Abstract
Chlamydia spp. are obligate intracellular bacteria that replicate inside the host cell in a bacterial-modified-unique compartment called the inclusion. As other intracellular pathogens, chlamydiae exploit host membrane trafficking pathways to prevent lysosomal fusion and to acquire energy and nutrients essential for their survival and replication. The Conserved Oligomeric Golgi (COG) complex is a ubiquitously expressed membrane-associated protein complex that functions in a retrograde intra-Golgi trafficking through associations with coiled-coil tethers, SNAREs, Rabs and COPI proteins. Several COG complex-interacting proteins, including Rab1, Rab6, Rab14 and Syntaxin 6 are implicated in chlamydial development. In this study, we analyzed the recruitment of the COG complex and GS15-positive COG complex-dependent (CCD) vesicles to Chlamydia trachomatis inclusion and their participation in chlamydial growth. Immunofluorescent analysis revealed that both GFP-tagged and endogenous COG complex subunits associated with inclusions in a serovar-independent manner by 8 h post infection and were maintained throughout the entire developmental cycle. Golgi v-SNARE GS15 was associated with inclusions 24 h post infection, but was absent on the mid-cycle (8 h) inclusions, indicating that this Golgi SNARE is directed to inclusions after COG complex recruitment. Silencing of COG8 and GS15 by siRNA significantly decreased infectious yield of chlamydiae. Further, membranous structures likely derived from lysed bacteria were observed inside inclusions by electron microscopy, in cells depleted of COG8 or GS15. Our results showed that C. trachomatis hijacks the COG complex to re-direct the population of Golgi-derived retrograde vesicles to inclusions. These vesicles likely deliver nutrients that are required for bacterial development and replication.
Keywords: COG complex, GS15, Golgi, Chlamydia
Introduction
Chlamydiae are obligate intracellular Gram-negative bacteria associated with a multitude of human disease states. Chlamydia trachomatis is a major cause of pelvic inflammatory disease, ectopic pregnancy, and infertility among women; and also the leading cause of preventable blindness in the world (Schachter, 1989). Chlamydiae have a unique biphasic developmental cycle with 2 morphologically different forms, elementary body (EB) and reticular body (RB) (Rockey et al., 2002; Scidmore et al., 1996). Throughout their entire time in the host cell, chlamydiae remain within the confinements of the cytoplasmic membrane compartment termed inclusion, which very early during infection becomes fusiogenic with a subset of exocytic vesicles originating from the Golgi region. Acquisition of sphingomyelin from Golgi is essential for chlamydial development; this acquisition is initiated very early in the cycle, a phenomenon driven by early chlamydial protein synthesis (Hackstadt et al., 1996). While different chlamydiae exhibit differences in host tropism, spectrum of disease, and some aspects of basic biology, the nature of the inclusion is common within the genus and unique relative to all other intracellular pathogens (Rockey et al., 2002). However, little is known about the mechanisms involved in the exit from endocytic and fusion with exocytic vesicle. Recent investigations in several labs identified a sub-population of Golgi-localized proteins, including CERT (Derre et al., 2011), Rab1 (Rzomp et al., 2003), Rab6 (Rejman Lipinski et al., 2009), Rab14 (Capmany and Damiani, 2011) and SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) Syntaxin 6 (Moore et al., 2011) which are specifically re-localized to chlamydial inclusions and are essential for bacterial survival and reproduction.
The Golgi apparatus is a hub for membrane trafficking pathways, organizing both anterograde and retrograde trafficking of molecules (for review see (Shorter and Warren, 2002)). It plays a key role in the intracellular trafficking, processing and secretion of glycoproteins and glycolipids (Warren and Malhotra, 1998). Studies in yeast and mammalian cells have led to the identification of several multi-subunit protein complexes that are thought to be involved in Golgi vesicle tethering and/or compartment function, including the COG (conserved oligomeric Golgi) (VanRheenen et al., 1998; VanRheenen et al., 1999), the TRAPP (Sacher et al., 2001) and the GARP (Vps51-54) (Conibear and Stevens, 2000) complexes [for review see (Lupashin and Sztul, 2005)] The COG complex is preferentially localized to the cis/medial cisternae of the Golgi apparatus (Kim et al., 1999; Spelbrink and Nothwehr, 1999; Ungar et al., 2002), and is involved in Golgi membrane traffic (Bruinsma et al., 2004; Ram et al., 2002; Suvorova et al., 2001; Suvorova et al., 2002; Zolov and Lupashin, 2005). COG complex is a peripheral membrane hetero-oligomer which consists of eight subunits named COG1~8 (Fotso et al., 2005; Kingsley et al., 1986; Ram et al., 2002; Suvorova et al., 2001; Suvorova et al., 2002; Ungar et al., 2002; Whyte and Munro, 2001). Based on yeast genetic studies and observations with electron microscopy (Ungar et al., 2002), the COG subunits have been grouped into two sub-complexes consisting of COG1 to COG4 for lobe A subcomplex and COG5 to COG8 for lobe B subcomplex. Mutations or depletions of the COG complex subunits lead to disruption of retrograde intra-Golgi trafficking of endogenous and exogenous proteins (Smith et al., 2009; Zolov and Lupashin, 2005), improper glycosylation of glycoproteins and glycolipids (Bruinsma et al., 2004; Shestakova et al., 2006), and in humans causes multiple organ system pathologies referred to as congenital disorders of glycosylation (CDG) (Foulquier, 2009; Wu et al., 2004). Intracellular accumulation of Golgi-derived transport vesicles that carry Golgi v-SNARE GS15/GOS15 is the earliest phenotype associated with the loss of COG complex subunits (Zolov and Lupashin, 2005). COG complex is orchestrating intra-Golgi vesicular trafficking through the functional interaction with coiled-coil tethers (Sohda et al.), SNAREs (Shestakova et al., 2007; Suvorova et al., 2002), Rabs (Smith et al., 2009; Sun et al., 2007; Suvorova et al., 2002; VanRheenen et al., 1998) and COPI proteins (Ram et al., 2002; Suvorova et al., 2002; Zolov and Lupashin, 2005).
In cells infected with chlamydiae, Golgi resident protein golgin-84, one of COG-interacting coiled-coil golgins, is not re-localized to the inclusion, but instead is targeted for the specific protease cleavage (Heuer et al., 2009; Rejman Lipinski et al., 2009), while several other COG-interacting proteins, including Rab1, Rab6, Rab14 and Syntaxin6 are actively recruited to inclusion (Capmany and Damiani, 2011; Moore et al., 2011; Rejman Lipinski et al., 2009; Rzomp et al., 2003), raising a question about possible involvement of the COG complex in chlamydial maturation.
Materials and Methods
Cell culture
HeLa cells (CCL-2; ATCC) were grown in Dulbecco modified Eagle’s medium (DMEM/F-12) (HyClone) supplemented with 10% heat inactivated FBS (Atlas Biologicals) and containing penicillin 10 units/ml. Medium for stably expressing GFP-GS15 (N-terminal GFP tag), YFP-COG3 (N-terminal YFP tag) and COG8-GFP (C-terminal GFP tag) HeLa cells (this lab) was supplemented with 0.2 mg/ml G418 sulfate. Cells were transferred to antibiotic-free medium 24 h before infection.
Chlamydia trachomatis stock, infection and enumeration
Chlamydia trachomatis, serovar D or L2 strain and Chlamydia muridarum were propagated in Mycoplasma-free McCoy cells grown in DMEM media supplemented with 100 µM nonessential amino acids (Invitrogen), 2 mM L-glutamine (Invitrogen), 10% FBS, 50 mg/ml gentamicin sulfate, and 0.5 mg/ml cycloheximide. Infectious elementary bodies were isolated from McCoy cells by sonication 48 h post infection, washed in PBS, resuspended in SPG buffer (250 mM sucrose, 10 mM sodium phosphate, and 5 mM L-glutamic acid, pH 7.2), and stored in aliquots at −80°C. Stocks were titrated as described (Caldwell et al., 1981). Infections of cells with C. trachomatis were carried as previously described (Prantner and Nagarajan, 2009). After chlamydiae were added at specific multiplicity of infection (MOI), cells were centrifuged at 1690 × g at 37°C for 1 h. Infections were performed at 1 MOI, except when indicated otherwise. After the centrifugation, the media was aspirated and replaced with fresh media. Infected cells on coverslips were processed for immunofluorescence or inclusion forming units enumeration on a fresh McCoy monolayer at indicated times post infection (Caldwell et al., 1981). Specifically cell lysates were prepared in 1.0 ml of SPG buffer, sonicated and centrifuged at 100 × g to remove the debris. Lysate were overlaid on a McCoy titration plate (black bottom) at multiple dilutions (1:100, 1:1000 and 1:10,000) in a total volume of 200 µl and infections were done by centrifugation as described earlier. Cells were fixed at 30 h and IFU were counted in 20 fields and calculated per ml. For all si-RNA knock down experiments, infections were performed 72 h after siRNA transfection.
DNA and siRNA transfection
Plasmid DNAs were isolated using the QIAprep Spin Miniprep Kit (Qiagen) and transfected into tissue culture cells with FuGene Transfection Reagent (Roche), according to the manufacturer's protocol. siRNA-duplexes for Rab6 (Sun et al., 2007), COG3 (Zolov and Lupashin, 2005), COG8 (Smith et al., 2009) and GS15 (Tai et al., 2004) and siCONTROL1 (UAGCGACUAAACACAUCAA), were obtained from Dharmacon. Transfection was performed using Lipofectamine RNAiMAX siRNA Transfection Reagent (Invitrogen), following manufacturer’s protocol. Knock down efficiency was confirmed by western blot and/or immunofluorescence.
Immunofluorescence microscopy
HeLa cells were grown on 12 mm glass coverslips (#1, 0.17 mm thickness) at 30% density one day before transfection. After knock down (KD) and infection with C. trachomatis, cells were fixed in 4% paraformaldehyde (16% stock solution; Electron Microscopy Sciences) and then treated with 1% Triton X-100 for one minute. Following incubation with 50 mM ammonium chloride for 5 min, cells were washed with PBS. For COG staining, the coverslips were subjected to freshly prepared 6M urea in PBS for 2 minutes, and washed with PBS. All coverslips were blocked twice for 10 min with 1% BSA, 0.1% saponin in PBS. Then, cells were incubated for 30–60 min at room temperature with primary antibody diluted in the 1% cold fish gelatin, 0.1% saponin in PBS (antibody buffer), washed four times with PBS and incubated for 30 min with fluorescently tagged secondary antibody (1:400 HiLyte Fluor; AnaSpec) in antibody buffer at room temperature. After that, coverslips were washed four times with PBS, rinsed 10 times with first PBS then ddH2O, and mounted on glass microscope slides using Prolong® Gold antifade reagent along with DAPI (Invitrogen). Cells were imaged with the 63X oil 0.8 numerical aperture (NA) objective of a LSM510 Zeiss Laser inverted microscope outfitted with confocal optics. Image acquisition was controlled with LSM510 software (Release Version 4.0) SP1. All images presented are single z –plane sections.
SDS PAGE and western blots
Protein samples were lysed in hot 2% SDS and separated by 10% SDS-PAGE. Next, the gel was transferred onto 0.22 µm nitrocellulose membrane at 100V for 1 h. Membranes were blocked using LiCOR Odyssey Blocking Buffer for 20 minutes, incubated first with primary antibodies for 1 h, washed 4 times with PBS, and incubated with a secondary IgG antibody conjugated with IRDye 680 or IRDye 800 dyes. The blots were scanned and analyzed with an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Antibodies
Antibodies used for immunofluorescent (IF) microscopy or western blotting (WB) were purchased through commercial sources, gifts from generous individual investigators, and/or generated by us via affinity purification. Antibodies (and their dilutions) were as follows: mouse monoclonal antibodies EVI H1 recognizing chlamydial LPS (IF 1:500), IncA (a gift from Dr. Daniel Rockey) (IF 1:50) and hCOG3 (this lab) (IF 1:1000), affinity-purified rabbit polyclonal antibodies hCOG3, hCOG6, hCOG8 (this lab) (WB 1:1,000 and IF 1:1,000), Giantin (Covance) (IF 1:3,000), GS15 (Synaptic Systems) (IF 1:300), Rab6 (Santa Cruz) (WB 1:400). IRDye 680 goat anti-rabbit, IRDye 800 goat anti-mouse for WB secondary antibodies were obtained from LI-COR Biosciences. Anti-rabbit HiLyte Fluor 488, anti-rabbit HiLyte Fluor 555, anti-mouse HiLyte Fluor 555, and anti-rabbit HiLyte Fluor 647 for IF were obtained from AnaSpec, Inc.
Transmission Electron Microscopy
Samples were treated according to Valdivia’s lab protocol (Cocchiaro et al., 2008) with some modifications. In short, cells were fixed for 20 min on ice with 2.5% glutaraldehyde and 0.05% malachite green (EMS) in 0.1M sodium cacodylate buffer, pH 6.8. Samples were post-fixed for 30 min at room temperature with 0.5% osmium tetroxide and 0.8% potassium ferricyanide in 0.1 M sodium cacodylate, for 20 min on ice in 1% tannic acid, and for 1 h in 1% uranyl acetate at room temperature. Specimens were dehydrated with a graded ethanol series, and embedded in Araldite 502/Embed 812 resin (EMS). Ultrathin sections were imaged at 80 kV on FEI Technai G2 TF20 transmission electron microscope. Digital images were acquired with FEI Eagle 4kX USB Digital Camera.
Results
Co-localization of COG complex subunits with chlamydial inclusion
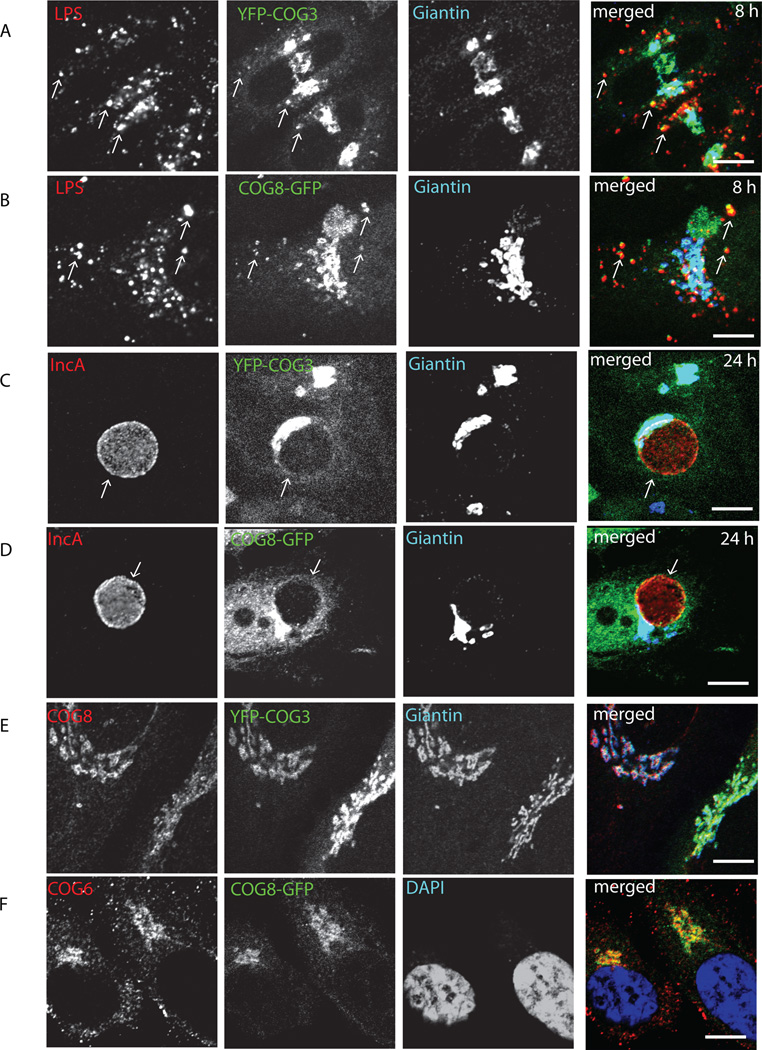
Chlamydial inclusion membrane acquires sphingomyelin and other nutrients from a host cell Golgi apparatus via a poorly defined mechanism (Moor et al, 2008), which is utilizing small GTPases Rab1, Rab6 (Rejman Lipinski et al., 2009; Rzomp et al., 2003), Rab14 (Capmany and Damiani, 2011) and trans-Golgi t-SNARE Syntaxin 6 (Moore et al., 2011). We and others have shown previously that COG complex, a peripheral membrane protein complex, implicated in tethering of the retrograde intra-Golgi vesicles (Fotso et al., 2005; Ungar et al., 2002; Ungar et al., 2006; Zolov and Lupashin, 2005), interacts with Rab1, Rab6 (Fukuda et al., 2008; Sun et al., 2007) and Syntaxin 6 (Laufman et al., 2011). Given the central role of vesicle tethering factors in orchestrating vesicle trafficking, we examined whether GFP-tagged COG complex subunits co-localize with the chlamydial inclusion in C. trachomatis serovar L2-infected HeLa. Antibodies to chlamydial LPS and IncA were used to detect early (4 h, mostly peripheral), mid-cycle (8 h, both peripheral and clustered perinuclear) and late (24 h, large perinuclear) chlamydial inclusions. Both YFP-COG3 (Lobe A subunit) and COG8-GFP (Lobe B subunit) were localized to the Golgi apparatus in uninfected cells (Figure 1E,F)) as observed previously (Shestakova et al., 2007), and were not detected on early inclusion at 4 h (data not shown). YFP-COG3 (Figure 1A) and COG8-GFP (Figure 1B) specifically colocalized with LPS-positive mid-cycle chlamydial inclusions 8 h after infection indicating that both Lobe A and Lobe B sub-complexes of the COG complex are diverted to the inclusion membrane. Significant colocalization for COG complex subunits was also observed on or around the late (24 h) IncA-positive chlamydial inclusions (Figure 1C, D). In agreement with previous reports, Golgi trafficking factor Giantin, that is responsible for the tethering of one of the subclasses of intra-Golgi vesicles (Malsam et al., 2005) did not localize to either mid-cycle or late chlamydial inclusions.
Fig. 1. Recruitment of fluorescently tagged COG3 and COG8 to the chlamydial inclusions.
Stable YFP-COG3 (A,C) and COG8-GFP (B,D) HeLa cells infected with Chlamydia trachomatis L2 (MOI 1.0) were fixed either 8 h (A,B) or 24 h (C,D) after infection. Cells were stained for chlamydial LPS using conjugated mAbs LPS (HiLyte Fluor -555) for 8 h infection or IncA (HiLyte Fluor -555) for 24 h infection, Giantin (HiLyte Fluor-649), and DAPI. Control uninfected YFP-COG3 (E) and COG8-GFP (F) cells were stained for COG8, COG6, Giantin and DAPI as indicated. Slides were visualized using a Zeiss LSM 510 Meta Laser confocal microscope. Arrows indicate the membrane of chlamydial inclusions. The merge images are shown on the right. Scale bar, 10 µm.
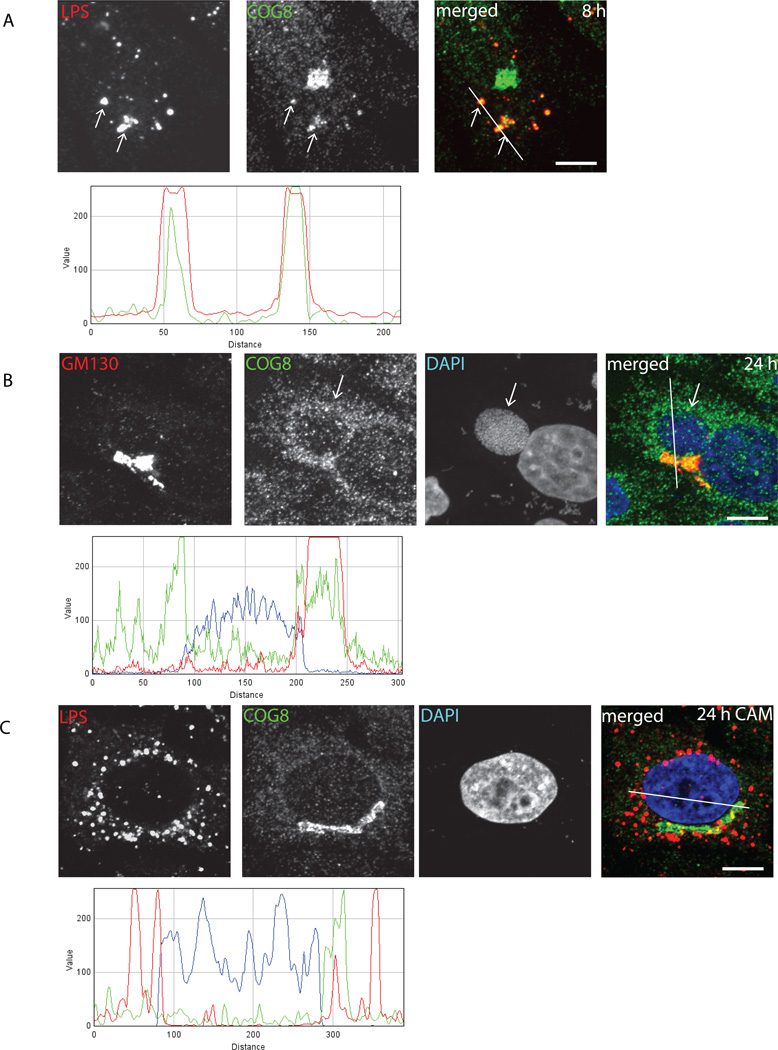
To confirm that the endogenous COG complex is being hijacked by chlamydial inclusion, localization of the endogenous COG complex subunits in infected cells was detected by indirect immunofluorescence (Figure 2). Indeed, almost all LPS-positive mid-cycle inclusions were co-labeled with anti-COG8 antibodies (Figure 2A) and significant COG8 signal was localized around the delimited membrane of IncA-positive large perinuclear inclusions (Figure 2B). Some of COG8-positive material was observed inside the inclusions. Importantly, recruitment of COG8 to chlamydial inclusion required active bacterial protein synthesis, since COG8 signal did not co-localize with LPS signal in chloramphenicol-treated cells (Figure 2C). These data verify antibody specificity and indicate that COG complex recruitment is facilitated by newly synthesized chlamydial proteins. Similar result was obtained with anti-COG3 and anti-COG6 staining (data not shown), supporting the hypothesis that the entire COG complex is partially mislocalized in Chlamydia-infected cells.
Fig. 2. Endogenous COG8 is associated with C. trachomatis inclusions.
HeLa cells infected with C. trachomatis serovar L2 (A,B) or serovar D (C) (MOI 1.0) were fixed either 8 h (A) or 24 h (B, C) post-infection and analyzed by confocal microscopy. In (C) cells were treated with chloramphenicol 3 h post-infection to block bacterial protein synthesis. Cells were stained for chlamydial LPS (HiLyte Fluor-555) and COG8 (HiLyte Fluor-488) (A), GM130 (HiLyte Fluor-555), COG8 (HiLyte Fluor-488) and DAPI (B), or LPS (HiLyte Fluor-555), COG8 (HiLyte Fluor-488) and DAPI (C). The merge images are shown on the right. The intensity profiles showed COG8 (green) specifically localized in a close proximity to inclusion membrane surrounding actively growing bacteria (LPS (red) in (A) and DAPI (blue) in (B)). Arrows indicate the membrane of chlamydial inclusions. Scale bar, 10 µm.
v-SNARE GS15 co-localize with late chlamydial inclusion
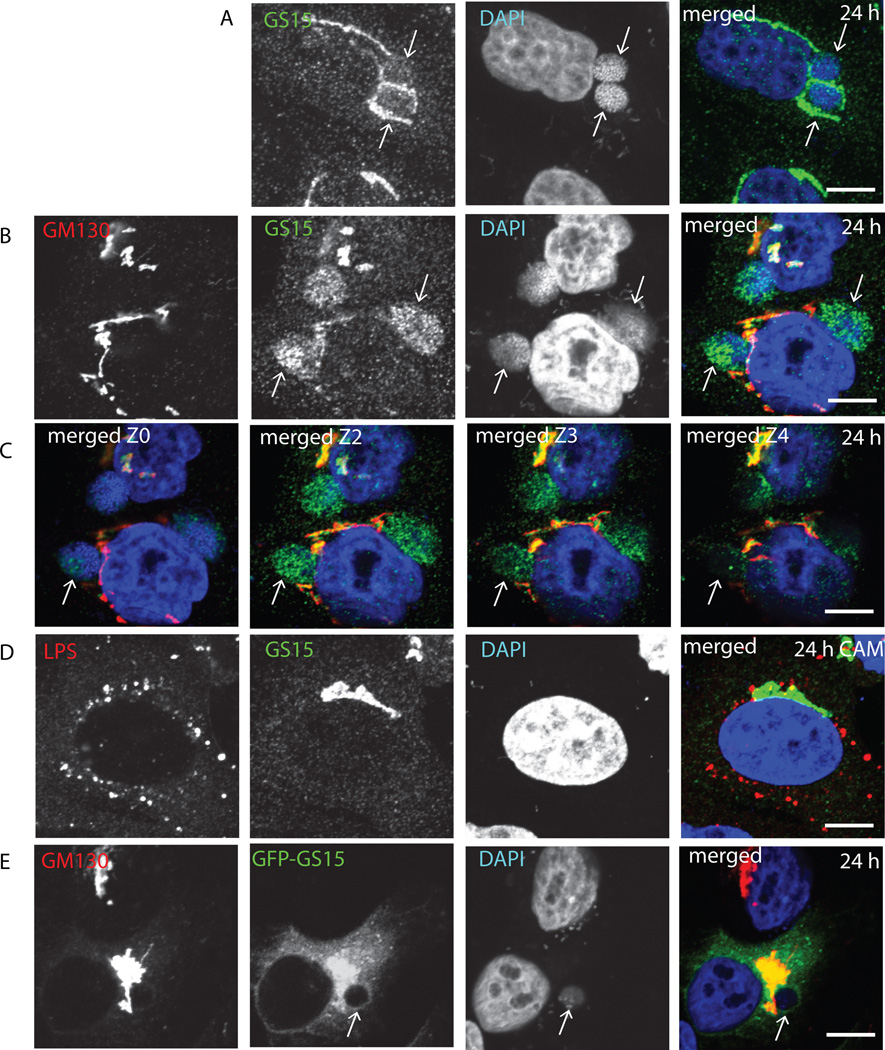
COG complex is one of the key regulators of the retrograde intra-Golgi trafficking. Research from our and other labs (Sohda et al., 2010; Zolov and Lupashin, 2005) demonstrated that the acute down-regulation of COG complex function in HeLa cells disrupts intra-Golgi trafficking and causes accumulation of multiple transport vesicles that carry Golgi v-SNARE GS15. We tested if localization of this Golgi SNARE is affected in cells infected with C. trachomatis and found that both endogenous (Figure 3A–C) and GFP-tagged GS15 (Figure 3E) are specifically diverted from the Golgi and co-localized with the membrane of the late chlamydial inclusions. Surprisingly, we observed that a fraction of GS15 immunoreactive material was accumulated inside inclusions (Figure 3B, C) indicating that GS15-positive vesicles may not only fuse with the inclusion membrane, but also accumulate within the inclusion. Again, as in the case of the COG complex, we found that the active bacterial protein synthesis is essential for the recruitment of GS15 to inclusion (Figure 3D).
Fig. 3. Colocalization of SNARE protein GS15 with C. trachomatis.
HeLa cells (A–D) or HeLa cells stably transfected with GFP-GS15 (E) infected with C. trachomatis L2 (B,C,E) or D (A,D) for 24 h were fixed and labeled with antibodies and DAPI as indicated. In (D) cells were treated with chloramphenicol 3 h after infection to block bacterial protein synthesis. The merge images are shown on the right. In (C) four Z sections of merged GM130/GS15/DAPI staining are shown. Arrows indicate the membrane of chlamydial inclusions. Scale bar, 10 µm.
COG complex and GS15 recruited to chlamydial inclusion in a serovar-independent manner
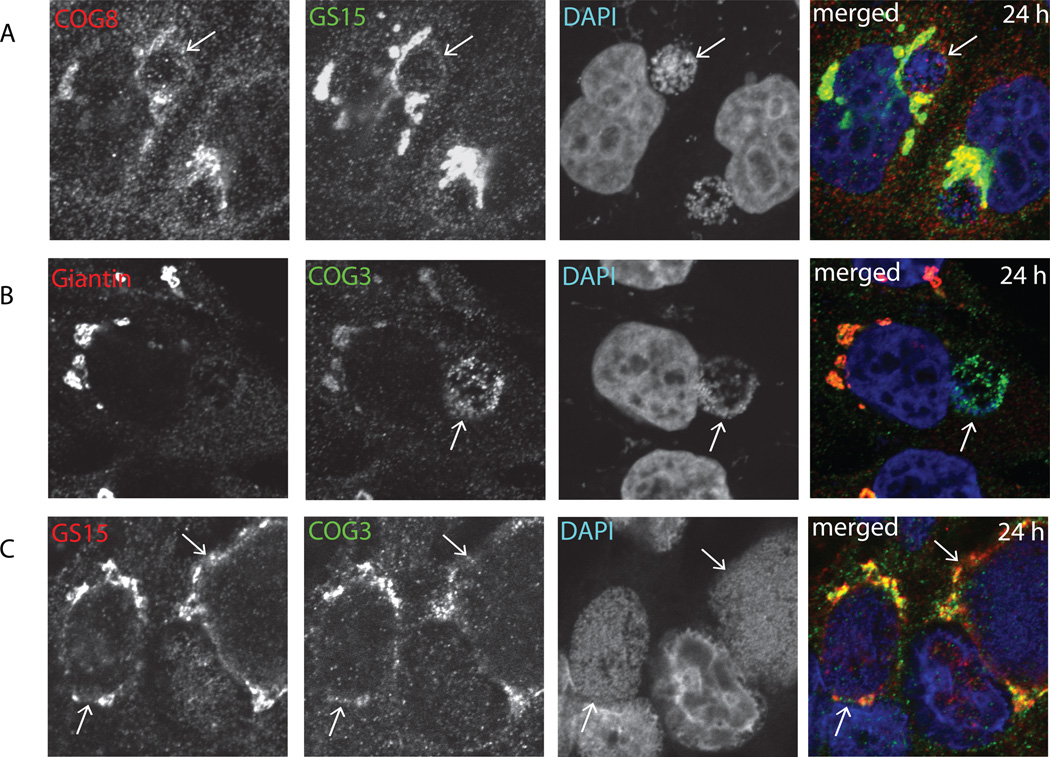
It has been shown previously that several host proteins, including the Rab6 effector Bicaudal D1 are associated with chlamydial inclusions in a serovar-specific manner (Moorhead et al., 2007). To test if this is the case for COG complex and GS15, localization of the endogenous COG3, COG8 and GS15 was determined in HeLa cells infected with C.trachomatis serovar D. We observed that both COG subunits and GS15 were specifically co-localized with late chlamydial inclusions (Figure 4A, B), indicating that they are recruited to the inclusion membrane in serovar-independent manner by the mechanism that is likely to be different to one used by Bicaudal D1. Similar association of COG3 and GS15 with inclusion membrane was also found in HeLa cells infected with C muridarum (Figure 4C).
Fig. 4. Recruitment of GS15 and COG complex subunits to inclusions formed by C. trachomatis serovar D and C. muridarum.
HeLa cells infected with C. trachomatis serovar D (A and B) or C. muridarum (C) for 24 h were fixed and labeled with antibodies against COG8 and GS15 (A), Giantin and COG3 (B), GS15 and COG3 (C) and DAPI. The merge images are shown on the right. Arrows indicate the membrane of chlamydial inclusions. Scale bar, 10 µm.
GS15 is localized to chlamydial inclusions in a COG-dependent manner
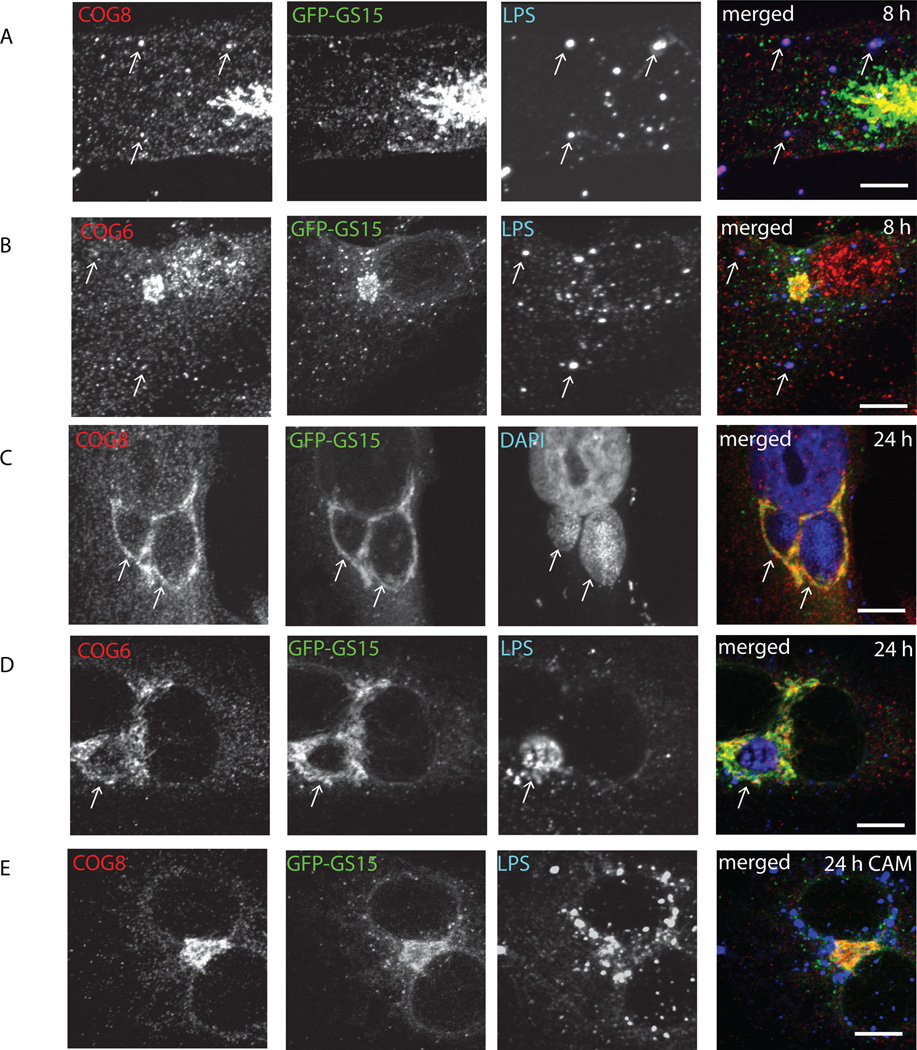
After establishing that both Golgi trafficking factors, COG complex and GS15 are actively recruited to chlamydial inclusion membrane, we asked the question which molecule is recruited first and if their recruitment is dependent on each other. We and others have shown previously that Golgi localization and stability of GS15 is regulated by COG complex (Oka et al., 2004; Zolov and Lupashin, 2005). We hypothesized that this scenario could be used in Chlamydia-infected cells. Indeed, in cells that stably express GFP-GS15, both COG8 (Figure 5A) and COG6 (Figure 5B) were recruited to LPS-positive small chlamydial inclusions 8 hours post-infection and these LPS/COG-positive structures did not possess any detectable GFP-GS15 signal. In contrast, both COG subunits and GFP-GS15 were co-localized with each other on the inclusion membrane 24 hours post-infection (Figure 5C, D, and Figure 6A)). Again, neither COG8 nor GFP-GS15 was localized to Chlamydia-positive membranes in cells treated with chloramphenicol (Figure 5E). Importantly in cells depleted for COG3 subunit of the COG complex, GFP-GS15 failed to localize on the inclusion membrane (Figure 6B), indicating that a functional COG complex is necessary for GS15 recruitment to chlamydial inclusions. Interestingly, in these cells COG6 signal was distributed diffusely in the cytoplasm without any concentration on the inclusion membrane, indicating that only a fully functioning COG complex is hijacked by chlamydial inclusion. Again, both COG6 and GFP-GS15 signals are co-localized with the inclusion membrane in control cells (Figure 6A). In cells depleted for GS15, the development of chlamydial inclusions was altered (see below), but these altered inclusions were still surrounded by COG6 and YFP-COG3 signals (Figure 6C), indicating that COG complex is recruited to inclusion in a GS15-independent manner.
Fig. 5. COG complex subunits localized to chlamydial inclusions before GS15.
Stably transfected GFP-GS15 HeLa cells infected with C. trachomatis serovar L2 (A–C) or serovar D (D–E) were fixed either 8 h (A, B) or 24 h (C, D, E) after infection. In (E) cells were treated with chloramphenicol 3 h after infection to block bacterial protein synthesis. Cells were stained for chlamydial LPS (HiLyte Fluor-555) and COG8 (HiLyte Fluor-647) (A, E), DAPI and COG8 (HiLyte Fluor-647) (C), or chlamydial LPS (HiLyte Fluor-555) and COG6 (HiLyte Fluor-647) (B,D). The merge images are shown on the right. Arrows indicate the membrane of chlamydial inclusions. Scale bar, 10 µm.
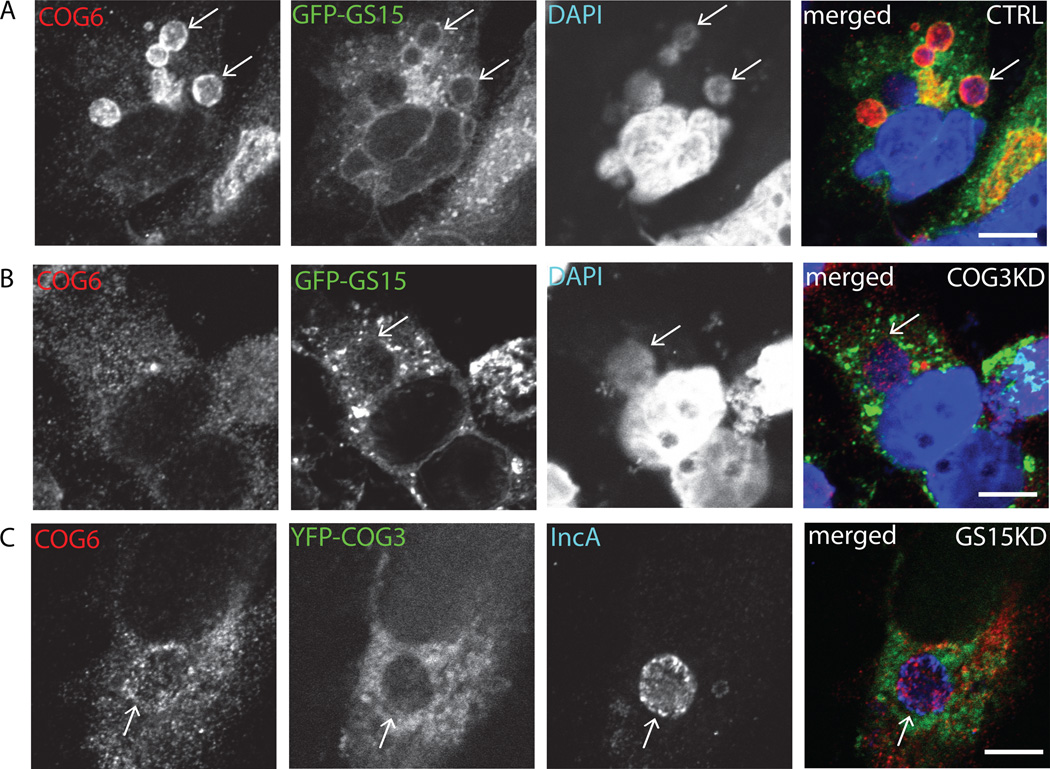
Fig. 6. Localization of GS15 to chlamydial inclusion is abrogated in COG3 KD cells.
HeLa cells stably expressing GFP-GS15 (A, B), or YFP-COG3 (C) were treated with control siRNA (A), COG3 siRNA (B) or GS15 siRNA (C) for 72 h and then infected with C. trachomatis serovar D and fixed 24 h post infection. Cells were stained for COG6 (HiLyte Fluor-555) and for DAPI (A,B) or IncA (HiLyte Fluor-488) (C). The merge images are shown on the right. Arrows indicate the membrane of chlamydial inclusions. Scale bar, 10 µm.
COG complex and GS15 are essential for chlamydial development
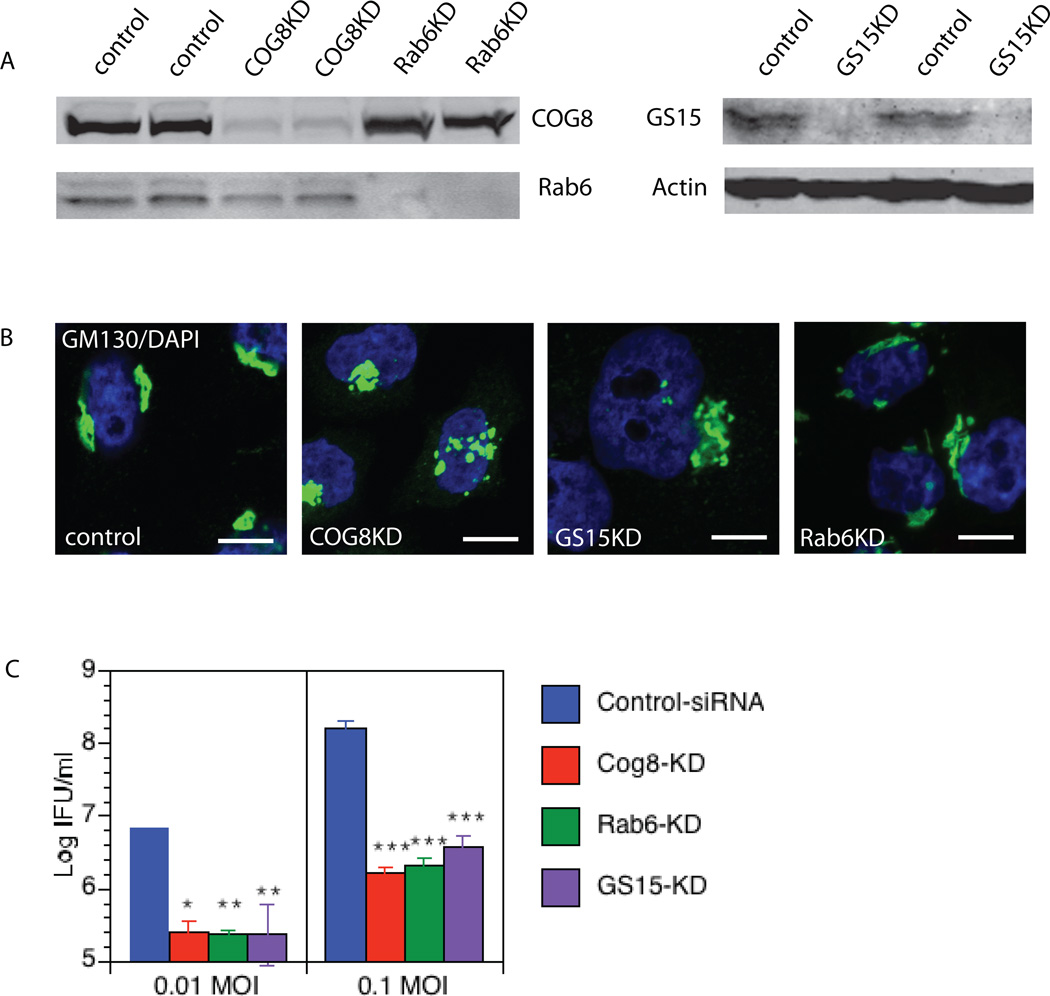
COG complex subunits were associated with only some nascent peripheral inclusions, while GS15 was found only on late inclusions, suggesting that these molecules are not involved in the entry process. Indeed, the number of initial inclusions was not altered in cells downregulated for COG3, COG8 or GS15 (data not shown). To analyze the role of these molecules in chlamydial development, growth and replication, HeLa cells were transfected with COG8 siRNA, GS15 siRNA, control siRNA (negative control) or Rab6 siRNA (positive control) (Rejman Lipinski et al., 2009) and infected with C. trachomatis, serovar D, 72h after siRNA transfection. At this point, downregulation of COG8, GS15 and Rab6 was ~90% complete, as assessed by western blot (Figure 7A) or fluorescent microscopy (data not shown). Localization of Golgi protein GM130 was largely unaltered in majority of siRNA treated cells (Figure 7B).
Fig. 7. GS15, COG8 and Rab6 are important factors for C. trachomatis development.
Western blot of control, GS15KD, COG8 KD and Rab6 KD HeLa lysates showing the efficiency of KD (A). COG8, GS15 and Rab6 were silenced by transfection of specific siRNAs. Cells were then fixed and stained with GM130 antibody and DAPI. Note that Golgi complex morphology has not been changed dramatically in knock-down cells (B). Down-regulation of COG8, Rab6 and GS15 affects infectious chlamydial yields significantly. HeLa cells were transfected with COG8siRNA, Rab6siRNA, GS15siRNA or control siRNA and infected 48h later with C. trachomatis, serovar D at 0.01 and 0.1 MOI. 48 h post infection, cells were harvested and infectious EBs obtained were determined by infecting a fresh McCoy cells layer and IFU determined. *p<0.05, **p<0.01, *** p<0.001 by one-Way ANOVA and post-hoc Tukey multiple comparison between control and specific KDs (C).
To address the effect of COG complex and GS15 depletion on bacterial growth and replication, infected HeLa cells were lysed at 48 h post-infection. The infectious EBs were titrated on a fresh McCoy monolayer by counting the Inclusions Forming Units (IFU) 30 h later. The amount of infectious bacteria recovered from COG8KD or GS15KD cells was significantly decreased compared to the control HeLa cells. The IFU assays demonstrated the negative impact of depletion of either COG complex or GS15 on chlamydial development (Figure 7C). Importantly, the extent of this negative effect was similar to the effect observed in Rab6 depleted cells.
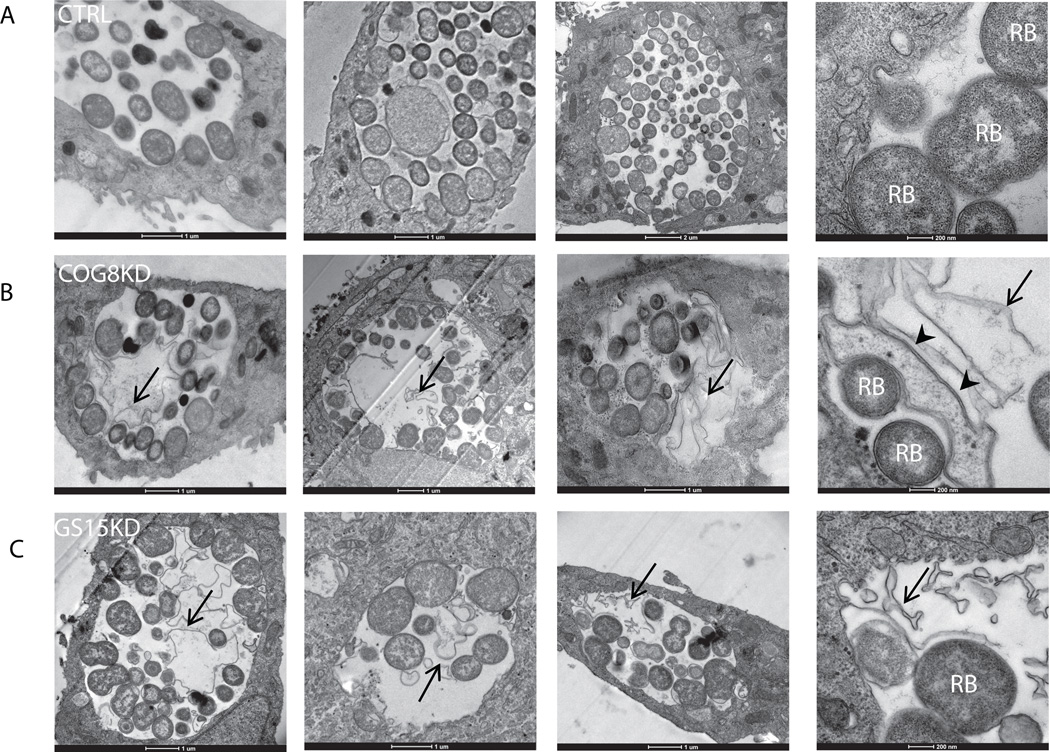
The development of bacteria in control, COG8KD and GS15KD cells was further assessed by TEM microscopy. To better preserve membrane and lipid structures inside inclusions, fixation in the presence of malachite green (Cocchiaro et al., 2008) was employed. Absolute majority of visualized inclusions in COG8KD (92%, n=26) (Figure 8B) and GS15 (87%, n=16) (Figure 8C) cells demonstrated a presence of multiple membranous structures and aberrant (partially lysed) bacterial forms (Figures 8B and C, arrows). In contrast, similar membrane structures were observed with very low frequency (25%, n=20) in infected HeLa cells treated with control siRNA (Figure 8A). The effect on chlamydial inclusion content and yield of infectious particles implies the role for the COG complex and Golgi v-SNARE GS15 in intracellular development and growth of Chlamydia spp. in mammalian cells.
Fig. 8. Ultrastructural analysis of chlamydial inclusions.
HeLa cells were transfected with control siRNA (A), COG8 siRNA (B), or GS15 siRNA (C) and infected with C.trachomatis serovar D 72 h (MOI=5) after transfection. Cells were fixed 24 h after infection and processed for TEM. Representative inclusions (out of ~20 random sections) are shown at 6500× magnification (Scale bar, 1 µm) and at 25000× magnification (Last image of each panel, Scale bar, 0.2 µm). Note that inclusions in COG8 KD and GS15 KD cells contained large amount of membranous material and bacteria ghosts (arrows) in addition to reticular bodies (RB). Al least some membranous material inside the inclusion in COG8KD cells is originated from the lysed RBs (D, arrowheads). Significantly more membranous material was observed in inclusions of COG8 KD (24/26 inclusions) and GS15 KD cells (14/16 inclusions) compared to control (5/20 inclusions). P<0.001 (control vs COG8 KD) and P=0.002 (control vs. GS15 KD) by Fisher exact test.
Discussion
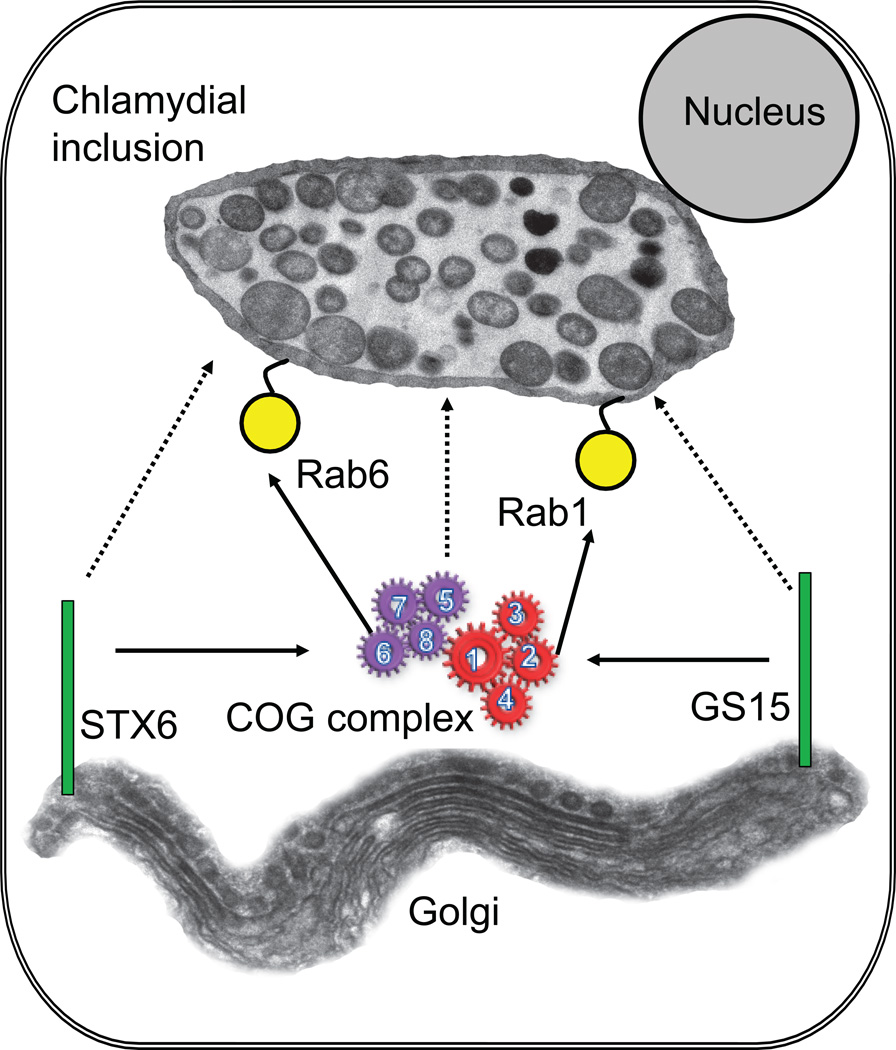
In this study, we demonstrate that the peripheral membrane complex COG, the master regulator of retrograde vesicular trafficking in the Golgi apparatus, as well as v-SNARE GS15 are recruited to chlamydial inclusion in a temporal manner and this association is required for optimal chlamydial development. Work in our and other labs demonstrated the COG complex, physically and functionally interacts with the majority of Rab and SNARE proteins that are recruited to chlamydial membrane (Fukuda et al., 2008; Laufman et al., 2011; Smith et al., 2009; Sun et al., 2007), suggesting that this complex could be recruited to inclusions as well. Indeed, we found that COG complex appeared on the inclusion membrane as early as 8 hour post-infection, indicating that this recruitment may be one of the earliest points of communication between bacterial inclusion and intra-Golgi vesicular trafficking. A schematic model that depicts the recruitment of the COG complex and COG-interaction proteins to chlamydial inclusions is shown on Figure 9.
Fig. 9. Model for the recruitment of the COG complex and COG-interacting proteins to chlamydial inclusions.
Solid arrows represent known protein-protein interactions. Dotted arrows represent recruitment to chlamydial inclusions. The model shows that recruitment of COG complex is essential for GS15 recruitment to the inclusion.
Chlamydia spp., like many other intracellular parasites have evolved diverse mechanisms to boost their survival and replication within mammalian cells (Hackstadt et al., 1997). Interaction with the Golgi-derived vesicular pathway has been proposed as one potential mechanism enabling chlamydiae not only to escape from lysosomal fusion, but also to receive cholesterol and sphingomyelin from the host cell (Carabeo et al., 2003; Moore et al., 2008; Wolf and Hackstadt, 2001). Number of Golgi-derived host molecules, including Bicaudal D1 (Moorhead et al., 2007), CERT (Derre et al., 2011), Rab1 (Rzomp et al., 2003), Rab6 (Rejman Lipinski et al., 2009), Rab14 (Capmany and Damiani, 2011) and Syntaxin 6 (Moore et al., 2011) are shown to be recruited to the chlamydial inclusion membrane, but the nature of this recruitment and the exact role of these molecules in chlamydial survival and replication is still unclear. COG complex is known to bind SNARE domain of Golgi SNARE proteins (Laufman et al., 2011; Shestakova et al., 2007). Further, at least three chlamydial Inc proteins, IncA, CT813 and CT233 showed similarities with SNARE motifs (Delevoye et al., 2008). Therefore, one intriguing possibility is that one of these proteins is involved in hijacking of the COG complex to the nascent inclusion membrane. In support of this possibility, C. trachomatis inclusion membrane protein CT229 has been shown to interact with GTPase Rab4 (Rzomp et al., 2006).
COG complex regulates trafficking of intra-Golgi vesicles that contain v-SNARE GS15. Furthermore, this SNARE protein is recruited to chlamydial inclusion later in development. Surprisingly, we observed that a fraction of GS15 and COG subunit immunoreactive material was accumulated inside inclusions indicating that COG complex and GS15-positive vesicles may not only fuse with the inclusion membrane, but also accumulate within the inclusions. Alternatively, intra-inclusion signal may indicate that COG subunits and GS15 aggregates are internalized by the inclusion. This intra-inclusion immunostaining is likely to be specific, since it was observed with several affinity-purified polyclonal (anti-GS15, anti-COG6, anti-COG8) and monoclonal (anti-COG3) antibodies, and these antibodies did not stain chlamydial inclusions that were blocked by chloroamphenicol. However, we could not exclude that some of this intra-inclusion staining could be due to cross-reactivity of antibodies used in this study with yet unidentified bacterial components at heavy bacterial loads 24 h post infection. Intriguingly, similar intra-inclusion accumulation of lipid droplets (Cocchiaro et al., 2008), sphingomyelin (Wolf and Hackstadt, 2001) and beta-catenin (Prozialeck et al., 2002) were reported previously. The biological significance of sequestration of GS15 inside the inclusion is unclear. One possible speculation is that GS15 positive vesicles inside the inclusion facilitate nutrient transfer directly across the bacterial membrane. Interestingly, GS15 recruitment to inclusion is abrogated in COG deficient cells, indicating that functional COG complex is necessary for re-direction of trafficking of the GS15-bearing intra-Golgi vesicles toward bacterial inclusion. GFP-tagged GS15 and COG molecules were largely not present inside the inclusion, indicating that exogenously expressed (and often overexpressed) fluorescently-tagged proteins only partially recapitulate the behavior of the endogenous protein.
Consistent with the role for Golgi trafficking machinery in acquisition of nutrients by chlamydiae, recruitment of COG complex and GS15 to the chlamydial inclusion is essential for optimal chlamydial development. In cells depleted of COG complex and GS15, inclusions are filled with bacterial ghosts and other membranous remnants, while the yield of infectious bacteria is significantly diminished. This may be partially due to deficient trafficking of essential nutrients, including sphingomyelin and cholesterol, from the host Golgi, since the content of sphingomyelin is significantly reduced in COG deficient cells (Spessott et al., 2010). One intriguing possibility is that COG8 KD and GS15 KD could induce a persistent state due to nutrient deprivation. However, knock down of both the COG complex or GS15 recruitment did not influence inclusion formation or inclusion numbers, suggesting that COG3, COG8, and GS15 are not essential for chlamydial entry and early events in escaping from the endocytic pathway. Conforming to this observation, no association of COG complex was observed at early time points (4 h) post infection (data not shown).
Previously, it has been shown that Rab6 is recruited to chlamydial inclusion in a species independent manner, interacting with all C. trachomatis strains (Rzomp et al., 2003). However, in contrast to Rab6 recruitment, Rab6 effector BiCD1 interacts exclusively with C. trachomatis L2 and not with serovar B, serovar D, C. muridarum or C. pneumoniae (Moorhead et al., 2007). Similar to Rab6, we observed that interaction of COG complex with chlamydial inclusion was observed with C. trachomatis D, L2 and C. muridarum, suggesting that these interactions are likely essential for all chlamydial strains. Furthermore, this would suggest involvement of a chlamydial Inc proteins conserved between C. trachomatis strains. However, further investigation is necessary using ocular C. trachomatis serovars, and other biovars such as C. pneumonia and C. psittaci strains, to determine the specificities of COG and GS15 interactions.
In summary, our work demonstrates an important role for COG complex and GS15 in chlamydial growth and development, right after the early inclusion is formed. The presence of aberrant RB and membranous structures inside the inclusion in the absence of a functional COG complex and GS15, further indicates the important role of these vesicle transport factors in nutrient transport. It is likely that C. trachomatis is hijacking host COG complex subunits to the inclusion membrane via direct binding to its SNARE-like Inc proteins. The specific chlamydial protein interacting with COG complex is certainly of interest to determine in the future to understand the specific nature of this association.
Acknowledgements
We would like to thank D. Rockey (Oregon State University), B. Storrie (UAMS), R. Willett (UAMS), and others who provided reagents and critical reading of the manuscript. We also would like to thank Daniel Prantner (UAMS) for assistance with initial experiments in the project and Tetyana Kudlyk (UAMS) for excellent technical support. This project described was supported in part by the National Science Foundation (MCB-0645163 and DBI-0959745) to V.L, R01GM083144 (NIGMS) to V.L. and AI067678 (NIAID) to U.N.
Abbreviations
- COG
conserved oligomeric Golgi
- EBs
Elementary Bodies
- IFU
Inclusions Forming Units
- KD
knock-down
- RBs
Reticular Bodies
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Conflict of interest statement
None declared.
References
- Bruinsma P, Spelbrink RG, Nothwehr SF. Retrograde transport of the mannosyltransferase Och1p to the early Golgi requires a component of the COG transport complex. J Biol Chem. 2004;279:39814–39823. doi: 10.1074/jbc.M405500200. [DOI] [PubMed] [Google Scholar]
- Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capmany A, Damiani MT. Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS One. 2011;5:e14084. doi: 10.1371/journal.pone.0014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol Biol Cell. 2000;11:305–323. doi: 10.1091/mbc.11.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Nilges M, Dehoux P, Paumet F, Perrinet S, Dautry-Varsat A, Subtil A. SNARE protein mimicry by an intracellular bacterium. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000022. e1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Swiss R, Agaisse H. The Lipid Transfer Protein CERT Interacts with the Chlamydia Inclusion Protein IncD and Participates to ER-Chlamydia Inclusion Membrane Contact Sites. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002092. e1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotso P, Koryakina Y, Pavliv O, Tsiomenko AB, Lupashin VV. Cog1p plays a central role in the organization of the yeast conserved oligomeric golgi complex. Journal of Biological Chemistry. 2005;280:27613–27623. doi: 10.1074/jbc.M504597200. [DOI] [PubMed] [Google Scholar]
- Foulquier F. COG defects, birth and rise! Biochim Biophys Acta. 2009;1792:896–902. doi: 10.1016/j.bbadis.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics. 2008;7:1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. Embo J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- Heuer D, Lipinski AR, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Kim DW, Sacher M, Scarpa A, Quinn AM, Ferro-Novick S. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol Biol Cell. 1999;10:3317–3329. doi: 10.1091/mbc.10.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Kozarsky KF, Segal M, Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid linked carbohydrate chains. J Cell Biol. 1986;102:1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufman O, Hong W, Lev S. The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J Cell Biol. 2011;194:459–472. doi: 10.1083/jcb.201102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin V, Sztul E. Golgi tethering factors. Biochimica Et Biophysica Acta-Molecular Cell Research. 2005;1744:325–339. doi: 10.1016/j.bbamcr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- Moore ER, Fischer ER, Mead DJ, Hackstadt T. The Chlamydial Inclusion Preferentially Intercepts Basolaterally Directed Sphingomyelin-Containing Exocytic Vacuoles. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Mead DJ, Dooley CA, Sager J, Hackstadt T. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology. 2011;157:830–838. doi: 10.1099/mic.0.045856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead AR, Rzomp KA, Scidmore MA. The Rab6 effector Bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect Immun. 2007;75:781–791. doi: 10.1128/IAI.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Ungar D, Hughson FM, Krieger M. The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins. Mol Biol Cell. 2004;15:2423–2435. doi: 10.1091/mbc.E03-09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prantner D, Nagarajan UM. Role for the chlamydial type III secretion apparatus in host cytokine expression. Infect Immun. 2009;77:76–84. doi: 10.1128/IAI.00963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Fay MJ, Lamar PC, Pearson CA, Sigar I, Ramsey KH. Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequesters beta-catenin in human cervical epithelial cells. Infect Immun. 2002;70:2605–2613. doi: 10.1128/IAI.70.5.2605-2613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram RJ, Li B, Kaiser CA. Identification of sec36p, sec37p, and sec38p: components of yeast complex that contains sec34p and sec35p. Mol Biol Cell. 2002;13:1484–1500. doi: 10.1091/mbc.01-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000615. e1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DD, Scidmore MA, Bannantine JP, Brown WJ. Proteins in the chlamydial inclusion membrane. Microbes Infect. 2002;4:333–340. doi: 10.1016/s1286-4579(02)01546-0. [DOI] [PubMed] [Google Scholar]
- Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun. 2003;71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, Ferro-Novick S. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell. 2001;7:433–442. doi: 10.1016/s1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections--past, present, future. J Am Vet Med Assoc. 1989;195:1501–1506. [PubMed] [Google Scholar]
- Scidmore MA, Rockey DD, Fischer ER, Heinzen RA, Hackstadt T. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect Immun. 1996;64:5366–5372. doi: 10.1128/iai.64.12.5366-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakova A, Zolov S, Lupashin V. COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic. 2006;7:191–204. doi: 10.1111/j.1600-0854.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Shestakova A, Suvorova E, Pavliv O, Khaidakova G, Lupashin V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J Cell Biol. 2007;179:1179–1192. doi: 10.1083/jcb.200705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- Smith RD, Willett R, Kudlyk T, Pokrovskaya I, Paton AW, Paton JC, Lupashin VV. The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic. 2009;10:1502–1517. doi: 10.1111/j.1600-0854.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, Oda K. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic. 2010;11:1552–1566. doi: 10.1111/j.1600-0854.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Spelbrink RG, Nothwehr SF. The yeast GRD20 gene is required for protein sorting in the trans- Golgi network/endosomal system and for polarization of the actin cytoskeleton. Mol Biol Cell. 1999;10:4263–4281. doi: 10.1091/mbc.10.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessott W, Uliana A, Maccioni HJ. Cog2 null mutant CHO cells show defective sphingomyelin synthesis. J Biol Chem. 2010;285:41472–41482. doi: 10.1074/jbc.M110.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Shestakova A, Hunt L, Sehgal S, Lupashin V, Storrie B. Rab6 regulates both ZW10/RINT-1 and conserved oligomeric Golgi complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18:4129–4142. doi: 10.1091/mbc.E07-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova ES, Kurten RC, Lupashin VV. Identification of a human orthologue of Sec34p as a component of the cis-Golgi vesicle tethering machinery. Journal of Biological Chemistry. 2001;276:22810–22818. doi: 10.1074/jbc.M011624200. [DOI] [PubMed] [Google Scholar]
- Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. Journal of Cell Biology. 2002;157:631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai G, Lu L, Wang TL, Tang BL, Goud B, Johannes L, Hong W. Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network. Mol Biol Cell. 2004;15:4011–4022. doi: 10.1091/mbc.E03-12-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar D, Oka T, Brittle EE, Vasile E, Lupashin VV, Chatterton JE, Heuser JE, Krieger M, Waters MG. Characterization of a mammalian Golgi-localized protein complex COG that is required for normal Golgi morphology and function. Journal of Cell Biology. 2002;157:405–415. doi: 10.1083/jcb.200202016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16:113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- VanRheenen SM, Cao X, Lupashin VV, Barlowe C, Waters MG. Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J Cell Biol. 1998;141:1107–1119. doi: 10.1083/jcb.141.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen SM, Cao X, Sapperstein SK, Chiang EC, Lupashin VV, Barlowe C, Waters MG. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J Cell Biol. 1999;147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Malhotra V. The organisation of the Golgi apparatus. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Whyte JR, Munro S. The Sec34/35 Golgi Transport Complex Is Related to the Exocyst, Defining a Family of Complexes Involved in Multiple Steps of Membrane Traffic. Developmental Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Wolf K, Hackstadt T. Sphingomyelin trafficking in Chlamydia pneumoniae-infected cells. Cell Microbiol. 2001;3:145–152. doi: 10.1046/j.1462-5822.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Steet RA, Bohorov O, Bakker J, Newell J, Krieger M, Spaapen L, Kornfeld S, Freeze HH. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10:518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. Journal of Cell Biology. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]