Abstract
Translational studies are becoming more common in schizophrenia research. The past couple of decades witnessed the emergence of novel ideas regarding schizophrenia pathophysiology that originated from both human and animal studies. The findings that glutamate and gamma-aminobutyric acid transmission are affected in the disease led to the hypothesis of altered inhibitory neurotransmission as critical for cognitive deficits and to an exploration of novel therapeutic approaches aimed at restoring excitation-inhibition balance. Much is to be done yet to elucidate the ultimate mechanisms by which excitation and inhibition are affected in this disorder; a comprehensive translational effort is necessary to address what may cause altered GABA function, for example. Here, we present an overview of the excitation-inhibition imbalance hypothesis in schizophrenia and discuss ongoing efforts aimed at determining whether cortical inhibitory interneurons are affected by oxidative stress during development.
Keywords: GABA, animal models, cognitive deficits, NMDA, adolescence, parvalbumin
Introduction
Schizophrenia is a common and devastating disorder for which our knowledge about its etiology and pathophysiology is limited. As a consequence, several aspects of the disease are not alleviated by current therapies that focus on dopamine receptor blockade. Although antipsychotics are effective against positive symptoms, they do not improve cognitive deficits, which are likely the result of altered cortical information processing. Determining the brain alterations that lead to cognitive disturbances will pave the way to novel therapeutic strategies, and this endeavor requires comprehensive translational approaches. Over the past couple of decades, human and animal work identified cortical glutamate and gamma-aminobutyric acid (GABA) neurotransmission as critically involved in cognitive deficits. As these circuits mature during adolescence and symptom onset does not typically occur until late adolescence, understanding the developmental trajectories of cortical circuits is essential to gain insight about mechanisms contributing to the disease and its treatment.
Excitation-Inhibition Balance
The balance between excitation and inhibition in cortical circuits is critical for network function and exhibits a protracted developmental trajectory. Although pyramidal neurons comprise more than 90% of cortical neurons and they project to target regions, local inhibitory interneurons shape pyramidal cell firing and their timing. The outcome of this interaction can be detected with oscillations in the electroencephalogram (EEG).1,2 Inhibitory interneurons in general are crucial for normal cognitive processes, and the subpopulation expressing parvalbumin (PV) is implicated in schizophrenia pathophysiology. Rodent studies have shown that PV fast-spiking interneurons (FSI) mature extensively during adolescence. For example, the modulation of FSI firing by dopamine exhibits a dramatic change at this late developmental stage, when dopamine D2 agonists switch from being mildly inhibitory to actually increasing firing in FSI.3 Furthermore, robust changes in cortical synchrony are evident in humans during adolescence. High-frequency gamma oscillations, for which FSI activity is essential,4 change throughout development and do not reach full maturity until adulthood.5 These data suggest that FSI control of cortical networks is substantially reorganized at this time. The ensuing refinement of excitation-inhibition balance renders adolescence as a critical period in normal development during which insults or preexisting conditions could be particularly deleterious. As full-blown schizophrenia emerges during this critical period, it is possible that symptom onset is driven by periadolescent developmental factors that alter the maturation of interneuron-dependent cortical dynamics.
Converging evidence from animal and human studies indicates that dysfunctional GABAergic inhibition and subsequent imbalance of excitation and inhibition are involved in cortical anomalies that produce cognitive deficits in animal models and in schizophrenia patients. One of the most replicated findings in postmortem analysis of schizophrenia brains is a decrease in GAD67,6,7 the main GABA-synthesizing enzyme in the brain. This reduction occurs most robustly in GABAergic interneurons that express PV. Furthermore, PV is also reduced in cortical regions from SZ patients, and this reduction was not due to loss of PV cells but to a reduction in PV labeling per neuron.8 An interneuron deficit is likely present in early stages of the disease, as interneuron-dependent EEG oscillations are altered in schizophrenia patients both in the chronic stage and at disease onset.9 Many animal models of schizophrenia show impaired interneuron function and/or loss of PV labeling in cortical regions, providing a strong agreement with observations in human subjects. These models include the neonatal ventral hippocampal lesion (NVHL),10 gestational administration of the antimitotic drug methylazoxymethanol acetate,11 neonatal N-Methyl-d-aspartate (NMDA) receptor antagonist administration,12,13 neonatal immune challenge in the hippocampus,14 and knocking down the DISC1 gene.15 The interneuron-dependent deficits observed in these models typically emerge during adolescence (see O'Donnell16 for review). These findings have lead to the commonly accepted idea that PV interneurons are dysfunctional in schizophrenia, but the causes and mechanisms of this dysfunction are still largely unknown. An emerging hypothesis is that PV interneuron dysfunction may be due in part to oxidative damage during development.16–18
Oxidative Stress
A role for oxidative stress in schizophrenia pathology has been suspected for some time. Several studies in schizophrenia patients have revealed abnormal antioxidant systems, which can create an imbalance between free radical generation and scavenging and ultimately lead to oxidative damage. These abnormalities include robust decreases in glutathione (GSH) levels in the brain and cerebrospinal fluid (CSF) of schizophrenia patients19 as well as in postmortem brains.20 GSH is a major intracellular antioxidant and redox regulator that scavenges reactive oxygen species, protecting cells from oxidative stress. Impairing GSH synthesis by knocking-out glutamate cysteine ligase in mice leads to oxidative stress and a selective decrease of PV immunoreactivity in the ventral hippocampus as well as reductions in PV interneuron-dependent high-frequency oscillations.21 The reduction in cortical PV labeling in animal models is not likely due to cell death but to loss of the ability to express PV in individual interneurons.22 These observations suggest that oxidative damage due to redox imbalance may cause interneuron deficits and schizophrenia-related phenotypes. An ongoing study conducted by our group in collaboration with Kim Do has found an increase in a marker of oxidized DNA (8-oxo-dG) in juvenile NVHL rats accompanied by a subsequent reduction in PV cell counts in the anterior cingulate cortex and deficits in prepulse inhibition of the acoustic startle response in adulthood.23 The fact that the elevations in 8-oxo-dG precede the loss of PV immunolabeling suggests that PV interneurons may have exhibited oxidative stress during development, and this may have led to the loss of PV and behavioral and electrophysiological abnormalities in adulthood. Furthermore, PV interneurons exhibit increased markers of oxidative stress and superoxide formation following NMDA receptor blockade with noncompeting antagonists, a widely used pharmacological model of schizophrenia.24 Thus, alterations in the antioxidant system that yield oxidative stress are associated with schizophrenia in humans and with interneuron deficits in animal models of the disorder.
If oxidative stress in interneurons is causal to schizophrenia-related phenomena in animals and some aspects of the disease in humans, treatments that reduce oxidative stress should be beneficial in patients and in animal models. Indeed, the efficacy of antioxidant treatment in schizophrenia has been addressed in several studies. Adjunct treatment with N-acetylcysteine (NAC), an antioxidant that works as a cysteine donor in the synthesis of GSH, improved negative symptoms25 and corrected mismatch negativity (MMN) deficits in schizophrenia patients.26 MMN is an auditory evoked potential (AEP) elicited when a sequence of repetitive standard sounds is interrupted infrequently by a deviant oddball stimuli and is consistently shown to be impaired in schizophrenia patients.27 This AEP is thought to reflect an automatic preattentive comparison process that is primarily generated within the auditory and prefrontal cortices28; thus, MMN is a way of assaying the functionality of cortical areas in a basic sensory task. The data from antioxidant studies suggest that adding NAC to an existing treatment regimen improves symptoms as evidenced by Positive and Negative Syndrome Scale rating improvements and rescues an AEP deficit. Despite these promising findings, other studies have not been conclusive. It is conceivable that the developmental trajectory of the deficits makes antioxidant treatment late in the disease less likely to be successful. Preliminary data from our lab indicate that early NAC treatment (during presymptomatic stages) in NVHL rats reverses the loss of PV labeling and restores deficits in prepulse inhibition of the acoustic startle response (P. O'Donnell, J. H. Cabungcal, K. Q. Do, unpublished data). This observation suggests that antioxidant treatment at early stages of the disease and even prior to symptom onset can prevent cellular and behavioral deficits characteristic of this model of schizophrenia. Furthermore, the decrease in PV staining and increased superoxide formation induced by NMDA antagonists could be prevented by pretreatment with apocynin, an NADPH-oxidase inhibitor that reduces the generation of reactive free radicals.24 Thus, ongoing work from rodent models suggest that preventing or rescuing oxidative damage can improve molecular, physiological, and behavioral phenotypes associated with schizophrenia pathology, but it is important to act early.
As oxidative stress may have a causal role in disease pathology, the most beneficial window for intervention could be prior the full manifestation of the disease. A handful of studies examining preventive treatments in prodromal or high-risk populations have yielded encouraging results. Administration of omega-3 fatty acids for 12 weeks reduced the risk of progression to psychotic disorder and led to symptomatic improvements during the year following treatment.29 Omega-3 fatty acids, in addition to potential effects on membrane fluidity, have been shown to increase GSH in temporal lobes of first-episode schizophrenia patients30; thus, protecting neurons from oxidative stress may be critical for the beneficial effects of omega-3 fatty acids in this population. These results are indeed promising. A crucial next step is to further examine antioxidant combinations in high-risk populations along with markers of biological processes that may be affected by oxidative stress. As the redox system is quite complex and has many hubs for intervention, more animal and human studies must be done to determine the most effective drug or combination of drugs to correct redox imbalances that could contribute to schizophrenia pathophysiology.
Biomarkers
It is possible that the presence or extent of redox imbalances in patients is heterogeneous and that there are patients who would benefit more from antioxidant treatment than others. It is imperative that biomarkers of oxidative stress can be identified along with their correlation with indicators of altered cortical function. For example, it would be advantageous if GSH levels in blood or CSF correlated with central nervous system GSH or with specific oscillatory abnormalities (ie, MMN deficits). Electrophysiological and imaging approaches could then be used to screen patients and determine who would be likely to benefit the most from a given therapeutic approach.
Conclusions
The protracted development of cortical inhibitory interneurons provides several windows of vulnerability during which risk factors can combine yielding an abnormal adult circuit that can be responsible for psychiatric symptoms. Oxidative stress may be a common final mechanism by which different developmental disturbances may affect cortical PV interneurons and lead to the development of schizophrenia (figure 1). In order to restore cognitive function in this disorder, we may need tools that restore inhibitory interneuron function in the cortex, and the search for such agents has yielded mixed results. It may be more important, however, to act before critical damage is present in cortical circuits. If oxidative stress during adolescence is important in yielding interneuron deficits, redox manipulations prior to symptom onset are essential. In this regard, the identification of at-risk populations that exhibit clear indications of interneuron dysfunction is important for this approach. The search for biomarkers of oxidative stress and interneuron dysfunction (either with blood samples, imaging, or electrophysiology) will advance the field considerably and eventually can be used to screen subjects that can receive antioxidant treatment.
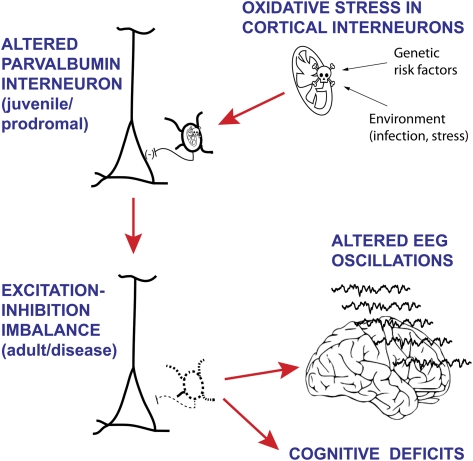
Fig. 1.
Since schizophrenia has a specific developmental trajectory, with onset in late adolescence or early adulthood, it is reasonable to hypothesize that a combination of genetic and environmental factors that lead to elevated oxidative stress in neurons could produce oxidative damage and subsequent interneuron dysfunction in the young adult cortex. The cartoon represents some of the proposed stages in this process. First, oxidative stress may be present in cortical interneurons during development as a consequence of a combination of predisposing gene variations and environmental factors that may include immune activation and stress. Pervasive oxidative stress in fast-spiking interneurons may impair their function, but as this cell population is not fully mature until late adolescence, the outcome may only be a mild set of cognitive deficits not too different from what can be frequently observed in healthy populations. It is during the protracted developmental trajectory of these interneurons that their altered state may become evident in full-fledge behavioral anomalies. As interneurons can become strongly activated in the transition to adolescence to adulthood, sick interneurons may yield a cortical circuit with altered excitation-inhibition balance. Such state can be evidenced in abnormal electroencephalogram oscillations and may yield schizophrenia symptoms, primarily in the cognitive domain.
Funding
National Institutes of Health (MH57683); NARSAD Distinguished Investigator Award (to P.O’D.)
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Cardin JA, Carlen M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traub RD, Pais I, Bibbig A, et al. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 7.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 11.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- 13.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feleder C, Tseng KY, Calhoon GG, O'Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa M, Kamiya A, Murai R, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Do KQ, Trabesinger AH, Kirsten-Kruger M, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steullet P, Cabungcal JH, Kulak A, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell P, Cabungcal JH, Piantadosi PT, Lewis E, Calhoon GG, Do KQ. Oxidative stress during development in prefrontal cortical interneurons in developmental animal models of schizophrenia. Schizophr Bull. 2011;37:111. [Google Scholar]
- 24.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 25.Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 27.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naatanen R, Paavilainen P, Reinikainen K. Do event-related potentials to infrequent decrements in duration of auditory stimuli demonstrate a memory trace in man? Neurosci Lett. 1989;107:347–352. doi: 10.1016/0304-3940(89)90844-6. [DOI] [PubMed] [Google Scholar]
- 29.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 30.Berger GE, Wood SJ, Wellard RM, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]