometry (DXA) of 28.7% and 42.5%, respectively, the same percentage of body fat at higher BMI cut points was found in Maori and Pacific Islander men (31.3, 34.1) and women (33.1, 35.0) (17).
Differences in body composition have been examined to explain variation in type 2 diabetes prevalence by ethnicity. In a logistic model predicting type 2 diabetes prevalence by ethnicity among Filipino, white, and African American women, adjustment for visceral fat did not explain the higher odds of diabetes associated with ethnicity, because Filipino women continued to have a statistically significant 7.51-fold and 2.30-fold increased odds of diabetes (16). In that study, ethnic differences in diabetes prevalence by ethnicity could not be completely explained by body composition and other measured lifestyle characteristics.
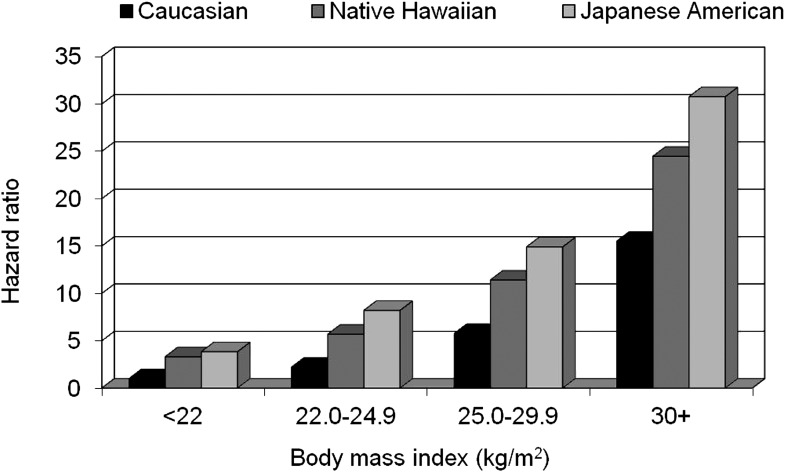
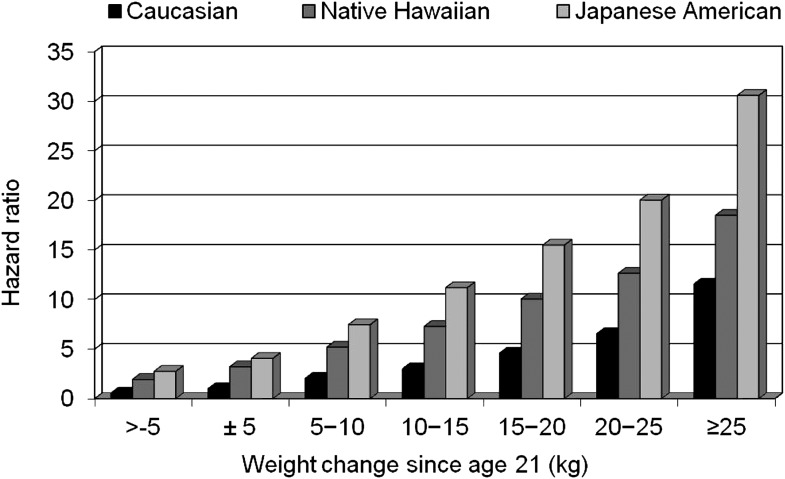
An analysis of the Hawaii component of the Multiethnic Cohort allowed a direct comparison of the influence of excess body weight and weight change on diabetes incidence in Caucasians, Native Hawaiians, and Japanese Americans (18,19). After a total follow-up time of 1,119,224 person-years, 11,838 incident diabetes cases were identified, with an annual incidence rate of 10.4/1,000 person-years. Native Hawaiians had the highest rate at 15.5, followed by Japanese Americans (12.5) and Caucasians (5.8); the adjusted hazard ratios (HRs) were 2.65 for Japanese Americans and 1.93 for Native Hawaiians compared with Caucasians (18). BMI was positively related to incidence in all ethnic groups (Fig. 1). Compared with the lowest category of BMI, the respective HRs at BMIs of 22.0–24.9, 25.0–29.9, and ≥30.0 kg/m2 were 2.10, 4.12, and 9.48. The risk was highest for Japanese Americans and intermediate for Native Hawaiians in each BMI category. Similar to the effects of BMI, diabetes risk was greatest for Japanese Americans, intermediate for Native Hawaiians, and lowest for Caucasians in each weight-gain category (Fig. 2). The partial population-attributable risk for BMI in men was 65, 60, and 38% for Caucasians, Native Hawaiians, and Japanese Americans, respectively, and 64, 67, and 38% for women, but the ethnic differences were not statistically significant.
Figure 1.
Diabetes risk by BMI category in the Hawaii component of the Multiethnic Cohort Study. The reference group is Caucasians in the lowest BMI category.
Figure 2.
Diabetes risk by weight change category in the Hawaii component of the Multiethnic Cohort Study. The reference group is Caucasians in the −5 to +5 kg (±5) weight change category.
DIABETES COMPLICATIONS
In previous reports, aggregate data for Asians and Pacific Islanders with health insurance coverage showed a significantly lower incidence of cardiovascular disease and lower-extremity amputation (LEA) and higher end-stage renal disease (ESRD) rates over 2 years of follow-up compared with Caucasians (20). To obtain disaggregated data, a prospective cohort study was done (1996–2006) of 64,211 diabetic patients enrolled in the Kaiser Permanente Northern California Diabetes Registry, including 40,286 Caucasians, 8,668 blacks, 7,763 Latinos, 3,572 Filipinos, 1,823 Chinese, 951 Japanese, 593 Pacific Islanders, and 555 South Asians (21). Incidence rates were calculated after 7.2 ± 3.3 years of follow-up, and Cox proportional hazard models were created, adjusted for age, educational attainment, English proficiency, neighborhood deprivation, BMI, smoking, alcohol use, exercise, medication adherence, type and duration of diabetes, HbA1c, hypertension, estimated glomerular filtration rate, albuminuria, and LDL cholesterol. Incidences of myocardial infarction (MI), congestive heart failure (CHF), stroke, ESRD, and LEA were age- and sex-adjusted. Presented here are the results of these analyses in the Asian and Pacific Islander groups, using Caucasians as the reference.
The average BMI was similar for Caucasians and Pacific Islanders but lower for the other four Asian subgroups (Chinese, Japanese, Filipino, and South Asian). The aggregated unadjusted Asian incidence rate (95% CI) for MI of 10.8/1,000 person-years (9.9–11.8) was lower than the rate in Caucasians of 17.1 (16.6–17.7) but obscured the wide range of rates across the four Asian groups, which were Chinese, 9.2; Japanese, 10.5; Filipino, 11.5, and South Asian, 12.3. Pacific Islanders had a much higher incidence rate for MI at 18.5 (14.3–23.5). The rates were attenuated after adjustment for age and sex, but Pacific Islanders still had the highest incidence of MI (10.1) of all groups. The rates among the other groups were Japanese, 3.3; Chinese, 3.6; Filipino, 4.7; South Asian, 5.9; and Caucasians, 5.9. These differences among the among groups persisted with multivariate adjustment for several potential explanatory factors.
The range of CHF unadjusted incidence rates among the four Asian groups was narrower than for MI (5.9–7.6), and the aggregated rate of 7.0 was lower than in Caucasians (13.0) and Pacific Islanders (9.2). Fully adjusted models showed no significant differences among these groups compared with Caucasians. The fully adjusted models for stroke showed a similar pattern as with CHF.
For ESRD, aggregating the Asian groups obscured the differences among the groups. The age- and sex-adjusted rate for the aggregate was 2.9 and slightly higher than in Caucasians (2.4), but the rates ranged from 1.1 in South Asians to 3.9 in Filipinos. In Pacific Islanders, the rate was even higher (5.0). The fully adjusted models showed higher rates than in Caucasians in all except for South Asians.
Asians had a ∼60% lower incidence of LEA compared with Caucasians or Pacific Islanders. The age- and sex-adjusted rates ranged from 0.7 to 1.3 in Asians, compared with 2.4 in Caucasians and 2.6 in Pacific Islanders. The fully adjusted models showed similar differences.
In summary, the incidence rates in Chinese, Japanese, and Filipinos were similar for most complications in this cohort with health care insurance. For the three macrovascular complications, Pacific Islanders and South Asians had rates similar to Caucasians. The incidence of complications varied dramatically among the Asian subgroups and highlights the value of a more nuanced ethnic stratification for public health surveillance and etiologic research.
TAILORING DIAGNOSIS, PREVENTION, AND TREATMENT TO REFLECT ETHNIC DIFFERENCES
Diagnosis
Current criteria for the diagnosis of diabetes include fasting plasma glucose ≥126 mg/dL, 2-h plasma glucose ≥200 mg/dL during an oral glucose tolerance test (OGTT), a random plasma glucose >200 mg/dL with classic symptoms, and HbA1c of ≥6.5%. In a cross-sectional study of Japanese Americans, OGTT assessment of 503 individuals with normal fasting glucose identified 176 with IGT and 20 with glucose levels in the diabetic range (22). These data raise the concern that diagnosis by fasting glucose alone fails to detect many Asian Americans with abnormal glucose tolerance who already have less favorable cardiovascular risk profiles. Similarly, the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia (DECODA) study, involving 11 population-based studies from Asia, showed that of 1,215 subjects diagnosed with diabetes by fasting plasma glucose or OGTT, only 449 met both criteria, a concordance of 37% (23). Because impaired fasting glucose (IFG) and IGT both demonstrate some features of β-cell defect and insulin resistance, but in differing degrees, the concordance between the two categories is expected to be limited (24).
In 2009, an international expert committee recommended the use of HbA1c ≥6.5% as a diagnostic criterion for diabetes (25). A study of Filipino Americans, Japanese Americans, and Native Hawaiians showed that the sensitivity and specificity of HbA1c ≥6.5%, compared with diabetes defined by OGTT, was 40.0 and 96.8%, respectively, and by fasting plasma glucose was 68.9 and 95.3%, respectively (26,27). These data indicate using HbA1c alone as a diagnostic test has high specificity but might delay the diagnosis of type 2 diabetes among AANHPIs.
These diagnostic considerations are particularly significant in Asian Americans because they have higher risks for diabetes, often present without overt signs of obesity, and may be misdiagnosed if a test with low sensitivity is used. Although more cumbersome and less replicable, the OGTT is the most robust but underused diagnostic standard for diabetes in Asian Americans.
Treatment
On the basis of clinical trials data and collective clinical experience, the American Diabetes Association/European Association for the Study of Diabetes and American Association of Clinical Endocrinologists have issued sets of recommendations for initiating and adjusting therapy for type 2 diabetes (28,29). A criticism of these algorithms—their failure to address fundamental pathophysiology—is especially relevant to a discussion of the treatment of diabetes in AANHPIs (30). The unique features of diabetes pathophysiology among subgroups within this very heterogeneous population may indicate a need for different treatment guidelines.
Moreover, the inherent methodologic issues of collection, analysis, and reporting of granular data in the AANHPI population means that there exist too few AANHPI subjects in clinic trials to determine the efficacy or safety of glucose-lowering drugs specific to the population (31). Some have advocated borrowing drug trial data from Asian countries in lieu of obtaining data from the Asian American population, but critics argue that despite identical genetic makeup between Asians and Asian Americans, environmental and epigenetic factors could alter physiologic responses.
Given these deficiencies, there are simply not enough data at present to develop separate treatment guidelines for this highly heterogeneous population. Nonetheless, some cultural factors, such as food, must be taken into account.
DIETARY GUIDELINES FOR AMERICANS, 2010 (DGA 2010): CAN THEY ACCOMMODATE ETHNIC AND TRADITIONAL FOOD PREFERENCES?
Food plays a significant role when considering the prevention and treatment of diabetes in AANHPIs. Food selection is often guided by religious beliefs, folklore, food availability, taste, and varying approaches to diet therapy. Environmental changes and increased acculturation can have a negative impact on the weight and health of AANHPIs (32,33).
The DGA 2010 (34) provide evidence-based recommendations for individuals aged 2 years and older that accommodate the food preferences, cultural traditions, and customs of the many diverse groups in the U.S. The two overarching concepts addressed in the guidelines are:
Maintain caloric balance over time to achieve and sustain a healthy weight.
To control the obesity epidemic and prevent associated risk factors, Americans are advised to decrease their intake of calories and increase physical activity. The strategies recommended are: 1) monitor food and beverage intake; 2) reduce portion sizes; 3) choose smaller portions or lower calorie options when eating out; 4) use food-preparation techniques that decrease fat intake such as baking, broiling or sautéing with minimal amounts of unsaturated oils in place of butter, ghee, lard, coconut oil, and milk (sources of saturated fatty acids), and trans-fats; and 5) limit television screen time. Although food ingredients may differ according to cultural practices, these recommendations are generally applicable to the AANHPI population. As already noted, the specific weight targets are lower for Asian Americans.
Focus on consuming nutrient-dense foods and beverages.
Americans are advised to consume less sodium, fat, refined grains, sugar, and alcohol-containing foods and beverages and replace them with vegetables, fruits, whole grains, lean meats, poultry without skin, seafood, very low-fat dairy, beans and peas, nuts, and seeds. If alcohol is consumed, the guidelines state up to two drinks per day for men and up to one drink per day for women. To reduce high blood pressure risk, the general recommended sodium intake is ≤2,300 mg/day. For individuals more sensitive to increased sodium intake, the recommendation is to lower it to 1,500 mg/day. High salt consumption is common in many Asian cultures because many foods are pickled or preserved in salt or in high-sodium sauces; therefore, the use of these condiments should be reduced.
The U.S. Department of Agriculture’s “MyPlate” is a practical tool to help Americans make appropriate food choices and control portion sizes. MyPlate illustrates the five food groups using a familiar mealtime visual, a plate setting. In MyPlate, half of the plate should be vegetables and fruits and the other half allotted to grains and proteins. An additional portion of dairy is also recommended. The limitation for this sizing approach is evident in some Asian cultures because bowls or multiple plates are used in place of one plate. Food is also often shared in communal plates from which individuals take bite sizes of food. These eating practices present major challenges to traditional teaching and counting of carbohydrates (35). In addition, many Asians may be more concerned with food texture, flavor, or aroma than what dietitians typically focus on such as the macronutrient composition or carbohydrate counting. AANHPIs also have strong beliefs on the healthfulness of foods in health and disease (36). These concepts are not familiar to Western culture. In summary, the principles outlined in DGA 2010 are applicable to the AANHPI community, but their practical implementation will require cultural adaptation.
DIABETES PREVENTION
No study of diabetes prevention has been specifically directed to Asians or Pacific Islanders in the U.S. However, a subset of 142 participants (4.4% of the cohort) in the Diabetes Prevention Program (DPP) was AANHPI (37). Although their sample size was small, results among AANHPIs are still worth noting. AANHPIs showed a higher conversion rate per year in the placebo group of 12.1% versus 10.3% in Caucasians, despite lower mean weight and BMI. However, compared with Caucasians, AANHPI showed greater risk reduction with lifestyle intervention (71% vs. 51%) and metformin (38% vs. 24%). Assessment of potential factors that could explain the differences in conversion rates and risk reductions, such as age, weight, physical activity level, family history, and visceral adiposity, could not be evaluated.
Other lifestyle intervention studies among Asians have been performed in Asia, and the results are reported in Table 1. Of interest are the varied diabetes conversion and intervention risk reduction rates among these different populations (38–40).
Table 1.
Diabetes prevention studies in Asians and Pacific Islanders
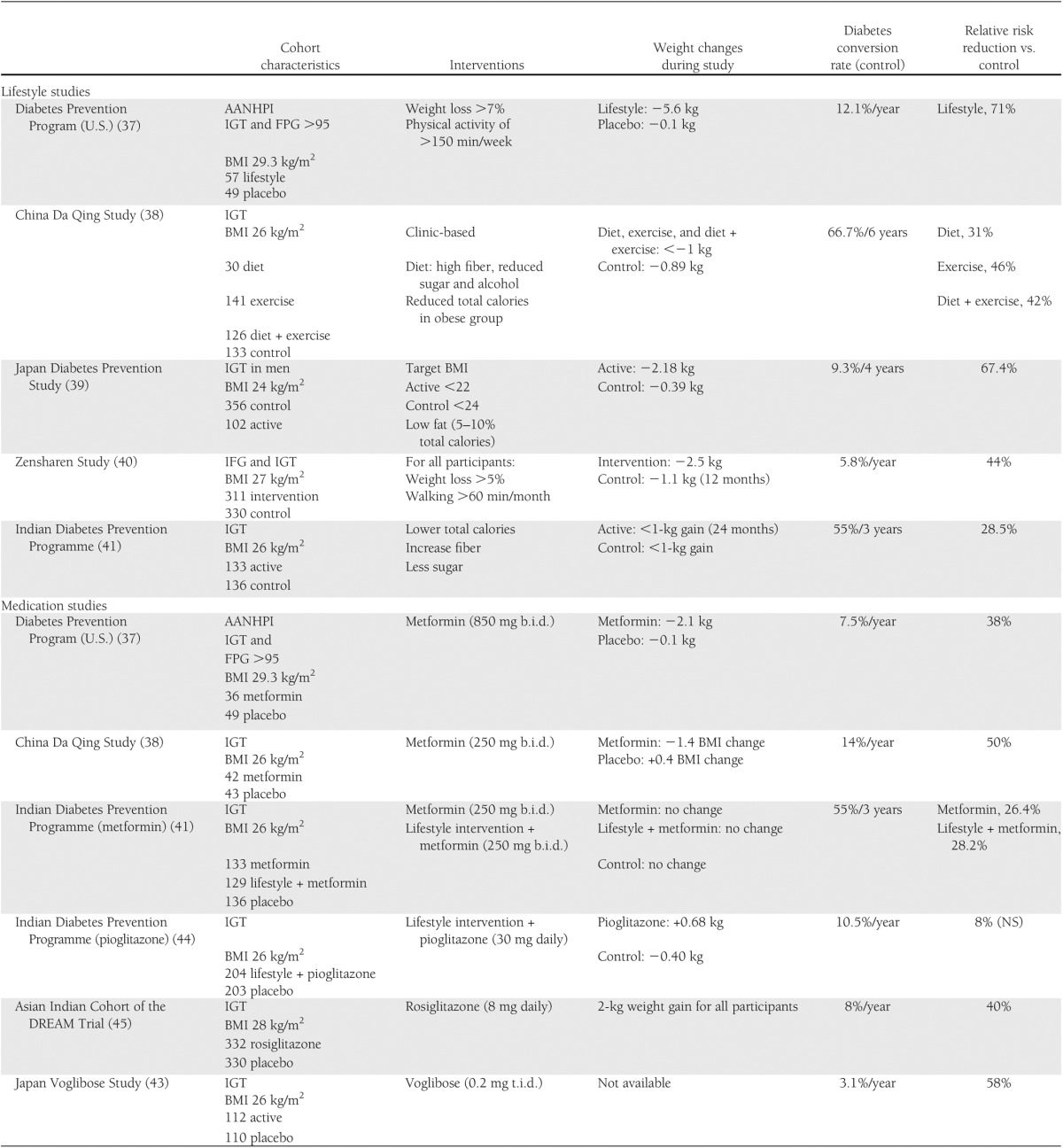
Responses to medication for diabetes prevention, including differences in side effects, also vary among Asians, perhaps due to differences in diabetes pathophysiology. In contrast to the response among AANHPI in the DPP, metformin failed to show a large reduction of diabetes incidence among Chinese and Indians (41,42), although the Indian Diabetes Prevention Programme showed that low-dose metformin reduced diabetes incidence by 26%, similar to lifestyle intervention. Interestingly, the addition of metformin to a lifestyle intervention failed to show further risk reduction. Because of the Asian custom of having high carbohydrate consumption with each meal, addressing postprandial hyperglycemia appears to be a unique and effective intervention among Asians. A diabetes prevention study in Japan using an α-glucosidase inhibitor showed a risk reduction of 58% (43). Mixed results have been noted with the use of thiazolidinediones. Pioglitazone did not further reduce diabetes incidence when added to lifestyle intervention in the Indian Diabetes Prevention Programme, and rosiglitazone treatment among the Indian cohort of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) Trial showed less risk reduction than other populations (40% vs. 60%) (44,45).
COMMUNITY-BASED INTERVENTION IN ASIAN INDIANS
Asian Indians (AIs) in the U.S. and other westernized countries have high rates of type 2 diabetes (46). Insulin resistance is highly prevalent in AI migrants, despite low rates of obesity (47). Increased urbanization and socioeconomic and lifestyle transitions are associated with the rapid rise of type 2 diabetes in urban and rural India as well as in AI immigrants to the U.S. (48).
To assess the prevalence of diabetes in AIs living in the U.S., a study of 1,038 randomly selected AI adults was undertaken at seven U.S. urban sites (46). Participants were a mean age of 45.7 ± 12.8 years, length of residence in the U.S. was 18.5 ± 11.04 years, and BMI was 25.4 ± 3.7 kg/m2. Most (60%) were not vegetarian, and 52% reported dietary acculturation since moving to the U.S. Family history of diabetes (siblings and parents) was reported by 57%. The prevalence of diabetes was 17.4% (14.0% with known diabetes and 3.4% undiagnosed diabetes), prevalence of prediabetes was 32.9%, and prevalence of the metabolic syndrome, using International Diabetes Federation criteria, was 38.2%. CVD risk factors were also observed, including high levels of triglycerides, total cholesterol, LDL cholesterol, homocysteine, and C-reactive protein, and low levels of HDL cholesterol.
Serving as a model for effective community-based intervention, a 7-month community-based participatory diabetes prevention and management program was initiated in South India (49). Trained community health workers provided an educational intervention to 703 village inhabitants, consisting of adults and youth, aged 10–92 years. Mean BMI in the adult population was 20.6 ± 3.8 kg/m2; 11.6% were overweight, and 16.4% were obese. Mean daily caloric intake was 1,636.1 ± 683.1 kcal in adults and 1,370.5 ± 536.2 kcal in youth. High-risk participants were provided one-on-one education and counseling for blood glucose management by a certified diabetes educator. Culturally and linguistically appropriate health education messages addressed diet, physical activity, and knowledge of the disease. The age-specific prevalences of diabetes and IFG were 5.1% and 13.5% among adults and 0% and 5.1% among youth, respectively.
Intervention successfully reduced fasting blood glucose levels among adults, youth, and high-risk groups of IFG and type 2 diabetes: 3% in the adults, 11% in IFG adults, 17% in IFG youth, and 25% among adults with type 2 diabetes. A significant reduction in all obesity parameters was also noted among adults and IFG adults, and improvements in dietary fiber and protein also occurred among adults (P = 0.001). Lower intake of dietary fiber, high blood pressure, and obesity significantly predicted higher blood glucose levels (R2 = 0.14, F = 4.54, P = 0.001). Although the intervention did not change dietary patterns in youth, there was an increase in the knowledge level of the participants (P = 0.013).
This study showed that a community-based participatory approach to lifestyle intervention successfully improved blood glucose levels, fiber intake, and obesity risk factors in rural India and may serve as a prototype for similar programs among AIs living in the US.
CULTURE-BASED DIABETES EDUCATION AND SELF-MANAGEMENT AMONG NATIVE HAWAIIANS AND PACIFIC ISLANDERS
Diabetes disproportionately affects Pacific people, such as Native Hawaiians, where prevalence rates can be four times higher than in the general U.S. population (50). Community organizations in collaboration with the University of Hawaii’s Center for Native and Pacific Health Disparity Research developed innovative diabetes self-management education programs that combine classroom teaching with reconnecting participants to the land. For Pacific people, relationship to land is a deep and enduring part of their identity, history, and spiritual beliefs. Pacific Islanders, such as Native Hawaiians, view the origin of man as arising from the land or from a product of the land, metaphorically described as a familial relationship between plants and humans and a filial relationship humans have with the land. The land is seen as the provider of substance for the body and spirit. Aloha ‘āina (love for the land) is a recurring theme in the poetry, dance, and music of Pacific people that can be traced back at least 1,000 years.
Culture-based education is the grounding of instruction in the values, norms, knowledge, beliefs, practices, experiences, and language of the students’ culture. Also known as culturally responsive schooling, it is a framework for teaching promoted by educational scholars and indigenous leaders for the past 40 years (51). The four community-based health organizations in Hawaii with diabetes educational programs centered around reconnecting to the land and values of aloha ‘āina included three community health clinics and a federally established Native Hawaiian Health Care System site.
The Healthy Eating and Lifestyle Program (HELP) and Mai ka Mala‘ai (MALA) are diabetes self-management classes for Pacific people that combine classroom education with reconnecting to the land to grow produce. The program participants have type 2 diabetes and were referred by their primary care physicians because of poor self-management. Most participants are from Polynesian or Micronesian island groups or nations, including Hawaii, Tonga, Samoa, Marshall Islands, and Chuuk. The classroom curricula for each program, developed by each organization’s nutritionist, were built around evidence-based findings for diabetes self-care education. HELP is a 6-month program, with a monthly 2-h classroom session and optional biweekly communal gardening. MALA is a 10-week program with weekly 90-min classroom sessions and optional backyard gardening. Both programs extensively incorporate aspects of culture-based education into their curricula and teaching strategies.
Clinical measurements (weight, blood pressure, HbA1c, and cholesterol) were made before and at the completion of each program. HbA1c showed a statistically significant decrease of 1.3% (P < 0.05), and systolic blood pressure dropped 5.0 mmHg (P < 0.05). There were no changes in cholesterol and weight. Both programs created an environment of strong social support, and mean retention of enrolled participants was 81%.
The improvements in clinical and metabolic parameters were attributed to a combination of increase in practical knowledge about diabetes management, reduction of stress levels, and increase in support systems. The merging of preferences and practices appears to be an effective way of reaching disparate populations. Culture-based health education is very appealing to ethnic communities because it validates their cultural identify and heritage. The appeal of a health education program to participants is important for enrollment and retention. A learning environment must be created in which participants feel comfortable and confident in their ability to achieve success through increased knowledge and appropriate behavioral change.
A NATIONAL PROGRAM FOR DIABETES PREVENTION AND COMMUNITY INTERVENTION
The NDEP (52) was established in 1997 as a federally funded program sponsored by the U.S. Department of Health and Human Services’ National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC) and includes more than 200 public and private partners at the federal, state, and local levels. NDEP formed an Asian American Pacific Islander Work Group (AAPI WG) in 1998 that has been very active in developing and promoting resources for AANHPI populations. A new NDEP group is the Strategic Directions Group (SDG), essentially a “think tank,” that will help with strategic planning. SDG membership includes representatives from the National Council of Asian Pacific Islander Physicians, the Association of Asian Pacific Community Health Organizations (AAPCHO), and the Pacific Chronic Disease Coalition.
The NDEP partnership focuses on the use of education and communication approaches to address diabetes prevention and control. It develops, tests, and disseminates science-based, audience-tested and -tailored, and culturally appropriate resources for a wide range of audiences, including people with and at risk for diabetes and their families, health care providers, community-based lay workers, businesses and work sites. Some of the resources on the importance of early glycemic control, diabetes and heart disease, diabetes self-management, and diabetes prevention are available in 13–15 AANHPI languages. To improve culturally appropriate outreach to communities, CDC/NDEP funded organizations serving ethnic/racial minority communities: AAPCHO, National Asian Women’s Health Organization, Khmer Health Advocates, and Papa Ola Lokahi. These organizations further adapted NDEP materials and determined culturally appropriate ways to reach the communities.
There are many other resources that are relevant to AANHPI populations such as tips for selecting foods at buffets, a grocery list for Asian foods, and a range of media articles. Materials for professionals serving AANHPI populations also included “Silent Trauma,” a white paper for health care professionals, community leaders, and policy makers. Its recommendations deal specifically with issues and barriers to refugees and include information on reducing the impact of diabetes in Southeast Asians in the U.S. Another resource is the “Capacity Building Tool Kit,” based on AAPCHO’s A Community Approach to Responding Early (CARE) model using stages of change and provides a framework to help build diabetes outreach capacity in community-based organizations serving AANHPI population.
CONCLUSIONS
The symposium from which data from this review was discussed (“Diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders: A Call to Action”) was “a call to action” to address diabetes among AANHPI populations and highlighted the specific differences, enormous diversity, and broad challenges that face health care providers as well as patients with diabetes (Table 2). With an emphasis on developing an action plan and policy through evidence-generated understanding of the problems of diabetes within the AANHPI, general consensus was attained on several items.
Table 2.
Suggested next steps from the Symposium
First and foremost is the need for better characterization of the burden of diabetes and diabetes-related complications such as ESRD and accurate and complete population-based data. A greater research effort and increased funding should be provided to improve our understanding of the differences in diabetes pathophysiology in AANHPI.
Next is the need to enrich clinical research with better representation by AANHPI individuals. The current standards used by health care providers and payers for diabetes management for the AANHPI population are based on guidelines derived from clinical trials that did not include very many AANHPI individuals. Their disproportionately higher diabetes rates warrant the need for greater inclusion of AANHPIs in research so that results can be meaningfully interpreted. Larger clinical trials should include minority groups beyond their national population representation because the disease is disproportionately prevalent.
Another consensus was that aside from the need for more epidemiologic and clinical data, our current understanding of diabetes in AANHPIs should be resonated across the country. Many AANHPI patients with diabetes receive their care from non-AANHPI physicians. Health care providers need to be informed about the physiologic and cultural characteristics of diabetes in this population: diabetes risk may be associated with lower BMI and weight; the association of smaller degrees of visceral adiposity with greater insulin resistance and metabolic syndrome; higher carbohydrate consumption requires greater need to address postprandial hyperglycemia; and various cultural barriers to therapy and nutritional recommendations.
A final outcome from this symposium was the importance and effectiveness of community-based interventions. The diversity among AANHPIs precludes the use of any “one shoe fits all” recommendation or intervention. Improving diabetes care and reducing risk factors that contribute to diabetes and diabetes complications need to be tailored and made specific to a given population. The two contrasting interventions in India and Native Hawaiian communities demonstrate the effectiveness of “including the people” in their own care. Developing these programs may be an enormous and potentially complex undertaking, given the diversity of communities within the AANHPI population, but could ultimately result in cost-saving. An example is diabetes prevention. It is clear from all the available clinical trials and translational efforts among AANHPIs that lifestyle intervention directed toward weight loss and increasing physical activities will reduce diabetes incidence. An AANHPI initiative that targets lifestyle intervention in at-risk individuals and offered through community-based programs should be seriously considered as a priority within a national program for reducing the burden of diabetes.
Acknowledgments
This article was made possible in part by Award P20MD000173 from the National Center on Minority Health and Health Disparities. This work was partly supported by National Institutes of Health Grants DK-31170, HL-49293, and DK-02654; by facilities and services provided by the Diabetes and Endocrinology Research Center (DK-17047), Clinical Nutrition Research Unit (DK-35816), and the General Clinical Research Center (RR-00037) at the University of Washington. The VA Puget Sound Health Care System provided support for E.J.B.’s involvement in this research.
W.C.H. is on the advisory board for Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
W.C.H. and R.A. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. E.J.B., W.Y.F., A.Kan., W.K., A.Kar., G.L.K., M.L., G.M., R.M., and F.T.-P. researched data and wrote, reviewed, and edited the manuscript.
The AANHPI Diabetes Coalition acknowledges the sponsors who have made the State of the Sciences Conference 2011: “Diabetes in Asian Americans, Native Hawaiians and Pacific Islanders: A Call to Action” in Honolulu in September 2011 a reality: American Diabetes Association–Asian Pacific American Diabetes Action Council; Asian & Pacific Islander American Health Forum; AAPCHO; Daichii Sankyo, Inc.; Joslin Diabetes Center Asian American Diabetes Initiative; Kaiser Permanente; National Council of Asian Pacific Islander Physicians; Novo Nordisk, Inc.; and sanofi-aventis U.S. LLC.
The authors are grateful to the King County Japanese-American community for their support and cooperation.
Footnotes
References
- 1.National Council of Asian Pacific Islander Physicians. 2011 Diabetes Conference in Honolulu a Success! Available from http://www.ncapip.org/conference2011.html Accessed 24 January 2012
- 2.Danaei G, Finucane MM, Lu Y, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 3.Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin Endocrinol Diabetes Obes 2011;18:248–251 [DOI] [PubMed] [Google Scholar]
- 4.Hagopian WA, Erlich H, Lernmark A, et al. TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park Y. Why is type 1 diabetes uncommon in Asia? Ann N Y Acad Sci 2006;1079:31–40 [DOI] [PubMed] [Google Scholar]
- 6.Soltesz G, Patterson CC, Dahlquist G, EURODIAB Study Group Worldwide childhood type 1 diabetes incidence—what can we learn from epidemiology? Pediatr Diabetes 2007;8(Suppl. 6):6–14 [DOI] [PubMed] [Google Scholar]
- 7.Liese AD, D’Agostino RB, Jr, Hamman RF, et al. SEARCH for Diabetes in Youth Study Group The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006;118:1510–1518 [DOI] [PubMed] [Google Scholar]
- 8.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 9.Li JK, Chan JC, Zimmet PZ, Rowley MJ, Mackay IR, Cockram CS. Young Chinese adults with new onset of diabetic ketoacidosis—clinical course, autoimmune status and progression of pancreatic beta-cell function. Diabet Med 2000;17:295–298 [DOI] [PubMed] [Google Scholar]
- 10.Ikegami H, Kawabata Y, Noso S, Fujisawa T, Ogihara T. Genetics of type 1 diabetes in Asian and Caucasian populations. Diabetes Res Clin Pract 2007;77(Suppl. 1):S116–S121 [DOI] [PubMed] [Google Scholar]
- 11.Hsu WC, Okeke E, Cheung S, et al. A cross-sectional characterization of insulin resistance by phenotype and insulin clamp in East Asian Americans with type 1 and type 2 diabetes. PLoS ONE 2011;6:e28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto WY, Boyko EJ, Hayashi T, et al. Risk factors for type 2 diabetes: lessons learned from Japanese Americans in Seattle. J Diabetes Invest [E-pub ahead of print: 10.1111/j.2040-1124.2012.00195.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyer D, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Subcutaneous thigh fat area is unrelated to risk of type 2 diabetes in a prospective study of Japanese Americans. Diabetologia 2011;54:2795–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl. 1):16S–19S [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006;30:1163–1165 [DOI] [PubMed] [Google Scholar]
- 16.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res 2005;13:1458–1465 [DOI] [PubMed] [Google Scholar]
- 17.Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr 2009;102:632–641 [DOI] [PubMed] [Google Scholar]
- 18.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes 2009;58:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morimoto Y, Schembre SM, Steinbrecher A, et al. Ethnic differences in weight gain and diabetes risk: the Multiethnic Cohort Study. Diabetes Metab 2011;37:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 21.Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of diabetes outcomes among asians and pacific islanders in the US: the diabetes study of northern california (DISTANCE). Diabetes Care 2011;34:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Shofer JB, Boyko EJ, et al. Abnormal glucose tolerance and increased risk for cardiovascular disease in Japanese-Americans with normal fasting glucose. Diabetes Care 2001;24:39–44 [DOI] [PubMed] [Google Scholar]
- 23.Qiao Q, Nakagami T, Tuomilehto J, et al. International Diabetes Epidemiology Group. DECODA Study Group Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000;43:1470–1475 [DOI] [PubMed] [Google Scholar]
- 24.Petersen JL, McGuire DK. Impaired glucose tolerance and impaired fasting glucose–a review of diagnosis, clinical implications. Diab Vasc Dis Res 2005;2:9–15 [DOI] [PubMed] [Google Scholar]
- 25.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araneta MRG, Grandinetti A, Chang HK. A1C and diabetes diagnosis among Filipino Americans, Japanese Americans, and Native Hawaiians. Diabetes Care 2010;33:2626–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavagnolli G, Comerlato J, Comerlato C, Renz PB, Gross JL, Camargo JL. HbA(1c) measurement for the diagnosis of diabetes: is it enough? Diabet Med 2011;28:31–35 [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam NS, Khan S, Kwon S, Jang D, Ro M, Trinh-Shevrin C. Methodological issues in the collection, analysis, and reporting of granular data in Asian American populations: historical challenges and potential solutions. J Health Care Poor Underserved 2010;21:1354–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franzen L, Smith C. Acculturation and environmental change impacts dietary habits among adult Hmong. Appetite 2009;52:173–183 [DOI] [PubMed] [Google Scholar]
- 33.Huang B, Rodriguez BL, Burchfiel CM, Chyou PH, Curb JD, Yano K. Acculturation and prevalence of diabetes among Japanese-American men in Hawaii. Am J Epidemiol 1996;144:674–681 [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington DC, U.S Government Printing Office. Available from http://www.dietaryguidelines.gov Accessed 24 January 2012 [DOI] [PMC free article] [PubMed]
- 35.Chesla CA, Chun KM. Accommodating type 2 diabetes in the Chinese American family. Qual Health Res 2005;15:240–255 [DOI] [PubMed] [Google Scholar]
- 36.Lin K. Chinese Food Cultural Profile. Harborview Medical Center, University of Washington. Available from http://ethnomed.org/clinical/nutrition/chinese_food_cultural_profile Accessed 24 January 2012
- 37.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 39.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–162 [DOI] [PubMed] [Google Scholar]
- 40.Saito T, Watanabe M, Nishida J, et al. Zensharen Study for Prevention of Lifestyle Diseases Group Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 42.Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabet Med 1999;16:477–481 [DOI] [PubMed] [Google Scholar]
- 43.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K, Voglibose Ph-3 Study Group Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–1614 [DOI] [PubMed] [Google Scholar]
- 44.Ramachandran A, Snehalatha C, Mary S, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 2009;52:1019–1026 [DOI] [PubMed] [Google Scholar]
- 45.Gerstein HC, Yusuf S, Bosch J, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 46.Misra R, Patel T, Kotha P, et al. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications 2010;24:145–153 [DOI] [PubMed] [Google Scholar]
- 47.McKeigue PM, Pierpoint T, Ferrie JE, Marmot MG. Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia 1992;35:785–791 [DOI] [PubMed] [Google Scholar]
- 48.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 2008;93(Suppl. 1):S9–S30 [DOI] [PubMed] [Google Scholar]
- 49.Balagopal P, Kamalamma N, Patel TG, Misra R. A community-based diabetes prevention and management education program in a rural village in India. Diabetes Care 2008;31:1097–1104 [DOI] [PubMed] [Google Scholar]
- 50.Mau MK, Sinclair K, Saito EP, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev 2009;31:113–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castagno A, Brayboy B. Culturally responsive schooling for indigenous youth: a review of the literature. Rev Educ Res 2008;78:941–993 [Google Scholar]
- 52.National Diabetes Education Program. Available from http://ndep.nih.gov Accessed 24 January 2012