Abstract
The idea that social motivation deficits play a central role in Autism Spectrum Disorders (ASD) has recently gained increased interest. This constitutes a shift in autism research, which has traditionally focused more intensely on cognitive impairments, such as Theory of Mind deficits or executive dysfunction, while granting comparatively less attention to motivational factors. This review delineates the concept of social motivation and capitalizes on recent findings in several research areas to provide an integrated picture of social motivation at the behavioral, biological and evolutionary levels. We conclude that ASD can be construed as an extreme case of diminished social motivation and, as such, provides a powerful model to understand humans’ intrinsic drive to seek acceptance and avoid rejection.
Introduction: Social motivation and social cognition, two competing accounts of autism
Over the last three decades, a number of theories have been put forward to account for the pervasive social impairments found in Autism Spectrum Disorders (ASD). Among the various attempts, the idea of a core deficit in social cognition (Theory of Mind, or ToM, in particular, see Glossary) has become one of the most prominent accounts of ASD. Concomitantly, the impact of motivational factors on the development of social skills and social cognition has received little attention. Recently however, social motivation has emerged as a promising research domain at the intersection of social psychology, behavioral economics, social neuroscience and evolutionary biology. This paper integrates these diverse strands of research and defends the idea that social motivation is a powerful force guiding human behavior and that disruption of social motivational mechanisms may constitute a primary deficit in autism. In this framework, motivational deficits are thought to have downstream effects on the development of social cognition and deficits in social cognition are therefore construed as a consequence, rather than a cause, of disrupted social interest.
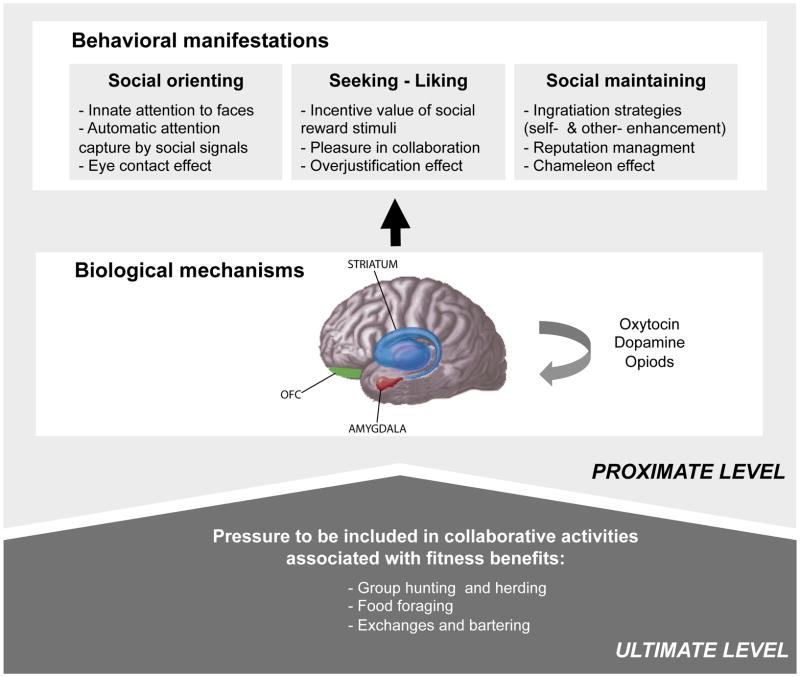
Providing a complete picture of social motivation requires both proximate and ultimate explanations. Proximate explanations pertain to how a behavior functions and ultimate explanations to why it was selected by evolution. At the proximal level, social motivation can be described as a set of psychological dispositions and biological mechanisms biasing the individual to preferentially orient to the social world (social orienting), to seek and take pleasure in social interactions (social reward), and to work to foster and maintain social bonds (social maintaining). At the ultimate level, social motivation constitutes an evolutionary adaptation geared to enhance the individual’s fitness in collaborative environments (see Figure 1).
Figure 1.
Social motivation constitutes an evolutionary adaptation geared to enhance the individual’s suitability for collaborative environments (ultimate level). The orbitofrontal-striatal-amygdala circuit, influenced by specific neuropeptides, underlies a range of behaviors including social orienting, social seeking and liking, and social maintaining (proximate level).
We first present evidence supporting this integrated model of social motivation in healthy individuals, and go on to review behavioral manifestations of diminished social orienting, social reward and social maintaining in ASD and the associated disruptions in the neural circuitry that typically underlie these behaviors. We then demonstrate that, as predicted by the evolutionary framework, some areas of social functioning are preserved in ASD. We conclude by arguing that deficits in social cognition are better explained within a social motivation framework, and acknowledge the limits of both socio-cognitive and social motivation theories in accounting for non-social deficits in ASD.
An integrated model of social motivation in typical development
Behavioral level
Behavioral manifestations of humans’ social interest are of at least three kinds: i) Objects with social importance are prioritized by attention; ii) Social interactions are rewarding; iii) Interpersonal behaviors are influenced by the desire to maintain and enhance relationships. We now review interdisciplinary evidence suggestive of this three-tiered disposition.
Social orienting
In very much the same way that negative signals (e.g., threats) capture attention, potentially beneficial or rewarding information is prioritized. Given their relevance for humans, social signals are therefore granted attentional priority: attention is rapidly captured by human faces and bodies [1], changes in faces are detected better than in other objects [2,3], and masked faces are detected faster and more accurately than masked objects [4]. This preference is expressed early in life, with infants preferentially attending to face-like stimuli rather than to scrambled or inverted faces [5,6]. Highly relevant social signals, such as direct gaze, are particularly powerful in capturing attention both in adults and newborns; they facilitate face-related tasks, such as gender discrimination or encoding of identities [7]; and, when artificially suppressed from conscious perception, they break through to consciousness faster than less salient social stimuli (such as inverted faces or averted gaze) [8,9].
Seeking and liking
Not only do people orient to the social world, they also find it rewarding. There are two components of reward –‘wanting’ and ‘liking’ [10], see Glossary– both of which apply to social signals. Behavioral economic studies have shown that adults exert effort to obtain social rewards [11], which highlights their incentive value, and that players in economic games report taking pleasure in mutual cooperation [12]. Similarly, when given the choice to access a reward collaboratively or individually, toddlers strongly prefer collaboration [13]. Importantly, social interactions have intrinsic motivational value. As the ‘overjustification effect’ (see Glossary) illustrates, people typically engage in prosocial behaviors not because they expect some kind of direct benefit to offset their efforts but because they find it inherently rewarding. Paying donors for giving blood, for example, actually decreases willingness to donate [14] and young toddlers are less prosocial after material incentives have been offered in exchange for a helping behavior [15]. Social psychologists have thus argued that the overjustification effect provides evidence that prosociality constitutes its own reward and is intrinsically motivated.
Social maintaining
Another important aspect of social motivation is individuals’ desire to engage with others over sustained periods of time. Maintaining strategies, which encompass behaviors by which people establish, maintain, and enhance their relationships with others, are therefore key manifestations of social motivation: people try to be viewed as likeable rather than unlikeable, as competent rather incompetent, as more rather than less physically attractive, etc. [16]. Concern for others’ acceptance is mostly expressed through ingratiating behaviors, such as flattery, which elicit positive attitudes in the recipient and thereby enhance the reputation of the ingratiator [17]. These behaviors emerge early in development with preschoolers spontaneously engaging in positive self-presentation, prosocial lies, and negative emotion concealment for politeness purposes [18–20]. Maintaining behaviors, far from being cold-hearted manipulations, often occur outside the individual’s conscious awareness. For instance, there is evidence that people unconsciously mimic others’ nonverbal manners and that they do so because perceived similarity is an important predictor of likeability, which can be exploited to enhance integration [21]. Consistent with this idea, more empathic individuals [22] and people scoring high in measures of social motivation [23] exhibit stronger mimicry.
Biological level
Social motivation is subserved by a network of brain regions including the amygdala, the ventral striatum, and orbital and ventromedial regions of the prefrontal cortex. Each region plays a greater role in specific aspects of motivation, but no region operates in isolation. Subcortical structures are most involved in the generation of reward utilities, but require cortical involvement for conscious hedonic representations [10]. More specifically, the amygdala plays an important role in guiding attention to biologically relevant stimuli, such as social information conveyed by eyes, faces, or biological motion [24], and in calculating and updating social orienting value [25]. Computing the salience value of social stimuli rests on strong interactions with the ventral striatum and orbitofrontal cortex (OFC) with which the amygdala shares dense connections [26] and which both respond to socially reinforcing stimuli. The ventral striatum, on the one hand, plays a specific role in representing rewards as a “decision utility” and in computing incentive salience and reward wanting for both non-social and social rewards (e.g., smiling faces [27], cooperation [28], or social approval [29]). Together with the OFC, it is also engaged when participants cooperate with a human partner vs. a computer partner, even when monetary gain is identical [30]. Additionally, the OFC plays a key role in transforming reward information into a common currency of subjective hedonic value that then informs executive systems and guides goal-directed action [25].
Interestingly, functional differences in the orbitofrontal-striatum-amygdala network correlate with individual differences in social motivation: higher social orienting is associated with enhanced amygdala and OFC activity in response to emotionally relevant stimuli [31] while anti-social traits are associated with weaker activations in these areas in response to uncooperative outcomes [32]. Socially anxious adolescents also show greater amygdala activation when anticipating evaluation from undesired peers [33] and amygdala damage affects subtle social skills such as people’s sense of personal space [34] or their use of eye contact during conversations [35], while OFC lesions disrupt emotion recognition and interpersonal maintaining behaviors [36].
Both human and animal research further suggests that these social motivational mechanisms are mediated, in part, by neuropeptide signaling. In particular, oxytocin (OXT), through interactions with dopamine, is thought to impact social orienting by modulating social salience and perceptual selectivity via the amygdala and social reward via the nucleus accumbens [37]. In line with this idea, OXT-receptor knockout mice exhibit a range of social deficits including fewer vocalizations in response to social isolation and impaired social discrimination [38]. In addition to OXT signaling, endogenous opioid, cannabinoid, dopaminergic, glutamatergic, and cholinergic mechanisms are thought to play important roles in mediating social affiliative behaviors, including the rewarding aspects of social play [39,40].
Evolutionary level
That nature selected and conserved mechanisms for orienting, rewarding and maintaining social interactions, indicates that these behaviors ultimately have important fitness benefits for the individual. Indeed, collaborative activities, such as exchanging information or helping one another, allow access to a range of benefits that would remain inaccessible were it not possible to engage in social relationships with others [41]. While many non-human animals live in groups, humans are indeed exceptional in the variety of collaborative activities that they pursue and in the benefits these bring about. In traditional societies, for example, important volumes of foods are pooled and shared, thereby making up for high variance in foraging luck [42]. To take one example, Aché hunter-gatherers return with no game on about 40% of their hunts, and the Hadza on over 90% of their hunts. In such contexts, individuals rely on others’ resources in times of need, and the value of cooperation far outweighs solitary alternatives [42]. Therefore, appearing as a good partner in the social group is, quite literally, vital.
In other apes, by contrast, food sharing either does not occur (i.e., food is foraged individually) or is not the result of a collaborative process (i.e., once the prey is killed, each hunter tries to secure as much meat as possible) [41]. In a study directly comparing chimpanzees and human children in their motivation to collaborate, it was recently found that, unlike chimpanzees, human children strongly prefer to engage in collaboration to forage food [13].
Importantly, under this specific evolutionary definition, the motivation for social affiliative interactions is distinct from other types of social motivations –such as those associated with sex, parenting, or dominance– that result from more ancient pressures and evolved into functionally and psychologically different systems [43]. Sexual arousal, for instance, is specifically geared to romantic relationships and is obviously inadequate to deal with family members; similarly, grossly uneven sharing may appear perfectly fine in a family context but be frowned upon among non-kins [43]. Thus, there are distinct motivations to deal with our conspecifics and each of these can vary across individuals or be selectively impaired (see e.g., [44] for hyposexuality; or [45] for disorders of mother-infant bonding). In what follows, we argue that ASD is characterized by a fairly specific disruption of motivation for social affiliation.
Social motivation in ASD
Behavioral level
Social motivation models of ASD posit that early-onset impairments in social attention set in motion developmental processes that ultimately deprive the child of adequate social learning experiences and that the resulting imbalance in attending to social and non-social stimuli further disrupts social skill and social cognition development [46–48]. As discussed in detail below, recent evidence demonstrates that social orienting, social seeking and liking, and social maintaining are all disrupted in ASD.
Social orienting
Core diagnostic criteria for ASD as well as descriptions of the first year of life include infrequent orienting to one’s own name, diminished eye-contact, and social aloofness [49]. In line with clinical descriptions, eye-tracking experiments have demonstrated impaired orienting to social stimuli: Children with ASD look more at the background than at the characters while watching static social photographs (e.g., friends chatting) [50] and adolescents and young adults freely viewing movie clips fixate less on people, faces and eyes than on other regions of interest [51,52]. Similarly, in the auditory modality, children with ASD do not exhibit a preference for socially salient sounds over non-social control noise [53,54] and display attention deficits for speech but not for non-speech sounds [55,56]. These differences in social attention are among the first manifestations of ASD [57] and preference for non-social patterns in toddlers has recently been identified as a robust predictor of ASD [58].
Seeking and liking
Half the adult population with ASD reports having no particular friends [59]. Yet, despite lower overall acceptance, greater loneliness is either not reported [60] or bears little relation to the individual’s actual degree of social involvement [61]. More generally, individuals with ASD score lower on the friendship questionnaire (which tests constructs such as pleasure in close friendships or enjoyment in interaction for its own sake) [62]. Experimental evidence also suggests that the preference for collaborative activities is diminished in ASD. Tasks aimed at assessing spontaneous collaborative engagement (e.g., helping an adult who accidentally dropped an object or bouncing a ball with two people moving each end of a trampoline synchronously) revealed that children with ASD are less likely to spontaneously help the experimenter [63] or to re-engage her when she interrupts the game. More generally, children with ASD lack declarative pointing [64], they are impaired at initiating [65] and responding to others’ bids for joint attention [66] and they are less responsive to social rewards, such as verbal praise [67]. Self-reported pleasure in social and non-social situations also reveals selective social anhedonia in adolescents with ASD and a correlation between degree of social anhedonia and ASD severity [68].
Social maintaining
Compared to TD populations, individuals with ASD display fewer maintaining strategies and appear to place less emphasis on preserving their reputation and managing their self image. They are less likely to offer spontaneous gestures of greeting and farewell [69], to adequately resort to maintaining strategies, such as hiding affect [70], presenting themselves strategically to convince a specific audience [71], or displaying social laughter [72] and social emotions –e.g., embarrassment, or coyness [73]. In a recent study testing reputation management more directly, the experimenter’s presence had little influence on the way children with ASD rated the quality of the experimenter’s drawing and this flattery index correlated negatively with levels of social anhedonia [74]. Similarly, a study on adults with ASD reported no ‘audience effect’ on charitable donations [75]. Anecdotally, these experimental findings echo reports of parents and caregivers who have long noted that individuals with ASD appear to be less influenced by considerations of impression management.
Biological level
The orbitofrontal–striatum-amygdala circuit has been repeatedly highlighted as abnormal in ASD[76], in particular in response to social stimuli such as faces [77], social approval [78], or social rejection [79]. One prominent hypothesis has been that social impairments result from a deficit in representing the reward value of social stimuli [48]. However, only few neuroimaging studies have directly addressed the basis of social vs. non-social reward processing in ASD and findings to date have not been entirely consistent (perhaps, in part, due to the lack of potent social reward paradigms) [78,80]. It therefore remains unclear whether aberrant reward processing in ASD is confined to social stimuli or reflects a more general deficit in stimulus-reward association. Finally, neuroimaging studies have yet to examine whether both components of social reward, i.e., ‘wanting’ and ‘liking’, are equally affected.
Research on neuropeptide signaling and ASD, though still at early stages, suggests that disrupted oxytocin regulation might play an important role in social reward dysfunctions in ASD [81] by impeding the accurate association of social stimuli and motivation values [37]. Consistent with this idea, associations between the OXT receptor gene and autism have been reported [82]. Furthermore, emerging animal models of ASD, with mutations in ASD-relevant neural cell adhesion molecules, have shown deficits in both the development of social affiliative behaviors and in glutamatergic synaptic structure in various brain regions, including circuits that may involve reward pathways [83,84].
Evolutionary level
Viewing the social motivation deficit in an evolutionary framework helps to explain the specificity of social affiliative impairments in ASD and why other interpersonal dispositions such as attachment or sexual drive are spared. Indeed, despite their unarguably social nature, these latter dispositions result from different pressures and are therefore distinct from the motivation for social affiliation. Consistent with this idea, researchers have long noted that attachment to parents and offspring and levels of sexual drive are spared in ASD. Children with ASD indeed show similar responses after separation from and reunion with their primary caregiver and have similar attachment styles to TD controls [85]. Similarly, interest in love and sexual relationships is spared in ASDs: autobiographies and parental journals indicate that people on the spectrum wish to develop intimate relationships and controlled surveys involving both parental and self-reports have confirmed that while the social skills needed to approach potential partners may be impaired (i.e., courtship skills), the desire for romantic and sexual partnership is present [86,87]. An evolutionary framework thus helps account for why affiliative but not sexual/romantic or familial drives are impaired in ASD.
What is the scope of the social motivation theory?
Although many questions remain (Box 3), the research reviewed here suggests that the social motivation theory provides a credible framework to account for social impairments in ASD. However, by concentrating on social deficits, the social motivation account faces similar shortcomings as the ToM account. Unlike non-social accounts (e.g., executive dysfunction or weak central coherence), both of these social theories indeed fall short of explaining non-social deficits in ASD, such as repetitive behaviors and restricted interests, as well as other important features of the disorder, such as its association with intellectual disabilities, co-morbidities (e.g., anxiety, depression, ADHD), or peaks of abilities (e.g., rote memory, systemizing, savant skills). Arguably, another challenge for social accounts is that both social cognition and social motivation deficits are not specific to ASD and can be found in other conditions (e.g., schizophrenia). It is important to note, however, that these shortcomings are only problematic if one considers that there ought to be a single explanation behind all the symptoms of ASD. On the contrary, if one agrees that ASD should be studied from a multiple-deficit perspective, it becomes more important to decide which of several competing theories provides the best account for a given set of deficits. In the case of social theories then, it is important to compare the explanatory power of social motivation vs. social cognition in accounting for social deficits.
BOX 3. Outstanding questions.
ASDs are notably heterogeneous. Does the social motivation account apply to all subtypes of ASDs (e.g., ‘aloof’ vs. ‘passive’ or ‘active-but-odd’)? In particular, individuals in the ‘active-but-odd’ subtype appear to display genuine signs of social motivation. It is therefore important to further characterize subgroups of ASDs that do or do not have diminished social motivation.
How can the social reward deficit be further characterized? Is the motivation deficit restricted to the social world or is there a more general stimulus-reward pairing deficit? Is social reward impaired at a general level or is the deficit circumscribed to one reward component only (e.g. ‘liking’ or ‘wanting’)? In addition to decreased prioritization of social signals, are non-social stimuli disproportionately prioritized in ASD?
What is the role of co-morbidities such as ADHD, depression or anxiety, which are known to have an impact on motivation and reward processing? In particular, does social anxiety (or social aversion) play a role in social motivation deficits in ASD?
What is the role of social motivation in learning? Does reduced motivation necessarily lead to impaired social skill learning? Given that humans learn a lot in the context of social interactions, how much might a social motivation deficit impact learning of non-social skills? Are there ways to extrinsically enhance social motivation to boost learning?
Are there developmental changes in social motivation in ASD, such that motivation to engage in social interactions increases spontaneously during adolescence and adulthood? If that is the case, how much do early deficits in social motivation have a long-lasting impact on social skills? Is there a critical period for the development of social skills and social cognition?
What are the implications of such a theoretical framework for intervention strategies? It appears that intervention can have a positive effect on social attention behaviors and that this, in turn can positively affect skills such as joint attention. Future research should determine how malleable social attention in childhood is and which intervention tools are most effective in boosting social attention.
Answering these outstanding questions will require the development of adequate tools to measure social motivation. At the moment, most research uses indirect measures, such as social attention, and as such relies on approximations to the direct measurement of social motivation. Future research should therefore focus on designing tools that measure social motivation directly, from the youngest age.
The key difference between social motivation and social cognition accounts is one of causality. In the social motivation framework, diminished social interest is thought to deprive the developing child of social inputs and learning opportunities, which, ultimately, leads to diminished expertise in social cognition. In the ‘mindblindness’ framework, social impairments are explained by the fact that individuals who struggle to understand the intricate workings of the social world are likely to end up losing interest in social interactions. Positing that deficits in social cognition are a consequence, rather than a cause, of diminished social motivation yields a number of predictions.
First, social cognition deficits, which constitute a downstream consequence of diminished exposure to the social world, might only appear in a subgroup of individuals, while social motivation deficits, which are primary, ought to appear in all or nearly all with ASD. In line with this, the general consensus has come to be that mentalizing deficits, though widespread in ASD, are by no means universal [88]. In particular, there is evidence that although some may fail to spontaneously use ToM, a significant proportion of children and adults with ASD do demonstrate an ability to represent others’ mental states in standard and advanced false belief tasks (FBTs) [89]. In this subpopulation of individuals with functional ToM skills, however, social motivation deficits remain (and in fact, constitute a fundamental part of the diagnosis). It is also important to highlight that there has been growing concern over the validity of FBTs, which served as a starting point for the ToM account and remain widely used as a test of ToM in autism. Research in developmental psychology has indeed revealed that very young infants succeed in age-appropriate versions of the FBT, hence indicating that failure in standard FBTs should not be taken as evidence for impaired ToM [90]. In the case of ASD then, it is equally unclear that failure to pass FBTs reflects lack of ToM.
Second, social motivation deficits should precede social cognition deficits in ontogeny. Following this second prediction, disrupted social orienting has, to this date, been evidenced much sooner than deficits in social cognition, with recent findings highlighting atypical social orienting from as early as 6 months in infants later diagnosed with ASD [57]. Arguably though, ToM in young ASD infants has yet to be assessed using adequate tools. Indeed, recent tasks designed to test ToM in young infants [90] have not been used in ASD and longitudinal studies assessing the development of social motivation and social cognition are still lacking.
Third, diminished social attention in development should be associated with diminished social cognition regardless of diagnoses (e.g., in non-ASD populations). The rationale is that diminished social motivation and attention starting in early childhood can ultimately deprive the child of crucial social inputs during what may be a sensitive period for the development of social cognition. Because the reward value of social interactions normally leads to a range of experiences that further allow social cognitive skills to develop, social exposure (mediated by social interest) should predict expertise in social cognition. In line with this idea, individual differences in social attention in TD infants correlates with preschool ToM abilities [91] and extreme social deprivation has been linked to the development of quasi-autistic symptoms (see, e.g., the case of institutionalized Romanian infants [92]).
Finally, if diminished social motivation and attention cause social cognition deficits, boosting social attention ought to enhance performance in social cognition. This fourth point is associated with the richest set of empirical findings which, as we demonstrate in Box 2, converge to suggest that boosting social attention has a positive impact on social cognitive performance in ASD. This suggests that ASD involves a lack of spontaneous interest in mobilizing social cognitive skills for social purposes but that underlying cognitive skills may be more spared than previously thought.
BOX 2. Boosting social motivation to enhance social cognition?
In the social motivation framework, impaired social cognition is seen as the consequence, rather than the cause, of impaired social attention. This predicts that boosting social attention in various ways (e.g., by providing explicit instructions to attend the social stimulus, increasing the relevance of the social stimulus to solve the task, or increasing the participant’s intrinsic interest for the stimulus) should lead to enhanced social cognitive performance.
Instructions
While high functioning adults with ASD do not spontaneously attribute mental states (as assessed in their looking times), they display control-like performances in verbally instructed versions of the FBT [89]. Similar results are observed with ironical utterances [100], speech sounds [101] and gaze following [102].
Relevance
There is robust evidence showing that gaze following is impaired in ASD. However, when gaze direction has a predictive value and is useful to solve the task, children with ASD do follow other people’s gaze [103]. This suggests that, despite a spontaneous disinterest in mutual gaze, they are not blind to eye direction.
Interest
Young children with ASD are better at matching facial and vocal expressions of emotion when these are portrayed by familiar, compared to unfamiliar, adults [104]. Similarly, activity in the Fusiform Face Area (FFA), which is often diminished in ASD [46], is enhanced when ASD participants are presented with familiar faces [105] or cartoon characters of specific interest to them (e.g., Digimon) [106], Figure I in this box.
Taken together, these findings suggest that the underlying competence to process social stimuli may be more spared than previously thought and that atypical performance can, at least in part, be accounted for by differences in spontaneous attentional patterns. This hypothesis is further supported by evidence showing that controlling for social attention has an important impact on observed performances [107].
This has important implications for intervention and suggests that boosting social motivation and attention might be a powerful lever for social learning. The most effective interventions might therefore be aimed at social motivation rather than at specific social skills. In this respect, OXT –which is known to enhance social salience [37]– can be seen as a promising therapeutic target and has been indeed been found to increase performance in a range of social cognitive tasks [81].
Conclusion
The social world summons our attention like no other domain: social signals are prioritized by attention, interactions are intrinsically rewarding, and social maintaining permeates interpersonal behaviors. Social motivation is subserved by dedicated biological mechanisms and can be seen as an evolutionary adaptation to humans’ highly collaborative environment: by enhancing attention to social information, by rewarding social interactions, and by promoting the desire to effectively maintain social bonds, social motivation smoothes relationships, promotes coordination and ultimately fosters collaboration. In ASD, by contrast, there appears to be an overall decrease in the attentional weight assigned to social information. Diminished social orienting, social reward and social maintaining, are all found in autism and can account for a range of behaviors, including cascading effects on the development of mature social cognitive skills. These deficits appear to be rooted in biological disruptions of the orbitofrontal-striatal-amygdala circuitry as well as in dysregulation of certain neuropeptides and neurotransmitters. ASD can thus be seen as an extreme case of early-onset diminished social motivation and provides a powerful model for understanding humans’ intrinsic drive to seek acceptance and avoid rejection.
Supplementary Material
Figure I.

Wilson, Tom Hank’s anthropomorphized companion in “Cast Away”
Figure I.
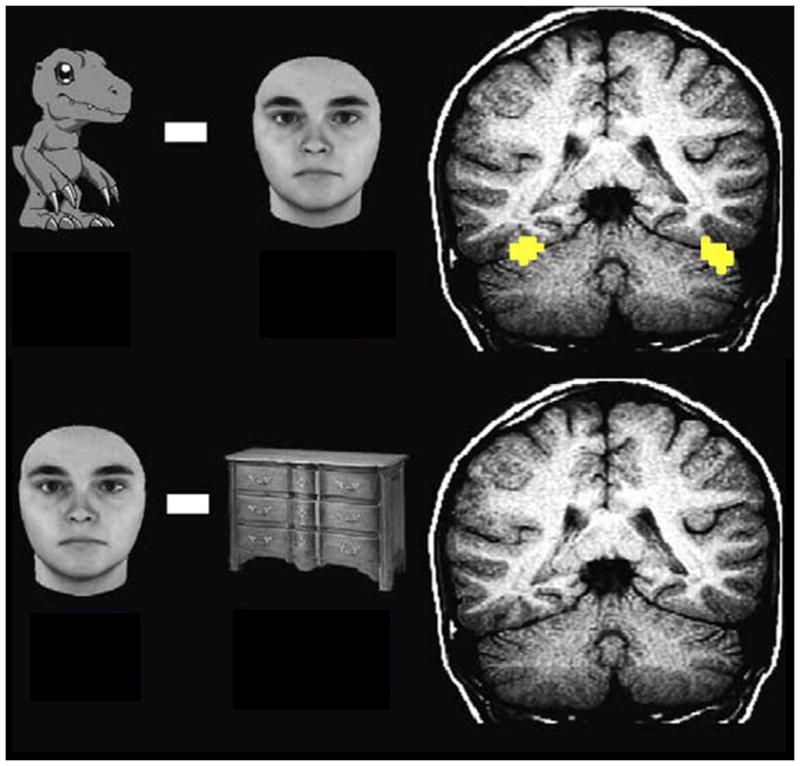
Activation of the FFA to Digimon (top panel), but not to faces (bottom panel) in patient DD. Right and left are reversed by radiological convention. Voxels are colored if the smoothed data have at ≥4 (which corresponds to P < .0001 uncorrected). Adapted from [145].
BOX 1. Social exclusion.
The adverse effects of social isolation on well-being are a natural consequence of the strength of social motivation. Economists and social psychologists have long emphasized that social bonds are indispensable for achieving happiness and epidemiologists have confirmed that lack of social support constitutes a major health risk, comparable in magnitude to well-established risk factors such as smoking and alcohol consumption [93]. People who lack positive relationships are likely to experience a range of negative psychological states ranging from loneliness to depression [94]. Social isolation or rejection can lead to a psychological state that is similar to physical pain and activates similar brain circuits [95]. It is thought that this aversive social pain signal evolved by coopting physical pain circuits to alert the excluded individual that her connections are weakening and to motivate her to repair them [94]. In line with this idea, the impact of social exclusion is manifest in every aspect of social motivation (orienting, seeking and liking, and maintaining). Chronic or induced loneliness enhances attention to social cues [96], sometimes to the extent of inventing humanlike agents (e.g., seeing faces in the clouds, or anthropomorphizing pets and objects, Figure I in this box) [97]; participants who have experienced social exclusion seek social interactions more and perceive others as more friendly [98]; and social exclusion leads to enhanced social maintaining, e.g., in the form of non-conscious mimicry [99].
Social motivation thus appears to function like other basic homeostatic systems: relative deprivation gives rise to negative feelings that signal to the individual that the her needs are not met, and a sophisticated psychological machinery is then triggered in an attempt to restore balance in the system (by increasing orientating, seeking, and maintaining behaviors).
GLOSSARY
- Audience effect
The audience effect refers to the influence of the presence of a spectator on a subject’s performance or decisions. This classic effect in social psychology has received robust experimental support. Behavioral economists have demonstrated that the presence of others enhances participants’ generosity in a range of games, such as the dictator game, the ultimatum game, and the public good game
- Overjustification effect
The overjustification effect refers to the fact that extrinsic incentives, such as money, can undermine intrinsically motivated behaviors, such as altruistic behaviors
- Theory of Mind (ToM)
ToM is the capacity to attribute mental states to others and oneself in order to explain and predict behavior. ToM is an evolved psychological ability –most highly developed in humans– specialized in the rapid attribution of beliefs, intentions, desires, or knowledge to others and oneself and in the spontaneous understanding that others have mental states that may differ from one’s own
- ‘Wanting’ and ‘liking
Reward has two dissociable psychological components: a ‘liking’ component, which refers to the hedonic value of rewards; and a ‘wanting’ component, which refers to the incentive salience of the reward (i.e., an incentive motivation promoting approach seeking and consumption of the reward) [10]. Because of the paucity of objective behavioral markers of ‘liking’ in humans, ‘liking’ and ‘wanting’ are typically confounded in behavioral studies of reward (e.g., lip licking after the consumption of a sweet beverage is often used as a behavioral marker of ‘liking’ in the animal literature but this overt expression of pleasure fades out after infancy in humans). In this respect, neuroimaging is especially useful because it enables researchers to disentangle neural mechanisms that are associated with the anticipation of a reward cue and mechanisms that are associated with the consumption of that reward
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fletcher-Watson S, et al. Rapid detection of person information in a naturalistic scene. Perception. 2008;37:571–583. doi: 10.1068/p5705. [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi Y, et al. Faces do not capture special attention in children with autism spectrum disorder: A change blindness study. Child Dev. 2009;80:1421–1433. doi: 10.1111/j.1467-8624.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 3.Ro T, et al. Changing faces: A detection advantage in the flicker paradigm. Psychol Sci. 2001;12:94. doi: 10.1111/1467-9280.00317. [DOI] [PubMed] [Google Scholar]
- 4.Pucell DG, Stewart AL. The face-detection effect: Configuration enhances detection. Atten Percept Psycho. 1988;43:355–366. doi: 10.3758/bf03208806. [DOI] [PubMed] [Google Scholar]
- 5.Gliga T, et al. Faces attract infants’ attention in complex displays. Infancy. 2009;14:550–562. doi: 10.1080/15250000903144199. [DOI] [PubMed] [Google Scholar]
- 6.Rosa Salva O, et al. The Evolution of Social Orienting: Evidence from Chicks (Gallus gallus) and Human Newborns. PLoS ONE. 2011;6:e18802. doi: 10.1371/journal.pone.0018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seju A, Johnson M. The eye contact effect: mechanisms and development. Trends Cogn Sci. 2009;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, et al. Processing of invisible stimuli: Advantage of upright faces and recognizable words in overcoming interocular suppression. Psychol Sci. 2007;18:349–355. doi: 10.1111/j.1467-9280.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 9.Stein T, et al. Eye contact facilitates awareness of faces during interocular suppression. Cognition. 2011;119:307–311. doi: 10.1016/j.cognition.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge KC, et al. Dissecting components of reward:’liking’,’wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden BY, et al. Economic principles motivating social attention in humans. P Roy Soc B-Biol Sci. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fer E, Camerer C. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Rekers Y, et al. Children, but Not Chimpanzees, Prefer to Collaborate. Curr Biol. 2011;21:1756–1758. doi: 10.1016/j.cub.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 14.Boles S. Policies designed for self-interested citizens may undermine“ the moral sentiments”: Evidence from economic experiments. Science. 2008;320:1605–1609. doi: 10.1126/science.1152110. [DOI] [PubMed] [Google Scholar]
- 15.Waneken F, Tomasello M. Extrinsic rewards undermine altruistic tendencies in 20-month-olds. Dev Psychol. 2008;44:1785–1788. doi: 10.1037/a0013860. [DOI] [PubMed] [Google Scholar]
- 16.Lery MR, Allen AB. Belonging Motivation Establishing, Maintaining, and Repairing Relational Value. Social Motivation 2010 [Google Scholar]
- 17.Higgins C, et al. Influence tactics and work outcomes: a meta analysis. J Organ Behav. 2003;24:89–106. [Google Scholar]
- 18.Talwar V, et al. White lie-telling in children for politeness purposes. Int J Behav Dev. 2007;31:1–11. doi: 10.1177/0165025406073530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu G, Lee K. Social grooming in the kindergarten: the emergence of flattery behavior. Dev Sci. 2007;10:255–265. doi: 10.1111/j.1467-7687.2007.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross H, et al. Shading the Truth: Self-Serving Biases in Children’s Reports of Sibling Conflicts. Merrill-Palmer Quart. 2004;50:61–86. [Google Scholar]
- 21.Lakin JL, et al. The chameleon effect as social glue: Evidence for the evolutionary significance of nonconscious mimicry. J Nonverbal Behav. 2003;27:145–162. [Google Scholar]
- 22.Chrtrand TL, Bargh JA. The chameleon effect: The perception–behavior link and social interaction. J Pers Soc Psychol. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- 23.Lain JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol Sci. 2003;14:334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- 24.Adlphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- 25.Klein JT, et al. Social attention and the brain. Curr Biol. 2009;19:R958–R962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghashghaei H, et al. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin A, et al. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neur. 2011;31:13039–13045. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taibnia G, Lieberman MD. Fairness and cooperation are rewarding. Ann NY Acad Sci. 2007;1118:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- 29.Izuma K, et al. Processing of the incentive for social approval in the ventral striatum during charitable donation. J Cognitive Neurosci. 2010;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- 30.Rilling J, et al. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–2243. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- 31.Schirmer A, et al. When vocal processing gets emotional: on the role of social orientation in relevance detection by the human amygdala. Neuroimage. 2008;40:1402–1410. doi: 10.1016/j.neuroimage.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Rilling JK, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiat. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Guyer AE, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiat. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy DP, et al. Personal space regulation by the human amygdala. Nat Neurosci. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spezio ML, et al. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. 2007;27:3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornak J, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 37.Bartz JA, et al. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. P Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trezza V, et al. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avale ME, et al. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 2011;25:2145–2155. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- 41.Toasello M. Human Culture in Evolutionary Perspective. Advances in Culture and Psychology. 2010;1:5–52. [Google Scholar]
- 42.Kaplan HS, et al. The evolutionary and ecological roots of human social organization. Philos T Roy Soc B. 2009;364:3289–3299. doi: 10.1098/rstb.2009.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenrick DT, et al. Renovating the pyramid of needs. Perspect Psychol Sci. 2010;5:292–314. doi: 10.1177/1745691610369469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brtto LA. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav. 2010;39:221–239. doi: 10.1007/s10508-009-9543-1. [DOI] [PubMed] [Google Scholar]
- 45.Brockington IF, et al. Severe disorders of the mother–infant relationship: definitions and frequency. Arch Women Ment Hlth. 2006;9:243–251. doi: 10.1007/s00737-006-0133-0. [DOI] [PubMed] [Google Scholar]
- 46.Scultz R. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Mudy P, Neal A. Neural plasticity, joint attention, and a transactional social-orienting model of autism. Int Rev Res Ment Ret. 2001;23:139–168. [Google Scholar]
- 48.Dawson G, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol. 2005;17:679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- 49.Osterling J, et al. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- 50.Riy DM, Hancock PJB. Viewing it differently: Social scene perception in Williams syndrome and autism. Neuropsychologia. 2008;46:2855–2860. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Klin A, et al. Visual Fixation Patterns During Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals With Autism. Arch Gen Psychiat. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 52.Nakano T, et al. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. P Roy Soc B-Biol Sci. 2010;277:2935–2943. doi: 10.1098/rspb.2010.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kln A. Young autistic children’s listening preferences in regard to speech: A possible characterization of the symptom of social withdrawal. J Autism Dev Disord. 1991;21:29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- 54.Kuhl P, et al. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8:1–12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 55.Ceponiene R, et al. Speech-sound-selective auditory impairment in children with autism: They can perceive but do not attend. P Natl Acad Sci USA. 2003;100:5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gervais H, et al. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- 57.Elsabbagh M, et al. Infant Neural Sensitivity to Dynamic Eye Gaze Is Associated with Later Emerging Autism. Curr Biol. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pierce K, et al. Preference for Geometric Patterns Early in Life As a Risk Factor for Autism. Arch Gen Psychiat. 2011;68:101–109. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howlin P, et al. Adult outcome for children with autism. J Child Psychol Psyc. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 60.Chamberlain B, et al. Involvement or Isolation? The Social Networks of Children with Autism in Regular Classrooms. J Autism Dev Disord. 2007;37:230–242. doi: 10.1007/s10803-006-0164-4. [DOI] [PubMed] [Google Scholar]
- 61.Baminger N, Kasari C. Loneliness and Friendship in High-Functioning Children with Autism. Child Dev. 2000;71:447–456. doi: 10.1111/1467-8624.00156. [DOI] [PubMed] [Google Scholar]
- 62.Baon-Cohen S, Wheelwright S. The Friendship Questionnaire: An investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. J Autism Dev Disord. 2003;33:509–517. doi: 10.1023/a:1025879411971. [DOI] [PubMed] [Google Scholar]
- 63.Liebal K, et al. Helping and cooperation in children with autism. J Autism Dev Disord. 2008;38:224–238. doi: 10.1007/s10803-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swinkels SHN, et al. Screening for autistic spectrum in children aged 14 to 15 months. I: The development of the Early Screening of Autistic Traits Questionnaire (ESAT) J Autism Dev Disord. 2006;36:723–732. doi: 10.1007/s10803-006-0115-0. [DOI] [PubMed] [Google Scholar]
- 65.Mundy P, et al. A parallel and distributed – processing model of joint attention, social cognition and autism. Autism Res. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lekam SR, Ramsden CAH. Dyadic orienting and joint attention in preschool children with autism. J Autism Dev Disord. 2006;36:185–197. doi: 10.1007/s10803-005-0054-1. [DOI] [PubMed] [Google Scholar]
- 67.Demurie E, et al. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psyc. 2011;52:1164–1173. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- 68.Chevallier C, et al. Brief Report: Selective Social Anhedonia in High Functioning Autism. J Autism Dev Disord. doi: 10.1007/s10803-011-1364-0. [DOI] [PubMed] [Google Scholar]
- 69.Hoson R, Lee A. Hello and Goodbye: A Study of Social Engagement in Autism. J Autism Dev Disord. 1998;28:117–127. doi: 10.1023/a:1026088531558. [DOI] [PubMed] [Google Scholar]
- 70.Babaro J, Dissanayake C. A Comparative Study of the Use and Understanding of Self-Presentational Display Rules in Children with High Functioning Autism and Asperger’s Disorder. J Autism Dev Disord. 2007;37:1235–1246. doi: 10.1007/s10803-006-0267-y. [DOI] [PubMed] [Google Scholar]
- 71.Scheeren AM, et al. Can you tell me something about yourself? Autism. 2010;14:457–473. doi: 10.1177/1362361310366568. [DOI] [PubMed] [Google Scholar]
- 72.Hudenko W, et al. Laughter Differs in Children with Autism: An Acoustic Analysis of Laughs Produced by Children With and Without the Disorder. J Autism Dev Disord. 2009;39:1392–1400. doi: 10.1007/s10803-009-0752-1. [DOI] [PubMed] [Google Scholar]
- 73.Hobson P, et al. Foundations for self-awareness: An exploration through autism: II. Background and methodological approach. Monogr Soc Res Child. 2006;71:29–47. doi: 10.1111/j.1540-5834.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 74.Chevallier C, et al. Diminished social motivation negatively impacts reputation management: Autism Spectrum Disorders as a case in point. PLoS ONE. 2012;7:e31107. doi: 10.1371/journal.pone.0031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izuma K, et al. Insensitivity to social reputation in autism. P Natl Acad Sci USA. 2011;108:17302–17307. doi: 10.1073/pnas.1107038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav R. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Schultz RT, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiat. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 78.Scott Van Zeeland A, et al. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masten CL, et al. An fMRI Investigation of Responses to Peer Rejection in Adolescents with Autism Spectrum Disorders. Dev Cog Neurosci. 2011;1:260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dichter GS, et al. Reward Circuitry Function in Autism During Face Anticipation and Outcomes. J Autism Dev Disord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm Behav. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacob S, et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peça J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bozdagi O, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:1–15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rutgers AH, et al. Autism and attachment: a meta-analytic review. J Child Psychol Psyc. 2004;45:1123–1134. doi: 10.1111/j.1469-7610.2004.t01-1-00305.x. [DOI] [PubMed] [Google Scholar]
- 86.Mezabin P, Stokes MA. Self-assessed sexuality in young adults with High-Functioning Autism. Res Autism Spect Dis. 2011;5:614–621. [Google Scholar]
- 87.Stokes M, et al. Stalking, and Social and Romantic Functioning Among Adolescents and Adults with Autism Spectrum Disorder. J Autism Dev Disord. 2007;37:1969–1986. doi: 10.1007/s10803-006-0344-2. [DOI] [PubMed] [Google Scholar]
- 88.Chevallier C. Theory of mind and autism: Revisiting Baron-Cohen et al.’s Sally-Anne study. In: Slater A, Quinn P, editors. Developmental Psychology: Revisiting the Classic Studies. Sage; 2012. [Google Scholar]
- 89.Senju A, et al. Mindblind Eyes: An Absence of Spontaneous Theory of Mind in Asperger Syndrome. Science. 2009;325:883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- 90.Baillargeon R, et al. False-belief understanding in infants. Trends Cogn Sci. 2010;14:110–118. doi: 10.1016/j.tics.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wellman HM, et al. Infant social attention predicts preschool social cognition. Dev Sci. 2004;7:283–288. doi: 10.1111/j.1467-7687.2004.00347.x. [DOI] [PubMed] [Google Scholar]
- 92.Hoksbergen R, et al. Post-Institutional Autistic Syndrome in Romanian Adoptees. J Autism Dev Disord. 2005;35:615–623. doi: 10.1007/s10803-005-0005-x. [DOI] [PubMed] [Google Scholar]
- 93.Holt-Lunstad J, et al. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kross E, et al. Social rejection shares somatosensory representations with physical pain. P Natl Acad Sci USA. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pickett CL, et al. Getting a cue: The need to belong and enhanced sensitivity to social cues. Pers Soc Psychol B. 2004;30:1095–1107. doi: 10.1177/0146167203262085. [DOI] [PubMed] [Google Scholar]
- 97.Epley N, et al. Creating social connection through inferential reproduction. Psychol Sci. 2008;19:114–120. doi: 10.1111/j.1467-9280.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 98.Maner JK, et al. Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem”. J Pers Soc Psychol. 2007;92:42–55. doi: 10.1037/0022-3514.92.1.42. [DOI] [PubMed] [Google Scholar]
- 99.Lakin JL, et al. I Am Too Just Like You: The effects of ostracism on non-conscious mimicry. Psychol Sci. 2008;19:816–822. [Google Scholar]
- 100.Wang A, et al. Reading Affect in the Face and Voice: Neural Correlates of Interpreting Communicative Intent in Children and Adolescents With Autism Spectrum Disorders. Arch Gen Psychiat. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whtehouse AJO, Bishop DVM. Do children with autism “switch off”to speech sounds? An investigation using event-related potentials. Dev Sci. 2008;11:516–524. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 102.Leekam S. Why do children with autism have a joint attention impairment? In: Eilan N, et al., editors. Joint attention: Communication and other minds. Oxford University Press; 2005. [Google Scholar]
- 103.Ristic J, et al. Eyes are special but not for everyone: The case of autism. Cognitive Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Kaana-Kalman R, Goldman S. Intermodal matching of emotional expressions in young children with autism. Res Autism Spect Dis. 2008;2:301–310. [Google Scholar]
- 105.Pirce K, Redcay E. Fusiform Function in Children with an Autism Spectrum Disorder Is a Matter of ’Who”. Biol Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grelotti D, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 107.Senju A, et al. Brief Report: Does Eye Contact Induce Contagious Yawning in Children with Autism Spectrum Disorder? J Autism Dev Disord. 2009;39:1598–1602. doi: 10.1007/s10803-009-0785-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.