Abstract
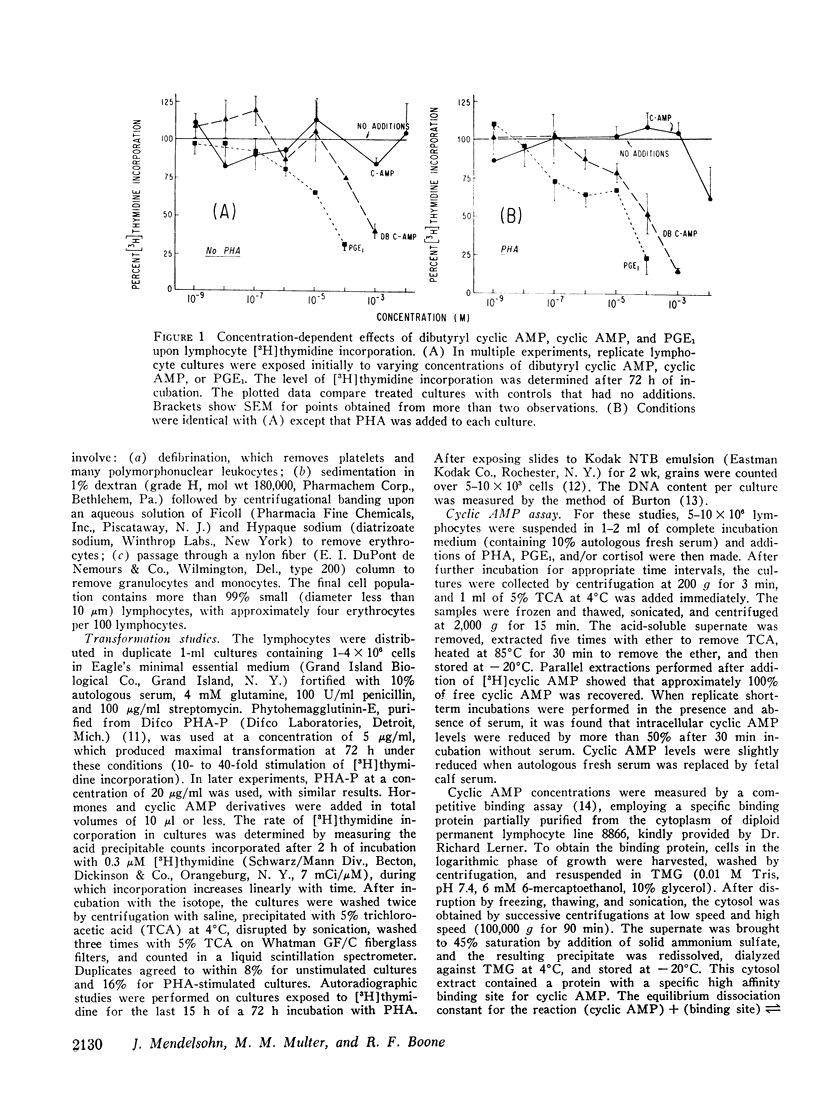
The combined effects of cortisol and agents acting through a cyclic AMP-mediated mechanism have been studied in cultures of highly purified human peripheral lymphocytes. Incubation with prostaglandin E1 (PGE1), dibutyryl cyclic AMP, or cortisol results in a concentration-dependent inhibition of [3H]-thymidine incorporation by both unstimulated and phytohemagglutinin (PHA)-stimulated lymphocytes, and PHA-induced morphologic transformation is prevented. When cortisol and PGE1 (or dibutyryl cyclic AMP) are added together to lymphocyte cultures, enhanced inhibitory effects are observed.
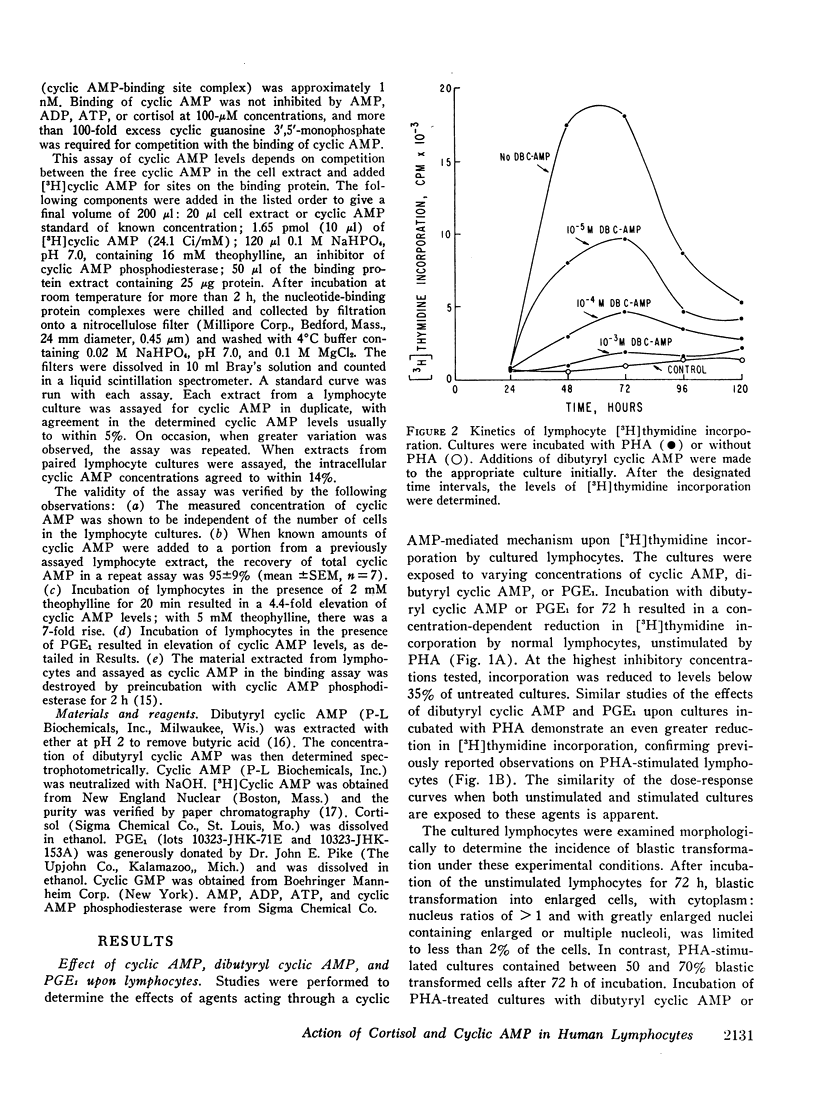
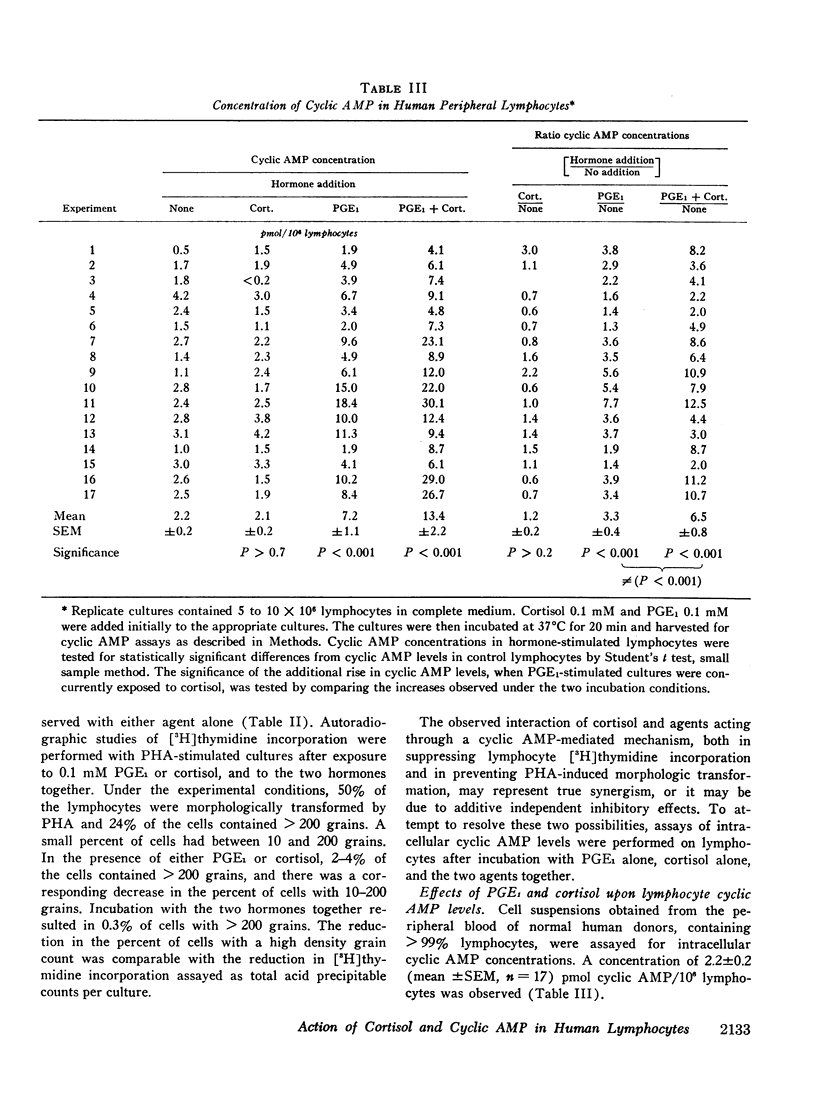
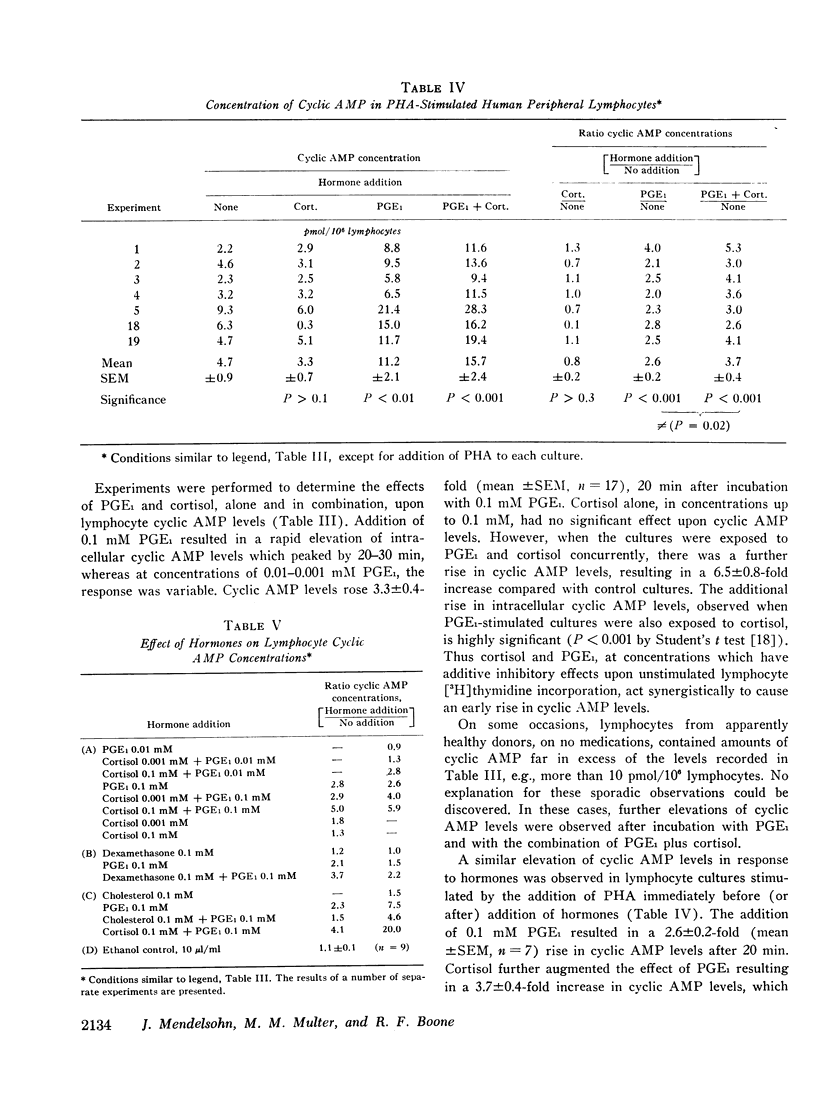
Incubation of unstimulated or PHA-stimulated lymphocytes with PGE1 results in an elevation of intracellular cyclic AMP levels within 20 min. The concentration of cyclic AMP gradually returns to base-line levels over a 1-6 h period of time. Cortisol alone does not significantly alter cyclic AMP concentrations. However, incubation with PGE1 in the presence of cortisol results in a greater stimulation of intracellular cyclic AMP levels than that observed with PGE1 alone. These findings suggest that cortisol may act synergistically with PGE1 to elevate lymphocyte cyclic AMP levels and to regulate [3H]thymidine incorporation and transformation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cross M. E., Ord M. G. Changes in histone phosphorylation and associated early metabolic events in pig lymphocyte cultures transformed by phytohaemagglutinin or 6-N,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Aug;124(1):241–248. doi: 10.1042/bj1240241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELVES M. W., GOUGH J., ISRAUELS M. C. THE PLACE OF THE LYMPHOCYTE IN THE RETICULO-ENDOTHELIAL SYSTEM: A STUDY OF THE IN VITRO EFFECT OF PREDNISOLONE ON LYMPHOCYTES. Acta Haematol. 1964 Aug;32:100–107. doi: 10.1159/000209561. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Weissmann G. Effect of cyclic 3',5'-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970 Apr;133(4):1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKINNEY A. A., Jr, STOHLMAN F., Jr, BRECHER G. The kinetics of cell proliferation in cultures of human peripheral blood. Blood. 1962 Mar;19:349–358. [PubMed] [Google Scholar]
- MICHALOWSKI A. TIME-COURSE OF DNA SYNTHESIS IN HUMAN LEUKOCYTE CULTURES. Exp Cell Res. 1963 Dec;32:609–612. doi: 10.1016/0014-4827(63)90202-7. [DOI] [PubMed] [Google Scholar]
- Manganiello V., Vaughan M. An effect of dexamethasone on adenosine 3',5'-monophosphate content and adenosine 3',5'-monophosphate phosphodiesterase activity of cultured hepatoma cells. J Clin Invest. 1972 Oct;51(10):2763–2767. doi: 10.1172/JCI107096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C. Inhibition of human leukocyte mitosis by prednisolone in vitro. Cancer Res. 1961 Dec;21:1518–1521. [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Effect of phytohemagglutinin and prostaglandins on cyclic AMP synthesis in rat lymph node lymphocytes. Biochim Biophys Acta. 1970 Aug 14;215(2):291–296. doi: 10.1016/0304-4165(70)90027-9. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. II. Independent nucleotide pools for nucleic acid synthesis. J Cell Physiol. 1971 Apr;77(2):241–248. doi: 10.1002/jcp.1040770213. [DOI] [PubMed] [Google Scholar]
- Polgar P. R., Kibrick S. Origin of small lymphocytes following blastogenesis induced by short-term PHA stimulation. Nature. 1970 Feb 28;225(5235):857–858. doi: 10.1038/225857a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Deisseroth A., Morganroth J., Carpenter C. B., Merrill J. P. Alteration of the cytotoxic action of sensitized lymphocytes by cholinergic agents and activators of adenylate cyclase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2995–2999. doi: 10.1073/pnas.69.10.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey D. C., Fudenberg H. H., Kamin R. M. Effect of prednisolone on synthesis of DNA and RNA by human lymphocytes in vitro. Nature. 1967 Jan 21;213(5073):281–282. doi: 10.1038/213281a0. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Garren L. D. An assay for adenosine 3',5'-cyclic monophosphate based on the association of the nucleotide with a partially purified binding protein. Biochemistry. 1970 Oct 13;9(21):4223–4229. doi: 10.1021/bi00823a026. [DOI] [PubMed] [Google Scholar]
- Weber T., Nordman C. T., Gräsbeck R. Separation of lymphocyte-stimulating and agglutinating activities in phytohaemagglutinin (PHA) from Phaseolus vulgaris. Scand J Haematol. 1967;4(1):77–80. doi: 10.1111/j.1600-0609.1967.tb01601.x. [DOI] [PubMed] [Google Scholar]


