Abstract
Objective
To estimate the prevalence, types and sociodemographic and biobehavioral correlates of antinuclear antibodies (ANA) in the United States (U.S.).
Methods
Cross-sectional analysis of 4,754 individuals from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. ANA by indirect immunofluorescence, including cellular staining patterns and specific autoantibody reactivities by immunoprecipitation in those with ANA.
Results
ANA prevalence in the U.S. population ages 12 years and older was 13.8% (95% CI, 12.2% to 15.5%). ANA increased with age (P = 0.01) and were more prevalent among females than males (17.8% vs. 9.6%, P < 0.001), with the female to male ratio peaking at 40–49 years of age. ANA prevalence was modestly higher in African Americans than whites (adjusted prevalence odds ratio [POR], 1.30; 95% CI, 1.00 to 1.70). Remarkably, ANA were less common in overweight and obese (adjusted POR, 0.74; 95% CI, 0.59 to 0.94) individuals than persons of normal weight. No significant associations were seen with education, family income, alcohol use, smoking history, serum levels of cotinine or C-reactive protein. In ANA-positive individuals, nuclear patterns were seen in 84.6%, cytoplasmic patterns in 21.8%, and nucleolar patterns in 6.1%, and the most common specific autoantibodies were anti-Ro (3.9%) and anti-Su (2.4%).
Conclusion
These findings suggest that over 32 million persons in the U.S. have ANA and the prevalence is higher among females, older individuals, African Americans and those with normal weight. These data will serve as a useful baseline for future investigations of predictors and changes in ANA prevalence over time.
Keywords: Antinuclear antibodies, epidemiology, NHANES
Autoantibodies to cellular constituents are the serologic hallmarks of autoimmunity and are frequently seen in systemic autoimmune diseases, including systemic lupus erythematosus (SLE), scleroderma, and polymyositis/dermatomyositis (1). They also are detected in patients with organ-specific autoimmune diseases, such as autoimmune thyroiditis and hepatitis (1), certain infections and neoplasms (2), and in some individuals without diagnosed disease (2, 3). The most common autoantibodies are antinuclear antibodies (ANA), which are traditionally assessed by indirect immunofluorescence and include antibodies to both nuclear and cytoplasmic components (4). The cellular staining patterns and specific autoantibodies detected in those with ANA are clinically useful as they are strongly associated with particular autoimmune diseases, such as the nucleolar staining patterns that are often seen in scleroderma and anti-Sm autoantibodies that are included in SLE classification criteria (1).
A variety of methods have been used to estimate ANA prevalence in selected populations, including blood donors (5, 6), hospital workers (6, 7), healthy volunteers (3, 8), or residents in small areas (9, 10), leading to a wide range of prevalence estimates (from 1.1% to 20%), which are difficult to compare. Factors associated with ANA production are largely unknown with the exception of some reports suggesting higher prevalence of ANA in females (8, 10–13) and older individuals (12, 14–16). A proportion of the ANA-positive population is thought to represent the preclinical stage of autoimmune diseases based on observations that autoantibodies are usually produced prior to clinical manifestations of disease (17). Thus, defining the prevalence and types of ANA, as well as characterizing factors associated with their production, may provide insight into the etiology of autoimmune diseases, which are thought to be increasing in frequency but are more difficult to characterize and study than ANA at the population level (18).
Therefore, we evaluated serum samples from the United States (U.S.) National Health and Nutrition Examination Survey (NHANES) from 1999–2004 to estimate ANA prevalence, cellular patterns and specific autoantibody reactivities, and to identify sociodemographic and biobehavioral factors associated with their production. We specifically assessed selected systemic autoimmune disease risk factors including smoking (19) and alcohol use (20). We also evaluated C-reactive protein (21) and obesity, the former being a marker and the latter an underlying cause of chronic inflammation (22) and a growing public health concern.
MATERIALS AND METHODS
Study design
NHANES is a continuous survey of the health and nutritional status of the US civilian, noninstitutionalized population, conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC). During the period under study, 5,000 persons of all ages were surveyed each year using a complex, multistage sampling strategy. Data are released in two-year cycles and our analyses combine data from the 1999–2000, 2001–2002, and 2003–2004 periods. The overall participation rates for the 1999–2000, 2001–2002, and 2003–2004 cycles were 76%, 80% and 76% respectively (http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm). Sampling rates were adjusted to reduce potential bias resulting from differences among respondents and nonrespondents.
The NHANES protocol was approved by a human subjects review board and written informed consent was obtained from all participants.
For each cycle encompassing the 1999–2004 NHANES, a representative sample of participants ages 12 years and older was selected by NHANES staff for a sub-study assessing serum levels of organochlorines (N = 7,106), a focus of our future investigations. Of these, 4,754 participants gave permission for sera storage and had samples available for analysis. There were no appreciable differences in demographic profiles in the larger sub-study and our study sample (data not shown).
Laboratory testing
Serum samples were evaluated by standard immunofluorescence ANA testing using commercial HEp-2 ANA slides (INOVA Diagnostics, San Diego, CA) with 1:80 dilutions of sera, followed by staining with DyLight 488-conjugated Donkey anti-human IgG (γ-chain specific) antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) (23). Antinuclear, anti-nucleolar and anti-cytoplasmic ANA patterns were identified by a standard classification method (2, 24) and the intensities of immunofluorescence staining were graded using a 0–4 scale based on comparisons with a standard reference gallery (see http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/SSANA_A.htm). ANA intensities of 3 and 4 were defined as positive based on findings from commercial ANA reference laboratories after the concurrent evaluation of CDC reference sera and 200 unknowns in our sample (25).
Results for all ANA assays were collected and stored as digital images. All readings were confirmed independently by at least two experienced evaluators. In less than 5% of the cases, the two independent evaluators disagreed on the ANA reading. In these cases, the evaluators would discuss the discrepant classifications and come to a consensus after re-reviewing the data. When consensus was not reached between the initial two reviewers, a third reviewer adjudicated. If the quality of the ANA staining was questionable, a repeat of the staining and reading would be performed.
Immunofluorescent ANA positive sera were tested by immunoprecipitation of 35S-methionine-labeled K562 cell extracts for determination of specific autoantibodies (26). Repeat testing of randomly-selected samples showed >98% concordance in immunofluorescence intensity, identification of ANA patterns and immunoprecipitation results.
Assessment of sociodemographic and biobehavioral measures
Sociodemographic data (race/ethnicity, sex, age, education of the head of the household, and household income that was used to calculate the family income to poverty level ratio), smoking history and alcohol consumption were based on self report (National Center for Health Statistics, National Health and Nutrition Examination Survey Questionnaires, datasets, and related documentation, Atlanta, Georgia: CDC; 2009; http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm).
Height and weight were measured and body mass index (BMI) calculated as weight (kilograms) divided by height (meters squared). For adults BMI was categorized using standard cut points (underweight < 18.5, normal weight 18.5 to < 25, overweight 25 to < 30, and obese 30+). For adolescents (age 12 to 19 years) American Medical Association recommended guidelines (27) and corresponding sex-specific BMI percentiles for age growth were used.
Tobacco smoke exposure was assessed by self report and by using serum cotinine measurements as previously described (28). Cotinine was categorized as not detectable (< the detection limit), second-hand smoke exposure (≥ the detection limit to 15 ng/mL), and active smoking (> 15 ng/mL). C-reactive protein was quantified by latex-enhanced nephelometry and standard cutpoints were used to categorize low (<1 mg/L), moderate (1 to 3 mg/L), high (>3 to 10 mg/L), and very high (>10 mg/L) levels (29).
Statistical analysis
To account for the complex sampling design used in NHANES and to assure unbiased variance estimates, we employed the appropriate SAS SURVEY procedures (version 9.2, SAS Institute Inc., Cary, NC) to estimate the prevalence of ANA positivity, 95% confidence intervals (CI), and corresponding P values and adjusted prevalence odds ratios (PORs). For specific ANA patterns and autoantibodies, we report prevalences for the subgroup of ANA positive participants, though based on variance estimates across the entire sample. Trend P values were calculated with SUDAAN version 10.0.1 (Research Triangle Institute, Research Triangle Park, NC) and figures were generated with R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria).
To account for sampling differences between the sub-study assessing organochlorines and our study sample, we adjusted the six-year weights (adjusting the NHANES 1999–2002 four-year weights by a factor of two thirds and the NHANES 2003–2004 two-year weights by a factor of one third (http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf) according to observed proportions of age, sex, and race/ethnicity. Statistical significance was based on P values <0.05.
RESULTS
Prevalence of ANA and associations with sociodemographic factors
The overall prevalence of ANA in the population was 13.8% (95% CI, 12.2% to 15.5%). ANA generally increased with age (P = 0.01) and were significantly higher in women than men (17.8% versus 9.6%, P < 0.001) (Table 1). Based on these findings, we estimate that 32.3 million people (95% CI, 28.5 million to 36.1 million) — 21.5 million females (95% CI, 18.7 million to 24.3 million) and 10.8 million males (95% CI, 8.6 million to 13.1 million) — had ANA in the U.S. during the period 1999–2004. ANA prevalence in the 50 to 59 year and 70+ year age groups was significantly higher than in younger age groups (P < 0.03). ANA prevalence was modestly higher among non-Hispanic blacks than other race/ethnic groups. ANA prevalence did not vary by education or family income to poverty level ratio. After adjustment for age, females had a two-fold increased odds of ANA (POR, 2.02; 95% CI, 1.57 to 2.60). In additional analyses, including further adjustments for race, sex, alcohol intake, smoking, BMI, and C-reactive protein, PORs for all variables analyzed were virtually unchanged (data not shown).
Table 1.
Estimated U.S. Prevalence of Antinuclear Antibodies (ANA) and Estimated Prevalence Odds Ratios for ANA Associations with Selected Sociodemographic Variables
| Characteristic | No.* | No.* ANA+ |
% ANA + (95% CI) |
Age-Adjusted POR (95% CI) |
|---|---|---|---|---|
| Total | 4754 | 670 | 13.8 (12.2–15.5) | |
| Age, years | ||||
| 12–19 | 1190 | 146 | 11.2 (7.8–14.6) | 1.00 (reference) |
| 20–29 | 686 | 90 | 13.1 (9.6–16.7) | 1.20 (0.74–1.93) |
| 30–39 | 642 | 93 | 13.4 (9.5–17.3) | 1.23 (0.75–2.02) |
| 40–49 | 581 | 66 | 11.5 (8.5–14.4) | 1.03 (0.72–1.46) |
| 50–59 | 478 | 87 | 17.4 (13.2–21.7) | 1.68 (1.13–2.48) |
| 60–69 | 525 | 68 | 13.8 (8.7–18.9) | 1.27 (0.77–2.08) |
| 70+ | 652 | 120 | 19.2 (15.0–23.4) | 1.88 (1.17–3.02) |
| P Value† | 0.01 | |||
| Sex | ||||
| Male | 2285 | 244 | 9.6 (7.6–11.6) | 1.00 (reference) |
| Female | 2469 | 426 | 17.8 (15.5–20.1) | 2.02 (1.57–2.60) |
| P Value† | <0.001 | |||
| Race/ethnicity | ||||
| Non-Hispanic white | 2118 | 293 | 13.7 (11.7–15.7) | 1.00 (reference) |
| Non-Hispanic black | 994 | 155 | 16.5 (13.5–19.4) | 1.30 (1.00–1.70) |
| Mexican American | 1246 | 168 | 12.8 (10.3–15.3) | 1.00 (0.78–1.29) |
| Other | 396 | 54 | 12.8 (8.5–17.2) | 0.96 (0.65–1.42) |
| P Value† | 0.35 | |||
| Educational attainment, years | ||||
| 0–8 | 697 | 106 | 13.6 (9.6–17.6) | 1.00 (reference) |
| 9–11 | 848 | 104 | 13.2 (10.5–15.9) | 1.01 (0.65–1.56) |
| HS diploma/GED | 1068 | 141 | 13.1 (10.4–15.7) | 1.02 (0.74–1.41) |
| Some college | 1152 | 171 | 14.7 (12.0–17.4) | 1.19 (0.81–1.74) |
| College or post graduate | 815 | 112 | 13.0 (10.1–16.0) | 1.01 (0.65–1.57) |
| P Value† | 0.89 | |||
| Family income to poverty level ratio | ||||
| At or above poverty ≥1 | 3370 | 477 | 13.7 (11.9–15.4) | 1.00 (reference) |
| Below poverty <1 | 982 | 125 | 13.9 (10.7–17.2) | 1.08 (0.84–1.39) |
| P Value† | 0.86 | |||
ANA = antinuclear antibodies; + = positive; POR = prevalence odds ratio; CI = confidence interval; HS = High School; GED = General Educational Development.
No. reflects the number of subjects within the sample, not an estimated count for the U.S. population.
For sex, race/ethnicity, and family income to poverty level ratio, the P Value for the Wald χ2 test investigates the general association among ANA+ and levels of the population characteristics. For age and educational attainment, the P Value for the trend test investigates the linear association among ANA+ and levels of the population characteristics.
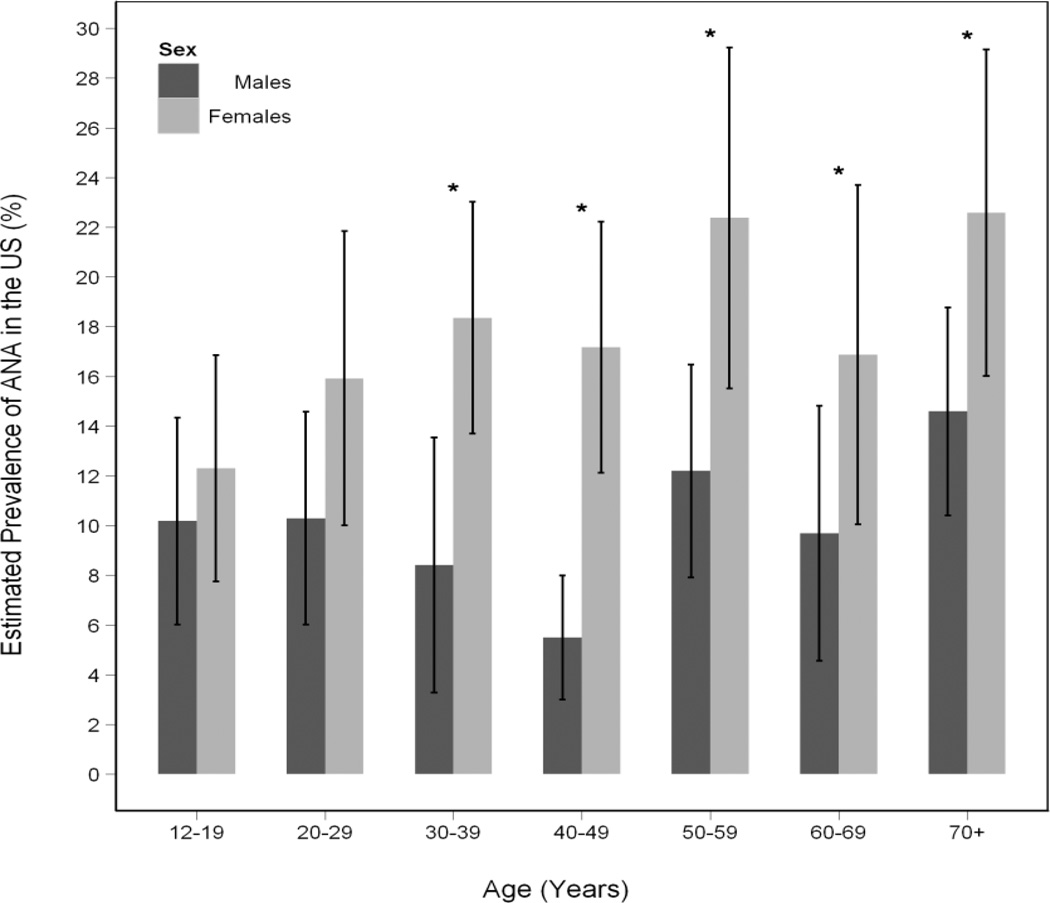
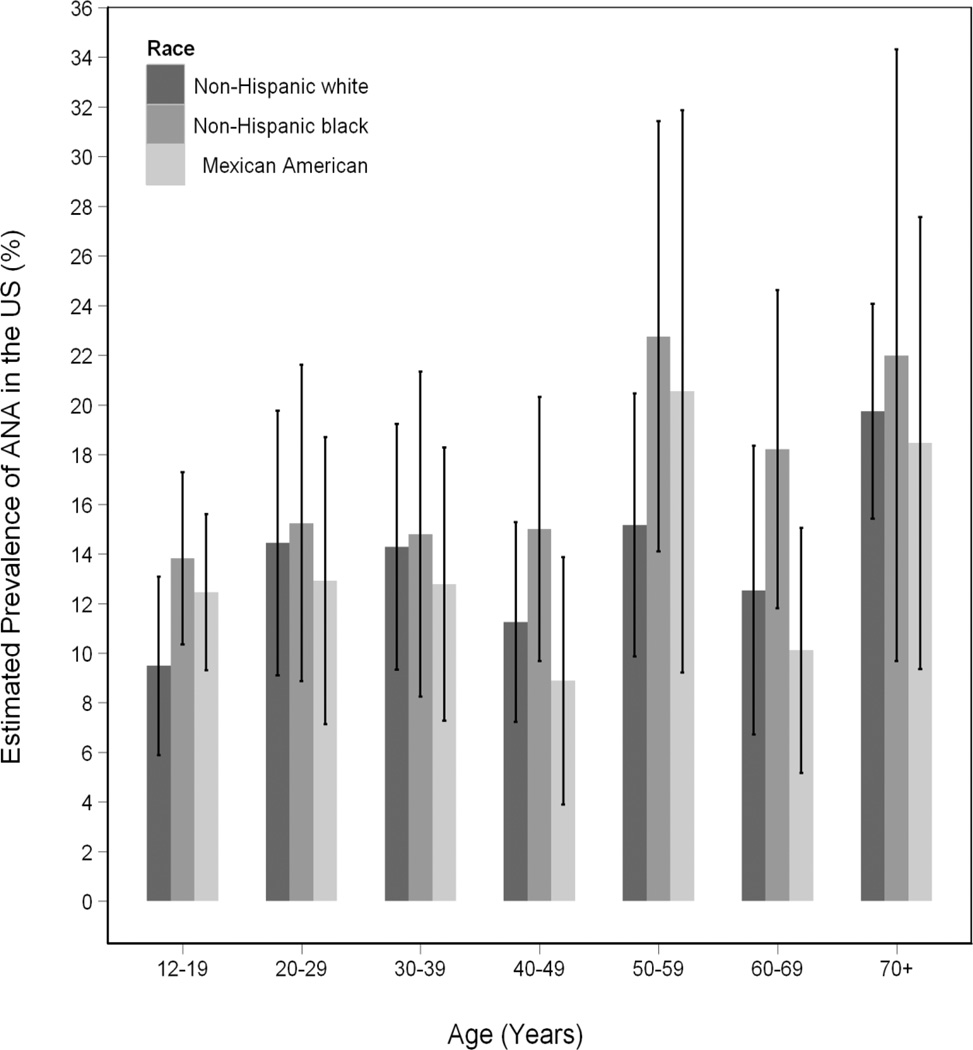
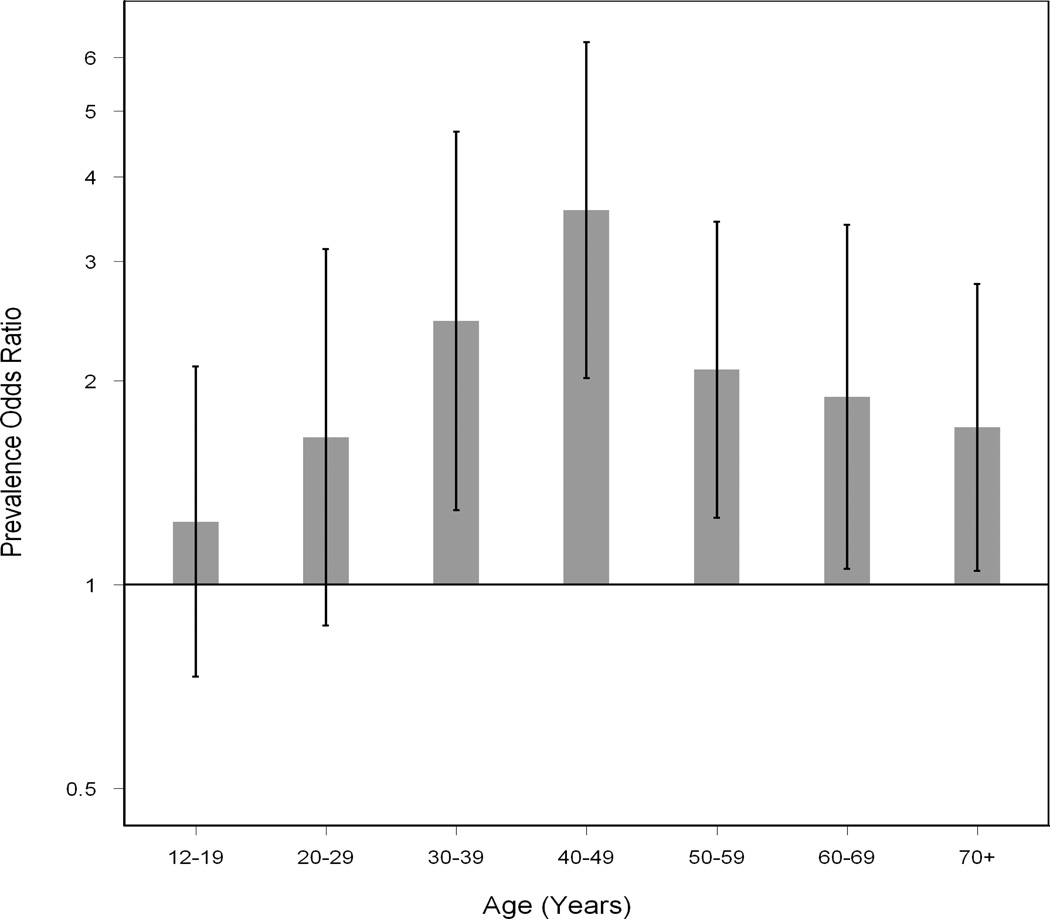
While there was an overall increase in prevalence of ANA with age, the pattern was not linear (Figure 1). When we explored different age groupings, and evaluated males and females and different ethnic groups separately (Figure 2), similar patterns were seen. The magnitude of the female to male PORs varied considerably across age groups (Figure 3). Female versus male differences were minimal under age 30 but rose at 30 to 39 years of age (POR, 2.45; 95% CI, 1.29 to 4.66), peaking at ages 40 to 49 years (POR, 3.57; 95% CI, 2.02 to 6.32) and then declined in older age groups.
Figure 1.
Estimated Prevalence (with 95% confidence intervals) of antinuclear antibodies (ANA) across age groups by sex
An asterisk (*) denotes significant differences in estimated ANA prevalences between females and males (P Value<0.05).
Figure 2.
Estimated prevalence of antinuclear antibodies (ANA), with 95% confidence intervals, across age groups by race/ethnicity*
* There were no significant differences among racial/ethnic groups in estimated ANA prevalence in the individual decades. The "Other" race/ethnic group is not displayed though it was included in significance testing.
Figure 3.
Estimated prevalence odds ratios for female compared to male ANA prevalence (with 95% confidence intervals) by age groups
Prevalence of ANA and associations with selected biobehavioral measures
ANA were less common in overweight (POR, 0.74; 95% CI 0.56 to 0.97) and obese (POR, 0.74; 95% CI, 0.59 to 0.94) individuals than in normal weight individuals (P for trend = 0.02) and these differences persisted after age-adjustment (Table 2). When stratified by gender, POR were slightly lower and no longer significant, with the exception of the comparison of obese to healthy weight women (age-adjusted POR 0.73; 95% CI, 0.55 to 0.97). To assess if educational attainment, which is strongly associated with BMI, could be influencing these results, we further adjusted for education in the BMI age-adjusted model. The resulting PORs were virtually identical, suggesting that educational attainment did not explain the BMI ANA findings.
Table 2.
Estimated U.S. Prevalence of ANA and Estimated Prevalence Odds Ratios for ANA Associations with BMI, Smoking Status, Alcohol Intake, and C-reactive Protein
| Characteristic | No.* | No.* ANA+ |
% ANA + (95% CI) |
Age-Adjusted POR (95% CI) |
|---|---|---|---|---|
| Total | 4754 | 670 | 13.8 (12.2–15.5) | |
| BMI‡,§,‖ | ||||
| Healthy | 1757 | 264 | 15.5 (12.8–18.2) | 1.00 (reference) |
| Underweight | 79 | 17 | 16.5 (6.6–26.5) | 1.11 (0.52–2.35) |
| Overweight | 1430 | 180 | 12.8 (10.3–15.3) | 0.74 (0.56–0.97) |
| Obese | 1370 | 188 | 12.6 (10.5–14.7) | 0.74 (0.59–0.94) |
| P Value† | 0.02 | |||
| Smoking status (cotinine), ng/mL | ||||
| No Detectable Smoke exposure, <0.015, 0.05¶ |
1246 | 191 | 14.5 (11.9–17.2) | 1.00 (reference) |
| Second-hand Smoke exposure, ≥ 0.05 –15¶ |
2439 | 349 | 14.4 (12.2–16.5) | 1.03 (0.81–1.31) |
| Active Smoking, >15 | 1040 | 125 | 12.2 (9.3–15.1) | 0.86 (0.64–1.16) |
| P Value† | 0.19 | |||
| Alcohol intake** | ||||
| Never and past | 1142 | 153 | 12.7 (9.9–15.6) | 1.00 (reference) |
| Current, light | 1473 | 232 | 15.9 (13.2–18.6) | 1.41 (0.99–2.00) |
| Current, moderate/heavy | 697 | 93 | 11.8 (8.7–14.9) | 1.02 (0.73–1.41) |
| P Value† | 0.73 | |||
| C-reactive protein, mg/L | ||||
| Low, <1 | 1625 | 221 | 13.7 (10.8–16.6) | 1.00 (reference) |
| Moderate, 1–3 | 1505 | 217 | 14.0 (11.3–16.6) | 0.92 (0.68–1.25) |
| High, >3–10 | 1176 | 164 | 13.5 (11.2–15.8) | 0.88 (0.64–1.19) |
| Very High, >10 | 448 | 68 | 14.9 (11.1–18.7) | 0.98 (0.70–1.38) |
| P Value† | 0.79 | |||
ANA = antinuclear antibodies; + = positive; POR = prevalence odds ratio; CI = confidence interval; BMI = body mass index.
No. reflects the number of subjects within the sample, not an estimated count for the US population.
P Value for the trend test investigates the linear association among ANA+ and levels of the population characteristics.
Body mass index was calculated as weight in kilograms divided by height in meters squared (kg/m2).
BMI categorization for children from birth to age 19 was based on the Centers for Disease Control and Prevention 2000 growth charts.
BMI categorization for adults age 20 and above was based on BMI values (kg/m2): Underweight (<18.5), Healthy (18.5 – <25.0), Overweight (25.0 – <30.0), Obese (≥30.0).
Cotinine detection limits (ng/mL) across the NHANES cycles were 0.05 (1999 – 2000), 0.015 and 0.05 (2001 – 2002), and 0.015 (2003 – 2004).
Alcohol use data for children 19 years old and younger was excluded from the analysis due to its restricted access to maintain confidentiality for these participants
Alcohol consumption, a smoking history, the total pack years of smoking (data not shown), current smoking status (based on serum cotinine) and C-reactive protein levels were not associated with ANA.
Prevalence of ANA patterns and specific antinuclear antibodies among individuals with ANA
Among the 670 NHANES participants who had ANA, nuclear staining was seen in 84.6% (Table 3). Nuclear patterns were less common as age increased (P for trend = 0.02) and less common among those with 0 to 8 years of education (65.5%) compared to those in other educational categories (81% to 90%). The prevalence of nuclear patterns did not vary by sex, race/ethnicity or family income to poverty level ratio, but did increase with increasing educational attainment (P for trend = 0.01).
Table 3.
Frequencies of ANA Patterns and Specific Autoantibodies among ANA-positive Individuals
| Characteristic | ANA Patterns |
Specific Autoantibodies§ |
||||
|---|---|---|---|---|---|---|
| All Nuclear No.* = 560 % (95% CI) |
Nucleolar No.* = 43 % (95% CI) |
All Cytoplasm No.* = 152 % (95% CI) |
Ro/La/Su/U1 No.* = 46 % (95% CI) |
Ro No.* = 23 % (95% CI) |
Su No.* = 20 % (95% CI) |
|
| Total (No.* = 670) | 84.6 (81.1–88.2) | 6.1 (3.6–8.6) | 21.8 (18.3–25.4) | 6.7 (4.3–9.1) | 3.9 (1.9–5.9) | 2.4 (1.0–3.8) |
| Age, years | ||||||
| 12–19 | 86.6 (78.7–94.5) | 10.2 (1.7–18.6)‡ | 18.1 (10.4–25.7) | 2.0 (0.1–3.9)‡ | 0.7 (0–1.5)‡ | 1.3 (0–2.9)‡ |
| 20–29 | 93.7 (88.6–98.8) | 11.2 (3.2–19.2)‡ | 16.0 (8.4–23.5) | 3.7 (0–8.0)‡ | 0 | 3.7 (0–8.0)‡ |
| 30–39 | 85.3 (76.4–94.3) | 1.1 (0–2.7)‡ | 21.7 (11.3–32.0) | 10.7 (2.0–19.4)‡ | 7.6 (0.3–14.8)‡ | 2.5 (0–6.5)‡ |
| 40–49 | 86.5 (76.6–96.5) | 6.9 (0–15.3)‡ | 16.1 (6.1–26.1)‡ | 3.1 (0–9.4)‡ | 3.1 (0–9.4)‡ | 0 |
| 50–59 | 77.9 (67.4–88.3) | 5.1 (0–10.3)‡ | 28.4 (18.0–38.7) | 13.5 (4.7–22.3)‡ | 6.4 (0.2–12.5)‡ | 6.5 (0.8–12.3)‡ |
| 60–69 | 81.7 (70.3–93.2) | 4.3 (0–9.7)‡ | 32.2 (19.8–44.5) | 3.5 (0–8.4)‡ | 2.6 (0–7.3)‡ | 0.9 (0–2.4)‡ |
| 70+ | 80.4 (72.7–88.1) | 4.8 (0.5–9.1)‡ | 22.9 (14.2–31.7) | 6.3 (1.2–11.5)‡ | 4.9 (0–10.0)‡ | 0.2 (0–0.5)‡ |
| P Value† | 0.02 | 0.15 | 0.04 | 0.05 | 0.03 | 0.33 |
| Sex | ||||||
| Male | 84.5 (77.9–91.1) | 8.5 (2.6–14.3)‡ | 22.8 (16.0–29.6) | 2.4 (0–5.1)‡ | 2.0 (0–4.6)‡ | 0.1 (0–0.3)‡ |
| Female | 84.7 (80.2–89.3) | 4.9 (2.6–7.2) | 21.3 (16.1–26.6) | 8.8 (5.5–12.2) | 4.9 (2.1–7.6) | 3.6 (1.5–5.7) |
| P Value† | 0.96 | 0.26 | 0.77 | 0.004 | 0.14 | 0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 86.3 (81.8–90.8) | 4.9 (2.3–7.5) | 20.2 (15.5–24.9) | 5.4 (2.5–8.3) | 4.1 (1.6–6.7)‡ | 0.8 (0–1.9)‡ |
| Non-Hispanic black | 79.5 (71.9–87.0) | 6.2 (1.9–10.5)‡ | 26.7 (18.6–34.7) | 10.0 (4.0–16.0) | 2.1 (0–5.1)‡ | 5.8 (1.3–10.3)‡ |
| Mexican American | 86.6 (80.2–93.0) | 7.5 (1.7–13.3)‡ | 20.3 (13.2–27.4) | 9.6 (4.7–14.4) | 2.9 (0–6.2)‡ | 6.7 (2.5–10.9)‡ |
| Other | 79.3 (65.7–92.9) | 12.9 (1.0–24.8)‡ | 27.3 (13.3–41.4) | 8.6 (0.1–17.1)‡ | 5.4 (0–12.2)‡ | 5.8 (0–13.3)‡ |
| P Value† | 0.38 | 0.44 | 0.41 | 0.26 | 0.67 | 0.01 |
| Educational attainment, years | ||||||
| 0–8 | 65.5 (52.0–79.0) | 3.1 (0.1–6.1)‡ | 46.4 (31.6–61.2) | 8.4 (1.1–15.7)‡ | 3.4 (0–7.2)‡ | 5.3 (0–11.1)‡ |
| 9–11 | 81.3 (72.8–89.7) | 6.0 (0–12.2)‡ | 24.4 (14.1–34.8) | 9.2 (1.7–16.6)‡ | 1.7 (0–4.1)‡ | 5.8 (0–12.3)‡ |
| HS diploma/GED | 89.3 (83.0–95.6) | 5.8 (2.5–9.0) | 18.9 (10.6–27.2) | 5.7 (1.7–9.6)‡ | 3.6 (0–7.3)‡ | 1.7 (0–3.4)‡ |
| Some college | 84.3 (78.1–90.5) | 7.0 (2.5–11.6)‡ | 20.6 (14.0–27.3) | 5.6 (0.7–10.5)‡ | 5.3 (0.3–10.3)‡ | 0.3 (0–0.8)‡ |
| College or post graduate | 89.7 (83.3–96.1) | 6.3 (0.9–11.6)‡ | 15.7 (8.5–22.9) | 4.2 (0.4–8.1)‡ | 3.2 (0–6.4)‡ | 1.0 (0–3.1)‡ |
| P Value† | 0.01 | 0.44 | 0.01 | 0.11 | 0.61 | <0.001 |
| Family income to poverty level ratio | ||||||
| At or above poverty ≥1 | 86.2 (82.0–90.5) | 5.3 (2.7–7.9) | 20.3 (15.7–24.8) | 6.1 (3.5–8.7) | 3.8 (1.6–6.0) | 1.4 (0.4–2.4)‡ |
| Below poverty <1 | 82.6 (71.8–93.4) | 7.8 (0.9–14.7)‡ | 22.8 (11.2–34.3) | 9.5 (0.9–18.0)‡ | 4.6 (0–11.5)‡ | 5.2 (0–10.5)‡ |
| P Value† | 0.56 | 0.49 | 0.71 | 0.47 | 0.83 | 0.15 |
ANA = Antinuclear antibodies; + = positive; U1 = U1RNP (ribonucleoprotein); CI = confidence interval; HS = High School; GED = General Educational Development.
No. reflects the number of subjects within the sample, not an estimated count for the U.S. population.
For sex, race/ethnicity, and family income to poverty level ratio, the P Value for the Wald χ2 test investigae the general association among ANA+ and levels of the population characteristics. For age and educational attainment, the P Value for the trend test investigates the linear association among ANA+ and levels of the population characteristics.
Estimate is considered unreliable because the relative standard error is greater than 30%.
Anti-Jo-1, -PL-7, -PL-12, -EJ, -SRP (signal recognition particle), -Ku, -PM-Scl, -Mi-2, -RNA polymerase I and III, and -U3RNP autoantibodies were not observed in any individuals in our ANA positive samples. Anti-Sm (N=2), -topoisomerase I (N=1), -U1RNP (N=7), -La (N=7), -ribosomal P (N=1), -RPA (replication protein A) (N=3), -OJ (N=1), and –NOR90 (nuclear organizer region antigen 90kDa; N=1) autoantibodies were rare in our sample.
The estimated prevalence of anti-Ro, -La, -Su, and -U1RNP autoantibodies in the general population are listed below, although the actual prevalence will likely be higher since not all specific autoantibody positive samples are classified as ANA+ by immunofluorescence.
Anti-Ro autoantibodies, 0.54% (female 0.86%, male 0.19%)
Anti-La autoantibodies, 0.21% (female 0.34%, male 0.08%)
Anti-Su autoantibodies, 0.33% (female 0.64%, male 0.01%)
Anti-U1RNP autoantibodies, 0.12% (female 0.20%, male 0.03%)
Nucleolar patterns were seen in 6.1% and cytoplasmic patterns in 21.8% of those with ANA, and, as expected, a number of individuals had more than one ANA staining pattern (data not shown). Nucleolar patterns were not significantly associated with sociodemographic characteristics, but cytoplasmic patterns increased with age (P for trend = 0.04) and decreased with higher educational attainment (P for trend = 0.01). However, the association of cytoplasmic patterns with age and education, while significant, were not linear.
Among individuals with ANA, the most common autoantibodies identified by immunoprecipitation were anti-Ro autoantibodies (3.9%), followed by anti-Su autoantibodies (2.4%). The combined prevalence of the most common autoantibodies (anti-Ro, -La, -Su, and - U1 ribonucleoprotein (RNP)), all of which are associated with multiple autoimmune diseases, was 6.7%. Further, the presence of these combined autoantibodies was more common among females (8.8%) than males (2.4%).
DISCUSSION
ANA are the most commonly measured biomarkers for autoimmunity and the easiest to assess at the population level. Estimating the prevalence and types of ANA in the U.S. is critical to understanding their etiology and changes over time. This study provides the first nationally-representative estimates of the prevalence of ANA in sociodemographic groups. Our investigation also included a determination of the patterns of ANA by standard immunofluorescent methods, as well as the identification of specific autoantibodies by a reliable immunoprecipitation assay in ANA-positive sera. Our finding of an overall ANA prevalence of 13.8% at a 1:80 serum dilution-level is similar to some small studies in selected healthy populations (3, 7, 8, 10), however, ANA prevalence in other reports at the same dilution level ranged from 1.1% to 20% (3, 5–8, 12, 13, 30). These differences likely relate to the different populations under study and variations in ANA assessments in different laboratories.
Our findings of higher ANA prevalences in females and older individuals are similar to several earlier reports (7, 10, 31). The reason for the female predominance in autoimmune diseases is not completely understood, nevertheless, the finding of a similar pattern of female dominance in ANA production suggests that hormonal or other factors in females play a role in this process (32, 33). While some reports suggest that aging is associated with autoimmunity and the prevalence of ANA is higher in the elderly (5, 14–16, 31, 34, 35), this trend was not apparent in other studies (3, 7, 9, 10, 36). Our finding of non-linear variations in ANA prevalence among different age groups could be the result of the differential exposure to factors related to development of ANA in certain age groups, non-linear intrinsic variations in the aging of the immune or endocrine systems, or sampling bias.
The variations in the female:male ratios of ANA in different age groups in our study are similar to patterns seen in systemic autoimmune diseases, which are strongly associated with ANA production (10, 31). One report suggested a lack of sex effects on ANA prevalence in subjects under 20 years of age (31), but the prevalence of many autoimmune diseases increases in females during the child-bearing years. For example, a female:male ratio of ~9:1 is seen in SLE patients with onset between 20 to 40 years, but this ratio is only ~2:1 in children with SLE (0–9 years old) and in elderly-onset SLE (≥ 60 years old) (37, 38). The female:male ratio in young to middle-aged adults who develop RA is ~4:1, but it is only ~1:1 in elderly-onset RA (39), and similar patterns are reported in scleroderma (40).
ANA prevalence in non-Hispanic blacks was modestly increased compared to non-Hispanic whites. This is consistent with the higher incidence of SLE (37, 38) and increased prevalence of certain lupus autoantibodies, such as anti-U1RNP, -Sm, and –Ku autoantibodies, in non-Hispanic blacks (41).
The lower prevalence of ANA in overweight and obese subjects, particularly in females, in the present study may be unexpected given the ability of adipose tissue to produce pro-inflammatory cytokines (22) and estrogens (42). Nonetheless, the effect of obesity on the immune system is complex, sometimes resulting in immunosuppression (43), and an inverse association of ANA frequency with obesity has been previously reported in women (44). Also, in a study of chronic obstructive pulmonary disease, ANA were not associated with smoking but their frequency was significantly higher in those with a low BMI (< 22 kg/m2) compared to subjects with a normal or high BMI (45). Additional investigations are needed to understand the cause of lower prevalence of autoantibodies in individuals with high BMI.
Despite some studies that suggest smoking as a risk factor for SLE, RA, and other autoimmune diseases (19), we did not find any evidence suggesting an association or dose effect of current or past smoking with ANA.
Although ANA pattern distributions among healthy individuals vary among studies, a nuclear pattern is usually the most commonly identified, followed by cytoplasmic and nucleolar patterns (5, 7, 8), as was observed in the present study. The most commonly identified autoantibodies in those who were positive for ANA were anti-Ro autoantibodies (3.9% among those with ANA and 0.53% among persons in the U.S.) and anti-Su autoantibodies (2.4% among those with ANA and 0.33% among persons in the U.S). The prevalence estimate of anti-Ro autoantibodies in this study is similar to that in an investigation of 5,000 blood donors (0.44%) (5), but lower than that seen in a Japanese report (2.66% in 2181 subjects) (10). Several investigations using reliable methods reported anti-Ro autoantibodies in 0.12 to 2% of blood donors or pregnant women (5, 46, 47), while other small studies using enzyme-linked immunosorbent assays reported an even higher percentage of anti-Ro positives among healthy individuals (48, 49). It is difficult to compare these data, however, due to variations in the sensitivities and specificities among the assays used (2). Because not all anti-Ro and anti-Su autoantibodies show strong immunofluorescence (2, 23, 50), some sera containing these autoantibodies may not have met the threshold for immunoprecipitation testing in our study. Thus, the actual prevalence of these autoantibodies in the general population is likely higher than our estimate. The prevalence of autoantibodies associated with multiple systemic autoimmune diseases did not increase with age, consistent with a study of anti-Ro autoantibodies in female blood donors (5).
In contrast to autoantibodies that are associated with multiple systemic autoimmune diseases, such as anti-Ro and -Su autoantibodies (2, 23, 50), autoantibodies that are highly specific for certain diseases or disease phenotypes (e.g., anti-Sm, -topoisomerase I, -RNA polymerase I/III, and -Jo-1 autoantibodies) (1, 2) were rarely if ever seen in this study, supporting their disease specificity and rarity.
Our study has limitations. First, the institutionalized U.S. population (e.g., nursing home residents) was not sampled by NHANES and this may have led to an underestimate of ANA prevalence, especially in the elderly. Also, small sample sizes of certain subgroups may have limited our power to detect differences in ANA prevalence for some factors. Furthermore, not all types of autoantibodies were assessed by our testing and there are other potential causes of autoantibody production in addition to autoimmune diseases, including certain infections, cancers and drugs (2). Due to limitations inherent in the NHANES data collection methodology, including cross-sectional sampling, we could not identify which ANA might be persistent versus transient, and we were unable to assess associations with specific autoimmune or other diseases. Finally, the prevalence of specific autoantibodies with less intense immunofluorescence may be underestimated since only ANA positive samples by immunofluorescence were tested by immunoprecipitation.
These findings demonstrate a high prevalence of ANA in the U.S., especially in females and older individuals. With the aging of the population, the number of individuals with ANA will likely increase beyond our estimate of 32 million persons. These first population-based estimates of ANA by indirect immunofluorescence, including their cellular staining patterns and specific autoantibody reactivities, resolve the uncertainties related to other published estimates from selected populations. These findings should be kept in mind by physicians when assessing ANA results and will serve as a useful baseline for future investigations of changes in ANA prevalence over time and the factors associated with their development.
Acknowledgments
This study was supported in part by the Intramural Program of the NIH, National Institute of Environmental Health Sciences (NIEHS Z01ES101074) and contract GS-23F-9806H to SRA.
The authors gratefully acknowledge the important administrative contributions of Renee Jaramillo as well as the statistical programming efforts and analytical insight of Dr. Zhanna Andrushchenko, both of SRA International, Inc. The authors also wish to thank Dr. Geraldine McQuillan of the CDC for administrative assistance and useful discussions, and Drs. Dale Sandler and Paivi Salo, National Institute of Environmental Health Sciences, and Dr. Mark Gourley, National Institute of Arthritis, Musculoskeletal and Skin Diseases, for their critical comments on the manuscript. We also thank the NHANES organizers and participants without whom this study would not be possible.
Footnotes
Financial disclosures: The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content. Dr. Miller had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study design: EKL Chan, Satoh, Ho, Rose, Cohn, Parks, Jusko, Walker, Germolec, Whitt, Crockett, Birnbaum, Zeldin, Miller
Acquisition of data: JYF Chan, Satoh, Pauley, EKL Chan, Ross
Analysis and interpretation of data: EKL Chan, Satoh, Pauley, JYF Chan, Ross, Ho, Rose, Cohn, Parks, Jusko, Walker, Germolec, Whitt, Crockett, Birnbaum, Zeldin, Miller
Statistical analysis: Ho, Rose, Crockett, Cohn
Project initiation, organization and funding: Miller
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the institutions to which they are affiliated.
References
- 1.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 2.Satoh M, Chan EKL, Sobel ES, Kimpel DL, Yamasaki Y, Narain S, et al. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert Rev Clin Immunol. 2007;3:721–738. doi: 10.1586/1744666X.3.5.721. [DOI] [PubMed] [Google Scholar]
- 3.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 4.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–1422. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 5.Fritzler MJ, Pauls JD, Kinsella TD, Bowen TJ. Antinuclear, anticytoplasmic, and anti-Sjogren's syndrome antigen A (SS-A/Ro) antibodies in female blood donors. Clin Immunol Immunopathol. 1985;36:120–128. doi: 10.1016/0090-1229(85)90045-5. [DOI] [PubMed] [Google Scholar]
- 6.Marin GG, Cardiel MH, Cornejo H, Viveros ME. Prevalence of antinuclear antibodies in 3 groups of healthy individuals: blood donors, hospital personnel, and relatives of patients with autoimmune diseases. J Clin Rheumatol. 2009;15:325–329. doi: 10.1097/RHU.0b013e3181bb971b. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004;50:892–900. doi: 10.1002/art.20096. [DOI] [PubMed] [Google Scholar]
- 8.Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:191–200. doi: 10.1002/art.30084. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg AM, Semchuk KM, McDuffie HH, Ledingham DL, Cordeiro DM, Cessna AJ, et al. Prevalence of antinuclear antibodies in a rural population. J Toxicol Environ Health A. 1999;57:225–236. doi: 10.1080/009841099157674. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi N, Koshiba M, Nishimura K, Sugiyama D, Nakamura T, Morinobu S, et al. Prevalence of disease-specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5-year period. Modern rheumatology / the Japan Rheumatism Association. 2008;18:153–160. doi: 10.1007/s10165-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 11.Uibo R, Talja I, Jogi R, Janson C, Bjornsson E, Boman G, et al. Autoantibodies in Estonia and Sweden, populations with different responses to allergens. Int Arch Allergy Immunol. 1998;117:126–130. doi: 10.1159/000023999. [DOI] [PubMed] [Google Scholar]
- 12.de Vlam K, De Keyser F, Verbruggen G, Vandenbossche M, Vanneuville B, D'Haese D, et al. Detection and identification of antinuclear autoantibodies in the serum of normal blood donors. Clin Exp Rheumatol. 1993;11:393–397. [PubMed] [Google Scholar]
- 13.Fernandez SA, Lobo AZ, Oliveira ZN, Fukumori LM, AM Pr, Rivitti EA. Prevalence of antinuclear autoantibodies in the serum of normal blood dornors. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:315–319. doi: 10.1590/s0041-87812003000600005. [DOI] [PubMed] [Google Scholar]
- 14.Ruffatti A, Rossi L, Calligaro A, Del Ross T, Lagni M, Marson P, et al. Autoantibodies of systemic rheumatic diseases in the healthy elderly. Gerontology. 1990;36:104–111. doi: 10.1159/000213183. [DOI] [PubMed] [Google Scholar]
- 15.Xavier RM, Yamauchi Y, Nakamura M, Tanigawa Y, Ishikura H, Tsunematsu T, et al. Antinuclear antibodies in healthy aging people: a prospective study. Mech Ageing Dev. 1995;78:145–154. doi: 10.1016/0047-6374(94)01532-q. [DOI] [PubMed] [Google Scholar]
- 16.Hurme M, Korkki S, Lehtimaki T, Karhunen PJ, Jylha M, Hervonen A, et al. Autoimmunity and longevity: presence of antinuclear antibodies is not associated with the rate of inflammation or mortality in nonagenarians. Mech Ageing Dev. 2007;128:407–408. doi: 10.1016/j.mad.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson DL, Gange SJ, Rose NR, Graham NMH. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 19.Klareskog L, Padyukov L, Alfredsson L. Smoking as a trigger for inflammatory rheumatic diseases. Curr Opin Rheumatol. 2007;19:49–54. doi: 10.1097/BOR.0b013e32801127c8. [DOI] [PubMed] [Google Scholar]
- 20.Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21:279–283. doi: 10.1097/BOR.0b013e32832a2e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7:282–289. doi: 10.1038/nrrheum.2011.37. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakymiw A, Ikeda K, Fritzler MJ, Reeves WH, Satoh M, Chan EK. Autoimmune targeting of key components of RNA interference. Arthritis Res Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiik AS, Hoier-Madsen M, Forslid J, Charles P, Meyrowitsch J. Antinuclear antibodies: A contemporary nomenclature using HEp-2 cells. J Autoimmun. 2010;35:276–290. doi: 10.1016/j.jaut.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Chan EK, Fritzler MJ, Wiik A, Andrade LE, Reeves WH, Tincani A, et al. AutoAbSC.Org -- Autoantibody Standardization Committee in 2006. Autoimmun rev. 2007;6:577–580. doi: 10.1016/j.autrev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Satoh M, Langdon JJ, Hamilton KJ, Richards HB, Panka D, Eisenberg RA, et al. Distinctive immune response patterns of human and murine autoimmune sera to U1 small nuclear ribonucleoprotein C protein. J Clin Invest. 1996;97:2619–2626. doi: 10.1172/JCI118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 28.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey: 1988 to 1991. Jama. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 29.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 30.Hilario MO, Len CA, Roja SC, Terreri MT, Almeida G, Andrade LE. Frequency of antinuclear antibodies in healthy children and adolescents. Clin Pediatr (Phila) 2004;43:637–642. doi: 10.1177/000992280404300709. [DOI] [PubMed] [Google Scholar]
- 31.Craig WY, Ledue TB, Johnson AM, Ritchie RF. The distribution of antinuclear antibody titers in "normal" children and adults. J Rheumatol. 1999;26:914–919. [PubMed] [Google Scholar]
- 32.Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr Opin Rheumatol. 2006;18:456–461. doi: 10.1097/01.bor.0000240354.37927.dd. [DOI] [PubMed] [Google Scholar]
- 33.Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther. 2009;11:252. doi: 10.1186/ar2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Candore G, Grimaldi MP, Listi F, Ferlazzo V, Colonna-Romano G, Motta M, et al. Prevalence of non organ-specific autoantibodies in healthy centenarians. Arch Gerontol Geriatr Suppl. 2002;35:75–80. doi: 10.1016/s0167-4943(02)00106-1. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson BO, Skogh T, Ernerudh J, Johansson B, Lofgren S, Wikby A, et al. Antinuclear antibodies in the oldest-old women and men. J Autoimmun. 2006;27:281–288. doi: 10.1016/j.jaut.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.White GL, Jr, Wood SD, Rom WN. Prevalence of antinuclear antibodies in a normal male population. Mil Med. 1983;148:536–538. [PubMed] [Google Scholar]
- 37.Hochberg MC. The incidence of systemic lupus erythematosus in Baltimore, Maryland, 1970–1977. Arthritis Rheum. 1985;28:80–86. doi: 10.1002/art.1780280113. [DOI] [PubMed] [Google Scholar]
- 38.McCarty DJ, Manzi S, Medsger TA, Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38:1260–1270. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel SE, Crowson CS, O'Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Steen VD, Oddis CV, Conte CG, Janoski J, Casterline GZ, Medsger TA., Jr Incidence of systemic sclerosis in Allegheny County, Pennsylvania. A twenty-year study of hospital-diagnosed cases, 1963–1982. Arthritis Rheum. 1997;40:441–445. doi: 10.1002/art.1780400309. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Satoh M, Kabir F, Shaw M, Domingo MA, Mansoor R, et al. Increased prevalence of autoantibodies to ku antigen in African American versus white patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:2367–2370. doi: 10.1002/1529-0131(200110)44:10<2367::aid-art400>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Experimental biology and medicine. 2010;235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez DA, De Leon AC, Rodriguez Perez MC, Coello SD, Gonzalez Hernandez A, Fuentes RC, et al. Inverse association between obesity and antinuclear antibodies in women. J Rheumatol. 2008;35:2449–2451. doi: 10.3899/jrheum.080322. [DOI] [PubMed] [Google Scholar]
- 45.Bonarius HP, Brandsma CA, Kerstjens HA, Koerts JA, Kerkhof M, Nizankowska-Mogilnicka E, et al. Antinuclear autoantibodies are more prevalent in COPD in association with low body mass index but not with smoking history. Thorax. 2011;66:101–107. doi: 10.1136/thx.2009.134171. [DOI] [PubMed] [Google Scholar]
- 46.Yamagata H, Akizuki M, Tojo T, Homma M. Anti-Ro/SSA and -La/SSB antibodies in patients with connective tissue diseases. Scand J Rheumatol Suppl. 1986;61:98–101. [PubMed] [Google Scholar]
- 47.Guzman L, Martinez P, Gaytan G, Arguelles E, Herrera-Esparza R. Anti- Ro antibodies in healthy mothers and their newborns. Clin Exp Rheumatol. 1987;5:93–94. [PubMed] [Google Scholar]
- 48.Garberg H, Jonsson R, Brokstad KA. The serological pattern of autoantibodies to the Ro52, Ro60, and La48 autoantigens in primary Sjogren's syndrome patients and healthy controls. Scand J Rheumatol. 2005;34:49–55. doi: 10.1080/03009740510017940. [DOI] [PubMed] [Google Scholar]
- 49.Metskula K, Salur L, Mandel M, Uibo R. Demonstration of high prevalence of SS-A antibodies in a general population: association with HLA-DR and enterovirus antibodies. Immunol Lett. 2006;106:14–18. doi: 10.1016/j.imlet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Satoh M, Langdon JJ, Chou CH, McCauliffe DP, Treadwell EL, Ogasawara T, et al. Characterization of the Su antigen, a macromolecular complex of 100/102 and 200 kDa proteins recognized by autoantibodies in systemic rheumatic diseases. Clin Immunol Immunopathol. 1994;73:132–141. doi: 10.1006/clin.1994.1179. [DOI] [PubMed] [Google Scholar]