Abstract
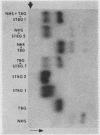
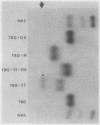
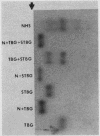
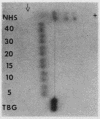
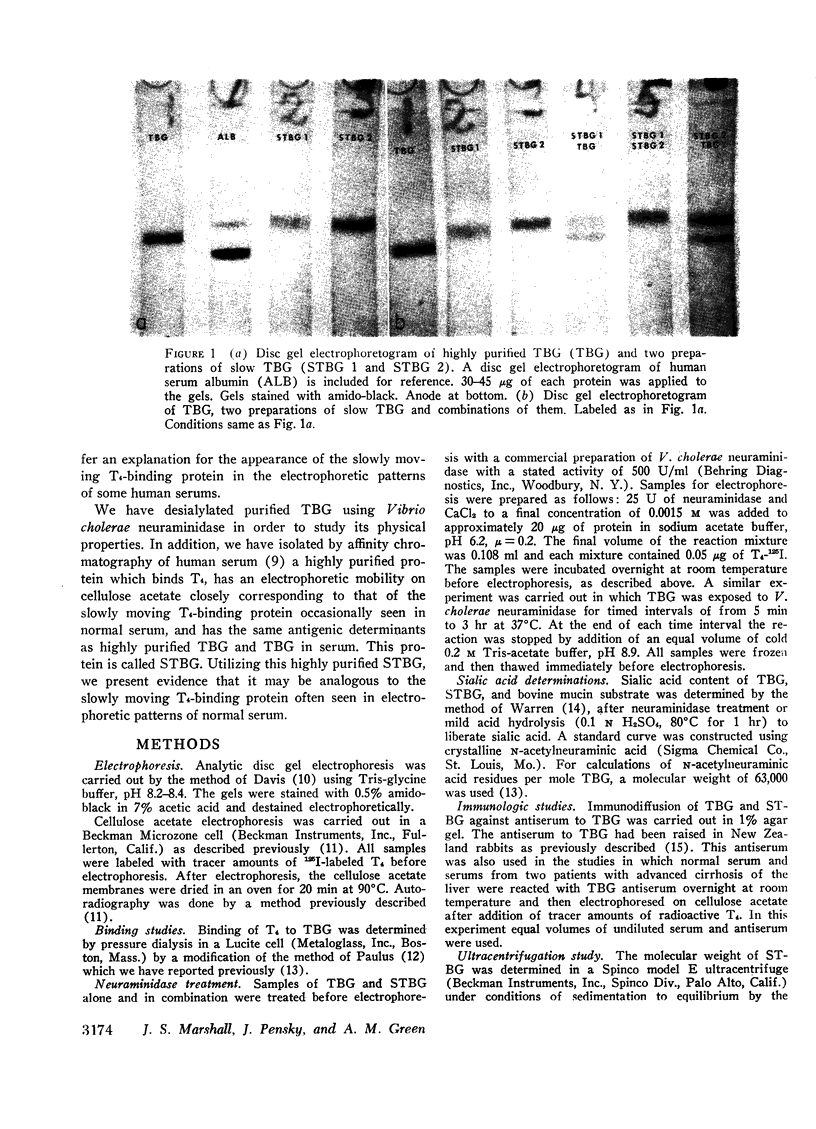
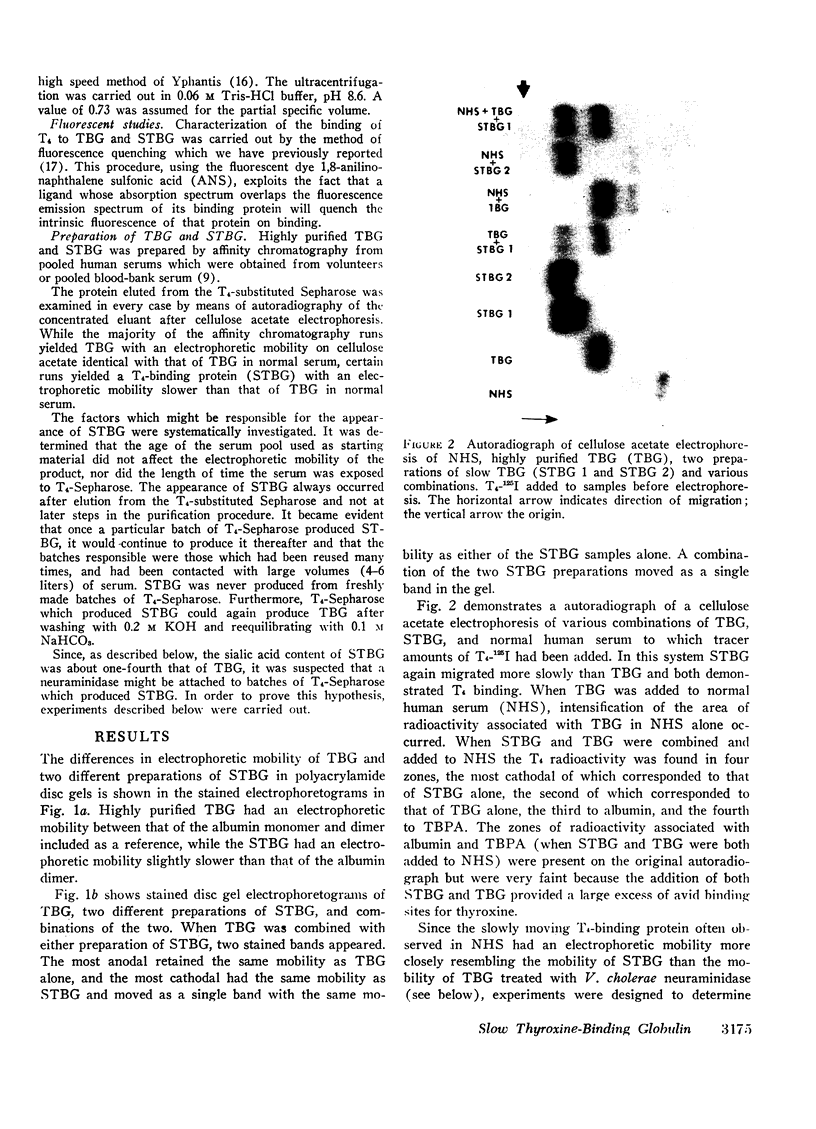
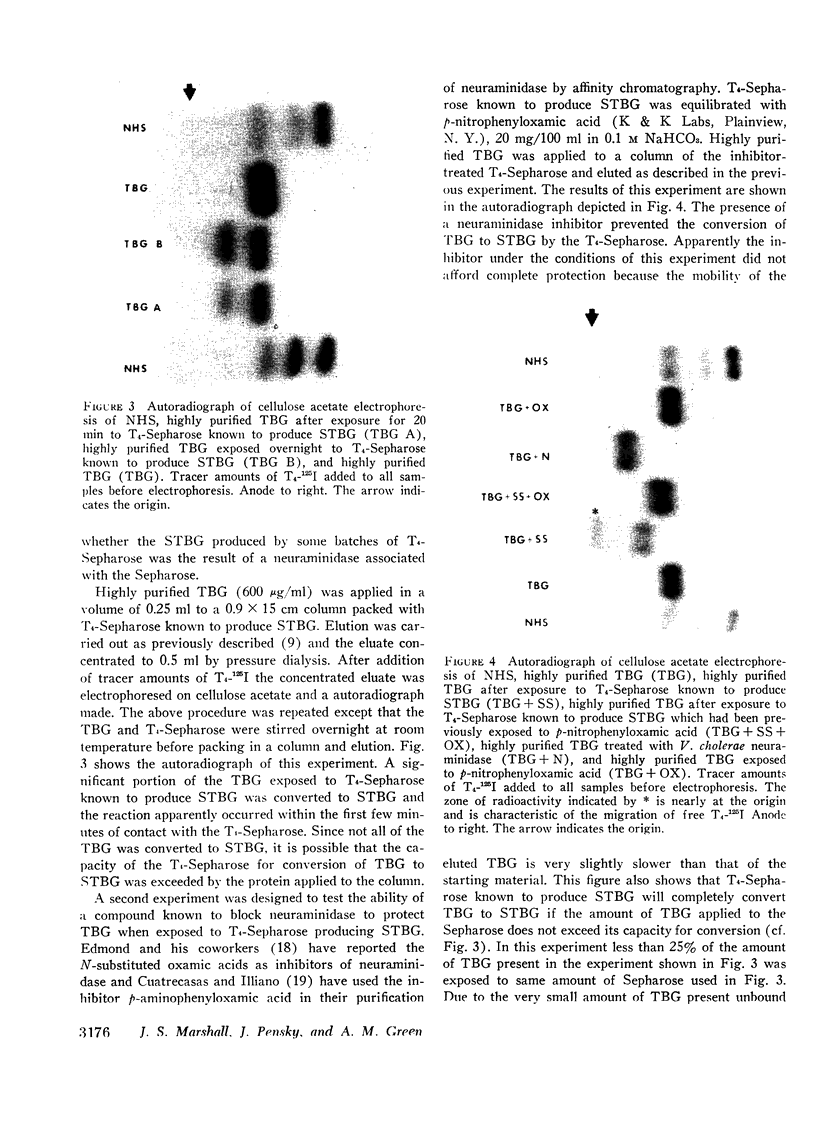
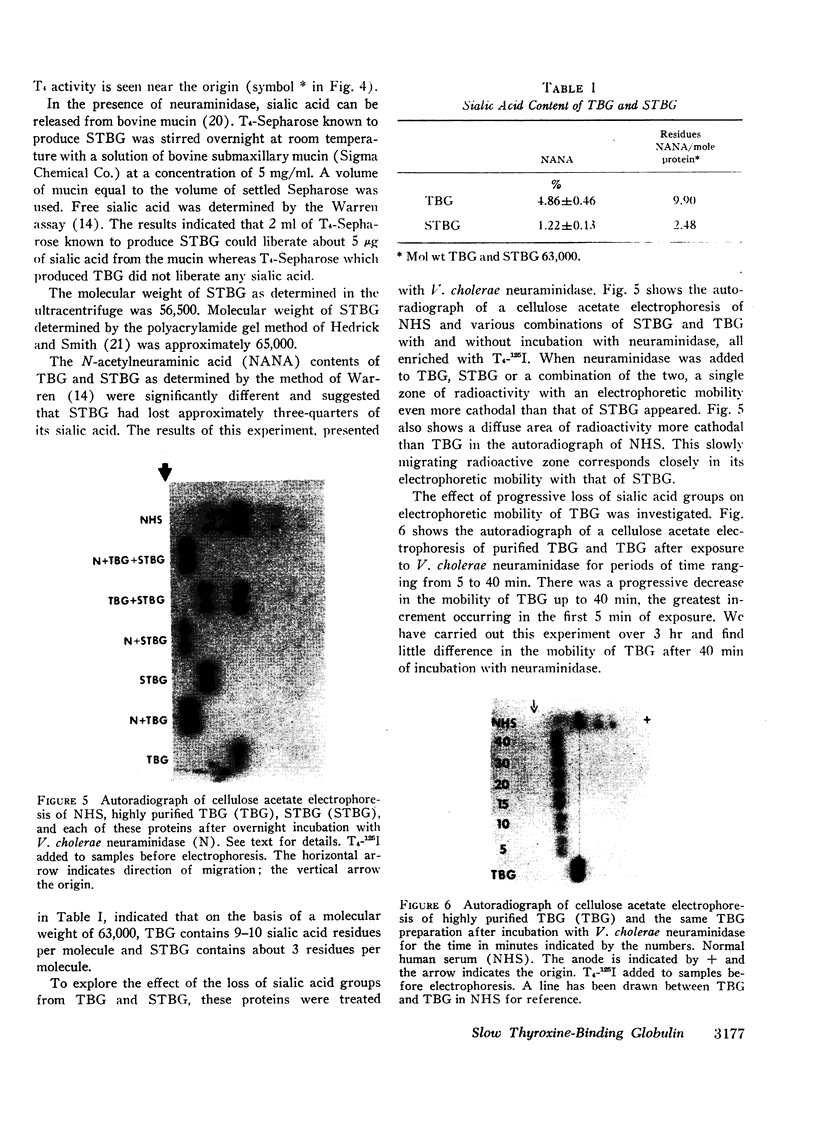
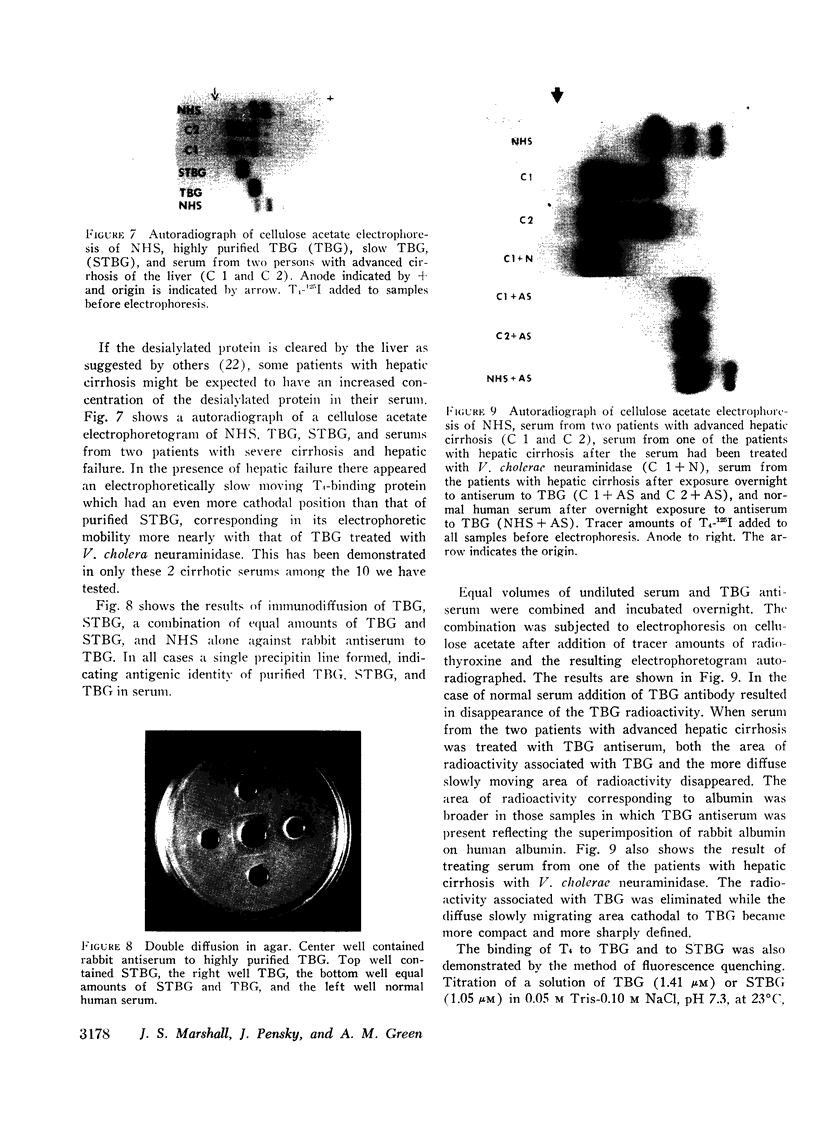
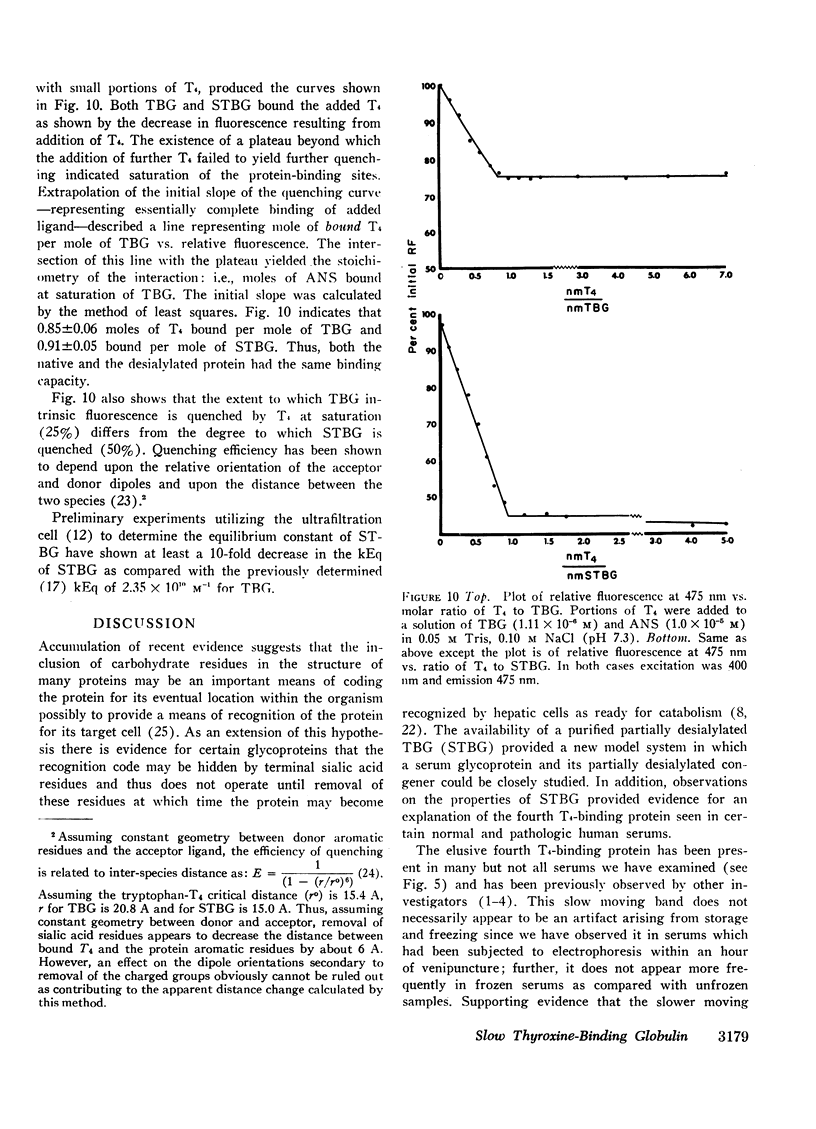
A model system utilizing a highly purified partially desialylated thyroxine-(T4) binding protein (STBG) was studied. STBG was prepared by the same affinity chromatographic method we have reported for preparation of highly purified T4-binding globulin (TBG). The necessary prerequisite for preparation of STBG was the use of T4-substituted Sepharose which had been repeatedly exposed to large volumes of serum for purification of TBG. STBG moved more slowly on cellulose acetate electrophoresis than TBG but had the same molecular weight and antigenic determinants as TBG. It bound T4 with a 1: 1 molar ratio but its affinity for T4 was about 10 times less than that of TB. STBG had about onefourth the sialic acid content of TBG and the electrophoretic mobility of this protein was similar to that of a T4-binding protein with a mobility slower than that of TBG which has been seen in the electrophoretic patterns of some normal human serums and in serums of patients with hepatic cirrhosis and which does not appear to be an artifact caused by storage and freezing of serum. This fourth slowly migrating T4-binding region in electrophoretograms of cirrhotic serums is completely abolished by prior incubation with rabbit antiserum to TBG. The in vitro production of partially desialylated TBG from T4-Sepharose which had been previously exposed to large volumes of serum may be due to adsorption of neuraminidases to the Sepharose either directly from serum or as the result of bacterial contamination. Partial desialylation of TBG in vivo may be an early step in the catabolism of this protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMBERG B. S., ROBBINS J. Thyroxine-serum protein complexes: single dimension gel and paper electrophoresis studies. Endocrinology. 1960 Sep;67:368–378. doi: 10.1210/endo-67-3-368. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Illiano G. Purification of neuraminidases from Vibrio Cholerae, Clostridium Perfringens and influenza virus by affinity chromatography. Biochem Biophys Res Commun. 1971 Jul 2;44(1):178–184. doi: 10.1016/s0006-291x(71)80175-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edmond J. D., Johnston R. G., Kidd D., Rylance H. J., Sommerville R. G. The inhibition of neuraminidase and antiviral action. Br J Pharmacol Chemother. 1966 Aug;27(2):415–426. doi: 10.1111/j.1476-5381.1966.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio N. A., Jr, Tabachnick M. Thyroxine-protein interactions. V. Isolation and characterization of a thyroxine-binding globulin from human plasma. J Biol Chem. 1968 May 10;243(9):2247–2259. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Levy R. P., Marshall J. S., Velayo N. L. Radioimmunoassay of human thyroxine-binding globulin (TBG). J Clin Endocrinol Metab. 1971 Mar;32(3):372–381. doi: 10.1210/jcem-32-3-372. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Pensky J. Studies on human thyroxine-binding globulin (TBG). I. Purification of TBG and immunologic studies on the relationship between TBG from normal persons and those with TBG "deficiency". J Clin Invest. 1969 Mar;48(3):508–515. doi: 10.1172/JCI106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Pensky J. Studies on thyroxine-binding globulin (TBG). 3. Some physical characteristics of TBG and its interaction with thyroxine. Arch Biochem Biophys. 1971 Sep;146(1):76–83. doi: 10.1016/s0003-9861(71)80043-7. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- NISIZAWA K., PIGMAN W. The composition and properties of the mucin clot from cattle submaxillary glands. Arch Oral Biol. 1959 Oct;1:161–170. doi: 10.1016/0003-9969(59)90008-1. [DOI] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Pensky J., Marshall J. S. Studies on thyroxine-binding globulin (TBG). II. Separation from human serum by affinity chromatography. Arch Biochem Biophys. 1969 Dec;135(1):304–310. doi: 10.1016/0003-9861(69)90544-x. [DOI] [PubMed] [Google Scholar]
- Perlman F. L., van Zyl A., Edelhoch H. The properties of thyroglobulin. XVI. Energy transfer to iodoamino acids. J Am Chem Soc. 1968 Apr 10;90(8):2168–2172. doi: 10.1021/ja01010a040. [DOI] [PubMed] [Google Scholar]
- Premachandra B. N., Perlstein I. B., Blumenthal H. T. Studies on obesity. II. Slow-moving thyroxine binding globulin in the sera of normal and obese subjects. J Clin Endocrinol Metab. 1970 Jun;30(6):752–762. doi: 10.1210/jcem-30-6-752. [DOI] [PubMed] [Google Scholar]
- SCHULTZE H. E. Influence of bound sialic acid on electrophoretic mobility of human serum proteins. Arch Biochem Biophys. 1962 Sep;Suppl 1:290–294. [PubMed] [Google Scholar]
- Thorson S. C., Tauxe W. N., Taswell H. F. Evidence for the existence of two thyroxine-binding globulin moieties: correlation between paper and starch-gel electrophoretic patterns utilizing thyroxine-binding globulin-deficient sera. J Clin Endocrinol Metab. 1966 Feb;26(2):181–188. doi: 10.1210/jcem-26-2-181. [DOI] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]