Abstract
Aims/hypothesis
Insulin delivery to muscle is rate-limiting for insulin's metabolic action and is regulated by insulin's own action to increase skeletal muscle blood flow and to recruit microvasculature. Microvascular dysfunction has been observed in insulin resistant states. We investigated the relation between insulin's action to recruit microvasculature and its metabolic action in type 1 diabetes.
Methods
Near euglycaemia was obtained by an overnight insulin infusion during 17 inpatient admissions of type 1 diabetes subjects. This was followed by a 2 h 1 mU.kg−1.min−1 hyperinsulinemic euglycaemic clamp. Microvascular blood volume (MBV) was assessed using contrast-enhanced ultrasound 10 min before and 30 min after starting the clamp.
Results
We observed that after overnight modest hyperinsulinemia (average ≈286 pmol/l) MBV was positively related to the steady-state insulin sensitivity measured during the subsequent clamp (r = 0.62, p = 0.008). The more marked hyperinsulinemia during the clamp (average steady-state insulin ≈900 pmol/l) increased MBV in the more insulin resistant subjects within 30 min but not in the insulin sensitive subjects. The change in MBV during the clamp was negatively correlated to the insulin sensitivity (r = −0.55, p = 0.022). As a result, MBV after 30 min of marked hyperinsulinemia was comparable between the insulin sensitive and resistant subjects.
Conclusions/interpretation
We conclude that moderate overnight hyperinsulinemia recruited microvasculature in the more sensitive subjects while higher levels of plasma insulin were needed for more insulin resistant subjects. This suggests that microvascular responsiveness to insulin is one determinant of metabolic insulin sensitivity in type 1 diabetes.
Keywords: microvascular recruitment, type 1 diabetes mellitus, insulin sensitivity, hyperinsulinemia, microvascular blood volume, contrast-enhanced ultrasound, insulin metabolic action
Introduction
In the early nineties, Baron et al. [1–3] established the concept that in lean subjects, euglycaemic hyperinsulinemia at high physiological concentrations stimulates blood flow in skeletal muscle tissue. More recently, it was shown that blood flow distribution stimulated by hyperinsulinemia mirrored changes in glucose utilization [4], suggesting that “insulin-induced alteration in blood flow patterns could be as important as direct signalling of cells by insulin in establishing the rate of glucose utilization in vivo” [5]. Insulin thus appears to regulate its metabolic action in part by acting on the vasculature to facilitate its own delivery to skeletal muscle [6], a step known to be rate-limiting for the insulin-mediated glucose disposal [7]. It has been recently highlighted that insulin acts on the vasculature not only to dilate resistance vessels (yielding increased blood flow), but also to relax pre-capillary arterioles and recruit microvasculature thus expanding the surface available for nutrient and insulin delivery [8, 9]. Insulin-mediated capillary recruitment occurs within 10 min and temporally precedes increases in total blood flow [9] and is a key part of the insulin action in vivo, accounting for as much as 50% of the insulin-induced increase in glucose uptake [8–11].
Laakso et al. reported that in insulin-resistant obese subjects, leg blood flow was stimulated by pharmacological but not by physiological hyperinsulinemia [12]. We have reported that metabolic insulin resistance in experimental animals [13] and humans [14] is associated with microvascular insulin resistance within skeletal muscle and that the latter contributes to metabolic insulin resistance [6, 15]. In these latter studies we used contrast-enhanced ultrasound (CEU) to measure the microvascular blood volume (MBV) in the basal state and during either insulin infusion (euglycaemic clamp) or meal stimulated insulin secretion [16]. Both obesity [14] and raising plasma non-esterified fatty acid concentrations [17] impaired insulin's ability to increase MBV and were associated with metabolic insulin resistance.
Most studies of type 1 diabetes have reported moderate degrees of insulin resistance relative to age and weight matched controls, even in type 1 diabetes adolescents [18, 19]. Recent studies have also shown that type 1 diabetes adults and adolescents have impaired flow-mediated dilation in conduit vessels [20, 21]. It is important to understand the effects of insulin on capillary recruitment in type 1 diabetes patients as they exclusively rely on exogenous insulin supplies to ensure glucose disposal and thus maintain safe glucose levels. In the present study we addressed whether a relation between insulin-induced microvascular recruitment and metabolic insulin sensitivity exists in type 1 diabetes as found in other insulin resistant states.
Because type 1 diabetes subjects are continuously dependent on exogenous insulin, and controlling blood glucose requires peripheral hyperinsulinemia, we measured MBV after an overnight insulin infusion which was selected to maintain a subject's blood sugar between 5.6 and 8.3 mmol/l based upon 30 min glucose measurements and adjustment of the insulin infusion rate. MBV was measured after this overnight, low-dose insulin infusion and subjects then received a primed, continuous infusion of insulin for two hours along with an exogenous glucose infusion to maintain glucose constant at the fasting concentration (euglycaemic hyperinsulinemic clamp). Thirty min into the euglycaemic clamp MBV was again measured using CEU imaging techniques. Steady-state insulin sensitivity was determined from the glucose infusion rate required to maintain euglycaemia over the last 30 min of the 2 h insulin clamp. The MBV measurements and insulin-induced change in MBV were related to the metabolic insulin sensitivity.
Methods
Study design and population
Maintenance of euglycaemia requires peripheral hyperinsulinemia in individuals with type 1 diabetes. If peripheral insulin concentrations in type 1 diabetes are reduced to levels comparable to non-diabetic individuals, hyperglycaemia ensues. In as much as hyperglycaemia per se substantially affects vascular function and responsiveness to insulin, we elected to treat the type 1 diabetes subjects overnight with a relatively low-dose insulin infusion to attain near euglycaemia and examine the effects of subsequent marked hyperinsulinemia using the clamp procedure. Seventeen clamps were performed on 14 patients with type 1 diabetes. For the 3 patients who were studied twice, the time interval between the clamps was at least 6 months, which allowed us to consider them independent. The study was approved by the University of Virginia Internal Review Board and performed at the General Clinical Research Center. All subjects gave informed consent.
All subjects had an outpatient screening visit which included a history and physical examination, with assessment for orthostatic hypotension. Laboratory testing included a urine microalbumin/creatinine ratio, HCG (females), hemoglobin A1c, hematocrit and a comprehensive chemistry panel. The subjects were on average 37.2 ± 11.8 year old, had type 1 diabetes for 19.8 ± 12.2 years and weighed 80.8 ± 12.1 kg. Their mean BMI was 26.3 ± 3.3 kg/m2. All subjects were treating their diabetes using an insulin pump or insulin injections; and had HbA1c of 8.0 ± 2.3 % measured prior to the clamp admission. We defined a complication index to assess the extent of a patient's diabetic microvascular complications as the number of complications the patient had developed among those related to the kidneys, nerves, feet, eyes, and digestive system. The complication index is equal to 0 for no complication and equal to 5 when all of them have developed. The mean complication index was 0.6 ± 1.0. Two subjects had nephropathy as assessed by a ratio of microalbumin over creatinine above 30 mgc/mg and one patient was on Cialis (prn) for sexual dysfunction. All subjects but one were maintaining regular physical activities. An exhaustive list of the patients' medications is given in Table 1.
Table 1.
Exhaustive list of the patients' medications and subject grouping resulting from the cluster analysis.
| Subjects | Group | Medications |
|---|---|---|
| 1 | 1 | Lantus, Humalog, Accupril, Avapro, HCTZ |
| 2 | 2 | Humalog |
| 3 | 1 | Humalog, Lipitor, Tricor, Estraderm Patch, Vitamin D, Prevacid, Multivitamin, Fish Oil, Calcium |
| 4 | 1 | Synthroid |
| 5 | 1 | Humalog, Lisinopril,multivitamin |
| 6 | 1 | Novolog, Amitriptyline, Atenolol, Lisinopril, HCTZ, Ranitidine, vitamins, Excedrin PM, Zocor |
| 7 | 1 | Lantus insulin, Novolog insulin, multivitamin |
| 8 | 2 | NPH insulin, Regular insulin, Wilsons Wonder (topical), Lotrimin (topical) |
| 9 | 2 | Humalog. Lipitor, Wellbutrin, Altace, baby aspirin, vitamin |
| 10 | 2 | Lantus, Humalog, SlowMag, Aspirin |
| 11 | 2 | Novolog, Lisinopril, Nuvaring, Multivt |
| 12 | 2 | Lantus, Novolog, Vytorin, Ambien |
| 13 | 2 | Humalog, Lipitor |
| 14 | 1 | Humalog, Elavil, Neurontin, Aspirin, Cozaar, Multivitamin, Cialis (prn) |
Prior to the admission, patients using long or intermediate acting insulin consulted with a study physician for insulin dose adjustment. Long-acting insulin was discontinued 60 h and intermediate acting insulin was discontinued 36 h prior to the clamp procedure. Only regular or rapid-acting insulin was allowed on the day of the admission. Patients were asked to try to keep their blood glucose between 5.6 and 8.3 mmol/l and to avoid hypoglycaemia, and to perform frequent fingerstick blood glucose measurements (10 per day, at least 30 min apart) for reference values. Subjects were admitted to the General Clinical Research Center on the evening prior to study. At 21:30, an intravenous infusion of regular insulin (Novolin R, Novo Nordisk, 0.1 unit/ml saline) was begun and titrated to maintain the subjects' blood glucose overnight between 5.6 and 8.3 mmol/l as measured every 30 min by YSI analyzer (YSI, Life Sciences, OH). This infusion was discontinued at 08:30 the following morning at the initiation of the clamp procedure. At time 0 an insulin infusion was given via Harvard pump (Harvard Apparatus, MA) as a 20 mU/kg priming over 10 min followed by a constant 1 mU.min−1.kg−1 infusion maintained for the next 110 min. Plasma glucose was measured at intervals of 5 min and clamped at basal levels via a variable-rate infusion of 20% dextrose using the equations of DeFronzo et al. [22]. Total plasma insulin concentration was measured by radioimmunoassay (RIA kits, Millipore, MA) at intervals of at most 10 min.
Contrast-enhanced ultrasound
CEU imaging was performed in the forearm with a SONOS 7500 ultrasound system (Philips Medical Systems, Bothell, WA) and a S3 probe. Pulse inversion imaging was performed at an ultrasound transmit frequency of 1.3 MHz and receive frequency of 3.6 MHz. A 3 ml suspension of octafluoropropane gas-filled lipid microbubbles Definity (Bristol-Myers Squibb Medical Imaging, North Billerica, MA) diluted in 57 ml saline and continuously infused at a rate of 1.5 ml/min was used as the contrast medium. CEU imaging was performed 10 min before and 30 min after starting the clamp. Images were obtained at increasing pulsing interval, from 1 to 20 cardiac cycles with at least 3 images acquired at each pulsing interval. This allowed us to quantify increasing microvascular replenishment with microbubbles between the pulses until the beam space was completely refilled. The sequences of ultrasound images were digitalized and stored as TIF files for offline analysis.
Measurement of microvascular blood volume
When exposed to high-energy ultrasound, the infused microbubbles are destroyed, resulting in a high-amplitude signal. By allowing progressively longer time intervals between pulses the reappearance of microbubbles within the muscle vasculature results in a time-dependent signal intensity increase. When the space defined by the beam thickness is completely filled, increasing the pulsing interval does not further affect the signal intensity. The replenishment curve, plotted as the mean intensity over a region of interest (ROI) against the pulsing interval in seconds, can be described by an exponential function [23]:
| (1) |
where y is the ROI mean acoustic intensity in decibels (dB) and t the pulsing interval in seconds. The constant c is the acoustic intensity that would be obtained if the pulsing interval was reduced to 0, that is, the background intensity reflected by the tissues per se. The parameter A represents the plateau of the background-subtracted intensity and β the rate at which the acoustic intensity rises with increasing pulsing intervals. As detailed in [23], MBV was estimated by the parameter A.
ROI selection and quantification of videointensity
To minimize/eliminate the dependence of the ROI selection on the operator and assure consistency in the ROI definition across different sequences, we developed a systematic procedure to select ROI [24]. Briefly, the method is based on the normalization of the intensity of the acoustic signal stored in the image files. The ROI is defined as the union of regions such that the intensity in that region does not exceed a threshold K1 across the frames taken at pulsing interval less than 2 heart beats (this is done to eliminate contributions from bones and large arteries and arterioles to the videointensity) and does not exceed a threshold K2 across all the frames of the sequence (this accounts for the movement of large arteries and arterioles). This method requires having the information of the pulsing interval measured in number of heart beats for each frame. We described in [24] an algorithm to reconstruct the pulsing interval in number of heart beats from the relative time (in seconds) when relative time is the only information stored in the file. For each sequence, the ROI was selected based on this systematic method and the mean acoustic intensity over the ROI was fitted to the exponential function described by equation (1) to obtain estimates of the parameters A, β and c.
Measures
The glucose infusion rate averaged during the steady-state of the hyperinsulinemic euglycaemic clamp, denoted M-value and expressed in μmol.min−1.kg−1, provides a measure of whole-body glucose uptake. Whole body insulin sensitivity (SI), expressed in μmol.min−1.kg−1/(pmol.l−1), was estimated over the last 30 min of the clamp when a steady-state glucose infusion rate was reached as the ratio of the M-value over the plasma insulin concentration as described by DeFronzo et al. [22]. Because the distribution of SI is skewed to the right, we performed a logarithm transform on SI and denoted the transformed variable logSI. Taking the logarithm of the data is a standard statistical transformation aiming at reducing the skewness of the data and increasing the validity of correlation analyses. We defined three measures of the plasma insulin concentration as follows: Ip,basal the insulin concentration before the start of the clamp (but after overnight insulin infusion), computed as the mean insulin concentration during the last 30 min preceding the clamp, Ip,priming the insulin concentration reached during the insulin priming, computed as the mean insulin concentration during the first 10 min of the clamp, and Ip,ss the insulin concentration reached during the last 30 min of the clamp, all three measures are expressed in pmol/l. Acquisition of the CEU replenishment curve takes approximately 5 min. Basal MBV (MBVbasal) was assessed 10 min before the start of the clamp and MBV during hyperinsulinemia was captured at 30 min after starting the clamp (MBV30min). Given the different baseline values, the insulin-induced capillary recruitment was assessed by the relative change in MBV from the basal state to the hyperinsulinemic state, expressed in percentage:
Statistical analyses
The relationships between logSI and basal MBV and between logSI and ΔMBV were examined using Pearson's correlation and linear regressions to control for other variables. The subjects were divided into two groups using a 2-means cluster analysis with respect to standardized logSI and ΔMBV. The standardization brings all parameters to the same order of magnitude by centring the parameters on their means and scaling them by their standard deviation. The distance between two points is defined as the Euclidean distance with respect to the standardized logSI and ΔMBV. The K-means analysis creates the groups by minimizing the sum, over all groups, of the within-group sums of point-to-group-centroid distances. In other words, subjects that are similar in both their logSI and ΔMBV are grouped together. Statistical comparisons between the two groups were then performed using an unpaired t-test. For all tests, statistical significance was declared at p < 0.05 (two-sided). The analyses were performed using MATLAB 7.10 (R2010a), The MathWorks™ and PASW Statistics 18, SPSS Inc. Data are presented in mean ± SD.
Results
Glucose disposal and microvascular responses to euglycaemic hyperinsulinemia
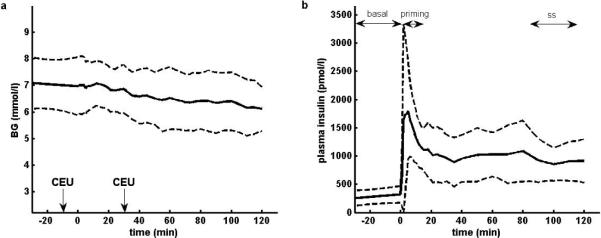
Steady-state plasma glucose was achieved after overnight insulin infusion as shown in Fig. 1. Plasma glucose was clamped at 6.6 ± 0.9 mmol/l for the 17 euglycaemic hyperinsulinemic clamps. The profiles of glucose and plasma insulin concentrations are shown in Fig. 2. The M-value, log SI, measures related to insulin concentration Ip,basal, Ip,priming and Ip,ss, blood glucose (BG) concentrations before the start of the clamp (BGstart) and during the last 30 min of the clamp (BGend), measures of capillary recruitment MBVbasal and MBV30 min, assessed from the basal and 30 min sequences respectively, and ΔMBV are shown in Table 2.
Fig. 1.
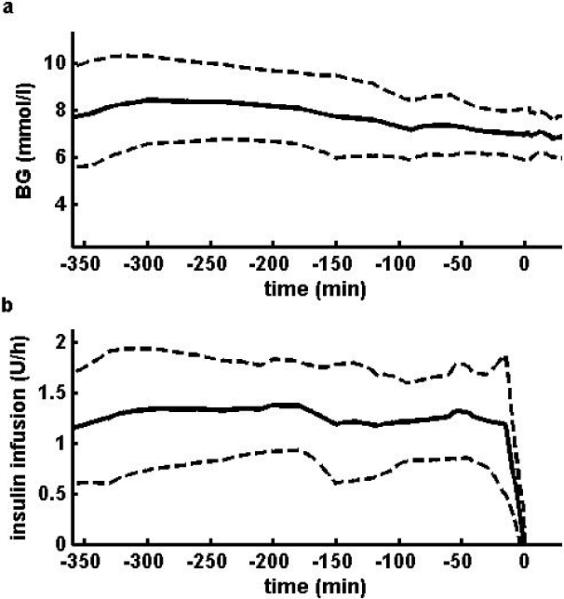
(a) Plasma glucose concentrations and (b) Insulin infusion rate during the 6 hours prior to the clamp. Data shown as mean ± SD (n = 17).
Fig. 2.
(a) Plasma glucose concentrations during the clamp. Microvascular recruitment was assessed through CEU imaging at basal plasma insulin levels (t = −10min) and at hyperphysiological insulin levels (t = 30min). (b) Plasma insulin concentrations before and during the clamp. The time intervals used to compute the insulin measures are indicated as “basal” for Ip,basal, concentration before the start of the clamp, as “priming” for Ip,priming, concentration during the insulin priming, and as “ss” for Ip,ss, concentration at steady-state of the clamp. All concentrations are shown as mean ± SD (n = 17).
Table 2.
Mean and SD of the M-value, logSI, plasma insulin concentration before the start of the clamp Ip,basal, during the insulin priming Ip,priming, and at steady-state of the clamp Ip,ss, , BG before the start of the clamp (BGstart) and during the last 30 min of the clamp (BGend), MBV at basal and high physiological levels of insulin MBVbasal and MBV30 min respectively, and the relative change in capillary recruitment ΔMBV (n = 17).
| mean | SD | |
|---|---|---|
| M-value (μmol.min−1.kg−1) | 24.7 | 9.1 |
| logSI | −3.61 | 0.57 |
| Ip,basal (pmol/l) | 285.8 | 132.4 |
| Ip,priming (pmol/l) | 1526.0 | 960.2 |
| Ip,ss (pmol/l) | 899.9 | 331.0 |
| BGstart (mmol/l) | 7.0 | 0.9 |
| BGend (mmol/l) | 6.3 | 1.0 |
| MBVbasal (dB) | 3.29 | 0.94 |
| MBV30min (dB) | 3.17 | 1.03 |
| ΔMBV (%) | 0.6 | 33.8 |
Relationship between basal MBV and insulin sensitivity
The relationship between MBVbasal and logSI is shown in Fig. 3. The correlation between MBVbasal and logSI is 0.62 (p = 0.008) and the linear regression of logSI on MBVbasal indicates that MBVbasal was a statistically significant predictor of logSI (p = 0.008). To control for the potential effect of the basal plasma insulin concentration on MBVbasal, we performed a linear regression of logSI on MBVbasal and Ip,basal and MBVbasal remained statistically significant (p = 0.014). The partial correlation between logSI and MBVbasal is 0.60, almost identical to the original correlation. Likewise, controlling for age, body weight, HbA1c and number of years since the diagnosis of type 1 diabetes did not change the significance of MBVbasal as a predictor of logSI. The positive relationship between logSI and MBVbasal shows that the subjects whose microvasculature was most expanded after the overnight insulin infusion were more insulin sensitive. This suggests that the ability to recruit capillaries at modest levels of peripheral hyperinsulinemia is a potential determinant to the sensitivity of a subject to insulin.
Fig. 3.
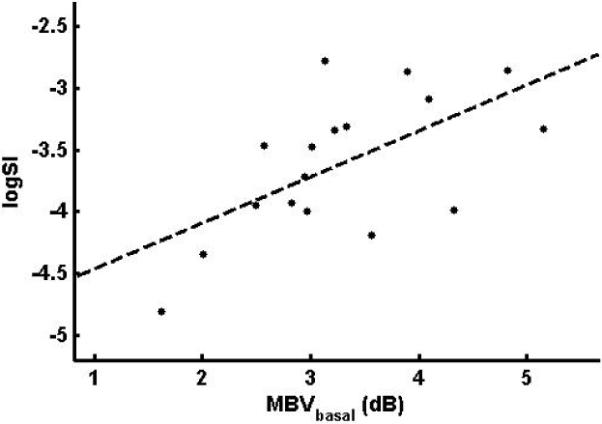
Relationship between MBVbasal and logSI (r = 0.62, p = 0.008)
Relationship between changes of MBV and insulin sensitivity during the clamp
The relationship between capillary recruitment induced by high concentrations of insulin attained during the clamp and logSI is shown in Fig. 4. The correlation between ΔMBV and logSI is −0.55 (p = 0.022). When performing a regression of ΔMBV on logSI, the variable logSI was significant in the linear model (p = 0.022), meaning that the insulin sensitivity is a significant predictor for the dependent variable ΔMBV. The model indicates that the more insulin sensitive patients, with higher MBV after the overnight insulin infusion, increased MBV less than the more insulin resistant ones. Controlling for age, body weight, HbA1c and number of years since diagnosed type 1 diabetes did not change the significance of logSI as a predictor of ΔMBV.
Fig. 4.
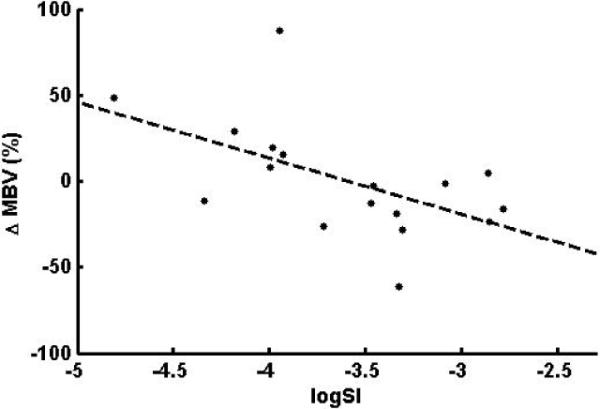
Negative relationship between ΔMBV and logSI (r = −0.55, p = 0.022)
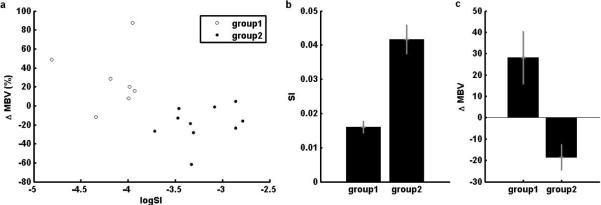
The subjects were divided into 2 groups using a K-means cluster analysis with 2 clusters based on standardized ΔMBV and logSI. The 2 groups obtained are shown in Fig. 5(a) and listed in Table 1. No significant differences were found between the two groups with respect to age, duration of type 1 diabetes, body weight, BMI, HbA1c measured prior to the clamp admission, and complication index. Insulin sensitivity and ΔMBV for the two groups are shown in Fig. 5(b) and 5(c) respectively and we observed a clear separation between the two groups on insulin sensitivity and on ΔMBV. Group 1 (n = 7) was characterized by lower insulin sensitivity but greater capillary recruitment during the clamp, while group 2 (n = 10) had higher insulin sensitivity, but less capillary recruitment. The mean insulin sensitivity of the 2 groups, 0.016 and 0.042 μmol.min−1.kg−1/(pmol.l−1) for group 1 and 2 respectively, were statistically different as assessed by an unpaired t-test (p = 0.0002). Likewise, the mean change in MBV of the 2 groups, 28.1% and −18.7% respectively, were statistically different one from the other (unpaired t-test p = 0.019). Therefore, capillary recruitment during the higher dose insulin infusion in type 1 diabetes was related to the insulin sensitivity of the subjects. More precisely, lower insulin sensitivity was related to higher capillary recruitment (ΔMBV for group 1 = 28.1 ± 32.0%, p = 0.060) while a higher sensitivity was associated with a decline in MBV (capillary derecruitment, ΔMBV for group 2 = −18.6 ± 18.7%, p = 0.012).
Fig. 5.
Cluster analysis based on ΔMBV and logSI. (a) Groups obtained from the cluster analysis; (b) Insulin sensitivity and (c) Relative change of MBV of the 2 groups
MBV after 30 min of hyperinsulinemia was not statistically different between the two groups (3.58 ± 1.26 dB for group 1 and 2.87 ± 0.77 dB for group 2, p = 0.170) and the correlation between log SI and MBV30min is almost null (r = 0.02, p = 0.95). Furthermore, MBV30min for group 1 (3.58 ± 1.26 dB) was not statistically different from MBVbasal for group 2 (3.61 ± 0.85 dB), indicating that microvascular recruitment in the more resistant group attained similar levels as those achieved in the sensitive group at basal levels of plasma insulin.
Discussion
We found that subjects who were more insulin sensitive had a greater MBV after the overnight insulin infusion, while more insulin resistant subjects increased MBV only after 30 min of marked hyperinsulinemia. To emphasize the relationship between insulin sensitivity and relative change in MBV, the subjects were grouped through a cluster analysis. The groups were thus defined based on the data rather than based on arbitrary thresholds. The first group included more insulin resistant subjects while the second one included more sensitive ones. The absolute MBV after the first 30 min of the insulin clamp was not statistically different between the two groups. Together, these observations suggest that insulin-induced capillary recruitment occurs at levels of plasma insulin that depend on the metabolic insulin sensitivity of the subject, and more precisely, that subjects whose microvascular volume is expanded after the overnight insulin infusion are subjects with higher whole-body glucose uptake at steady-state of the euglycaemic clamp. Conversely, subjects for whom higher insulin concentrations are needed to induce capillary recruitment will not be as insulin sensitive.
We have previously reported a dose-response relationship between insulin-induced capillary recruitment and MBV in rodents. In those studies we observed that insulin infusions of 3 mU.min−1.kg−1 produced near maximal capillary recruitment and increasing plasma insulin further did not augment capillary opening [25]. Here we computed insulin sensitivity based on the steady-state glucose consumption during the last 30 min of the clamp and found that patients with higher MBV after overnight insulin infusion are the patients with higher insulin sensitivity. This suggests that greater vascular insulin sensitivity predicts greater metabolic insulin sensitivity. It is important to note that at 30 min after the start of the clamp, the plasma insulin concentration reached steady-state levels and MBV was similar between the insulin sensitive and insulin resistant groups. If the effect of time on MBV is negligible compared with the effect of plasma insulin levels over the duration of the clamp, one would predict that at the time at which insulin sensitivity was measured (last 30 min of the clamp), the endothelial surface available for insulin transfer to muscle would be similar, and differences in metabolic insulin sensitivity would be attributable to either delays in insulin crossing the endothelium [6] or resistance at the myocyte per se [26].
We previously observed that a hyperinsulinemic euglycaemic clamp (insulin infusion rate = 1 mU.min−1.kg−1) increased MBV (measured by CEU) in lean but not in obese humans [14]. MBV at baseline was comparable in the lean and the obese groups (18.7 ± 3.3 and 20.4 ± 3.6 respectively) despite higher plasma insulin concentrations in the obese. These obese subjects were quite insulin resistant [27], as assessed from the whole-body glucose infusion rate during hyperinsulinemia. Because type 1 diabetes patients require continuous insulin treatment, basal MBV in the current study corresponds to MBV measured after an overnight insulin infusion, while baseline MBV in the obese/lean study corresponds to MBV measured before the start of the clamp, with no insulin infusion. In consequence, the plasma insulin concentrations in the type 1 diabetes population before (285.8 ± 132.4 pmol/l) and during the clamp (899.9 ± 331.0 pmol/l) were substantially higher than in either the obese or lean subjects studied previously both before (91.7 ± 12.5 pmol/l for the obese and 43.8 ± 7.0 pmol/l for the lean) and during the clamp (≈ 450 pmol/l for the obese and 415 pmol/l for the lean (see Fig. 2A. in reference [14]). Our study suggests that plasma insulin concentrations higher than 450 pmol/l might be needed to trigger capillary recruitment in the obese subjects.
Laakso et al. studied the insulin dose-response of leg blood flow in overnight fasted humans before and during insulin infusion [12]. Leg blood flow was similar in lean and obese subjects in the postabsorptive state and increased about twofold in a sigmoidal fashion in both groups as a function of insulin concentration. Maximum leg blood flow rates were similar in obese and lean subjects. However, the plasma insulin concentration which half-maximally increased leg blood flow (ED50) was 3.6 times higher in obese subjects than in lean subjects (1107.7 vs 307.7 pmol/l), demonstrating vascular insulin resistance in these obese subjects. We have not studied the insulin dose-response for MBV in obese humans. However, if the insulin dose-response curve for capillary recruitment parallels the one of total blood flow, the higher ED50 for obese subjects compared with lean subjects would be consistent with the absence of insulin-induced capillary recruitment in the obese subjects at the levels of plasma insulin observed in [27]. Of interest in the current study of type 1 diabetes we observed a negative correlation (r = −0.44, p = 0.081) between BMI and logSI in these type 1 diabetes subjects, indicating that more insulin resistant subjects were heavier in this population as well. A rightward shift for the dose-response of insulin-mediated capillary recruitment is consistent with the results obtained in our study.
In the circulation, insulin stimulates the release of both endothelin-1 (ET-1), a potent vasoconstrictor, and nitric oxide (NO), a vasodilator; the proportion of one to the other determining the hemodynamic effect of insulin [28]. It is of interest that the insulin sensitive type 1 diabetes subjects not only failed to further increase MBV during the clamp but MBV on average declined. Others have pointed out that prolonged insulin infusion (>6 h) can actually lead to an inhibition of NO-mediated vasodilation [29]. In our study insulin's effect to increase ET-1 may have become dominant when the insulin clamp followed the overnight infusion of insulin in the insulin sensitive group, but not in the resistant group.
In conclusion, we studied the action of insulin on muscle microvascular recruitment and its relation to metabolic insulin sensitivity in subjects with type 1 diabetes. The data indicate that microvascular perfusion is positively related to the insulin sensitivity with insulin sensitive subjects having an expanded MBV after an overnight insulin infusion while 30 min of greater hyperinsulinemia was required to recruit capillaries in more insulin resistant subjects. These findings indicate that the moderate insulin resistance seen in type 1 diabetes extends to the vascular responsiveness to insulin and that the vascular and metabolic insulin resistances are linked in type 1 diabetic subjects as has been reported for obese insulin resistant subjects.
Acknowledgements
This study is supported by the NIH/NIDDKRO1 DK 51562 grant and the University of Virginia General Clinical Research Center M01 RR 000847 grant.
Abbreviations
- BG
Blood glucose
- CEU
Contrast-enhanced ultrasound
- dB
Decibels
- ET-1
Endothelin-1
- MBV
Microvascular blood volume
- NO
Nitric oxide
- ROI
Region of interest
- SI
Insulin sensitivity
Footnotes
Clinical trial registration: ClinicalTrials.gov, clinical trial number NCT00943787, protocol entitled “Counter-regulatory impairment and the effect of microvascular insulin transfer in type 1 diabetes mellitus” (BPK003)
Contribution statement All the authors, Alice Chan, Eugene J. Barrett, Stacey M. Anderson, Boris P. Kovatchev, Marc D. Breton contributed to the conception and design, or analysis and interpretation of the data, the drafting the article or revising it critically for important intellectual content and the final approval of the version to be published.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Baron AD, Brechtel-Hook G, Johnson A, Hardin D. Skeletal muscle blood flow. A possible link between insulin resistance and blood pressure. Hypertension. 1993;21:129–135. doi: 10.1161/01.hyp.21.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. The Journal of Clinical Endocrinology and Metabolism. 1991;73:637–643. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD. Hemodynamic actions of insulin. The American Journal of Physiology. 1994;267:E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 4.Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P. Direct Measurements of the Permeability Surface Area for Insulin and Glucose in Human Skeletal Muscle. Journal of Clinical Endocrinology & Metabolism. 2003;88:4559–4564. doi: 10.1210/jc.2003-030434. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN. Insulin Action and Distribution of Tissue Blood Flow. Journal of Clinical Endocrinology & Metabolism. 2003;88:4556–4558. doi: 10.1210/jc.2003-031431. [DOI] [PubMed] [Google Scholar]
- 6.Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. The Journal of Clinical Investigation. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes/Metabolism Research and Reviews. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- 9.Vincent MA, Dawson D, Clark ADH, et al. Skeletal Muscle Microvascular Recruitment by Physiological Hyperinsulinemia Precedes Increases in Total Blood Flow. Diabetes. 2002;51:42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Coggins M, Lindner J, Rattigan S, et al. Physiologic Hyperinsulinemia Enhances Human Skeletal Muscle Perfusion by Capillary Recruitment. Diabetes. 2001;50:2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 11.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- 12.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. Journal of Clinical Investigation. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular Recruitment Is an Early Insulin Effect That Regulates Skeletal Muscle Glucose Uptake In Vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 14.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 15.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 16.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity Blunts Microvascular Recruitment in Human Forearm Muscle After a Mixed Meal. Diabetes Care. 2009;32:1672–1677. doi: 10.2337/dc09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing Lipid Raises Plasma Free Fatty Acids and Induces Insulin Resistance in Muscle Microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982;31:795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- 19.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. The Journal of Clinical Endocrinology and Metabolism. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmud FH, Van Uum S, Kanji N, Thiessen-Philbrook H, Clarson CL. Impaired endothelial function in adolescents with type 1 diabetes mellitus. The Journal of Pediatrics. 2008;152:557–562. doi: 10.1016/j.jpeds.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Sophie Peña A, Wiltshire E, Gent R, Hirte C, Couper J. Folic acid improves endothelial function in children and adolescents with type 1 diabetes. The Journal of Pediatrics. 2004;144:500–504. doi: 10.1016/j.jpeds.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo R, Tobin J, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 23.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 24.Chan A, Kovatchev BP, Anderson SM, Breton MD. Systematic method to assess microvascular recruitment using contrast-enhanced ultrasound. Application to insulin-induced capillary recruitment in subjects with T1DM. Computer Methods and Programs in Biomedicine. 2011;102:219–26. doi: 10.1016/j.cmpb.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Vincent MA, Richards SM, et al. Insulin Sensitivity of Muscle Capillary Recruitment In Vivo. Diabetes. 2004;53:447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- 26.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. Journal of Clinical Investigation. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prager R, Wallace P, Olefsky JM. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. The Journal of Clinical Investigation. 1986;78:472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardillo C, Nambi SS, Kilcoyne CM, et al. Insulin Stimulates Both Endothelin and Nitric Oxide Activity in the Human Forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- 29.Arcaro G, Cretti A, Balzano S, et al. Insulin Causes Endothelial Dysfunction in Humans: Sites and Mechanisms. Circulation. 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]