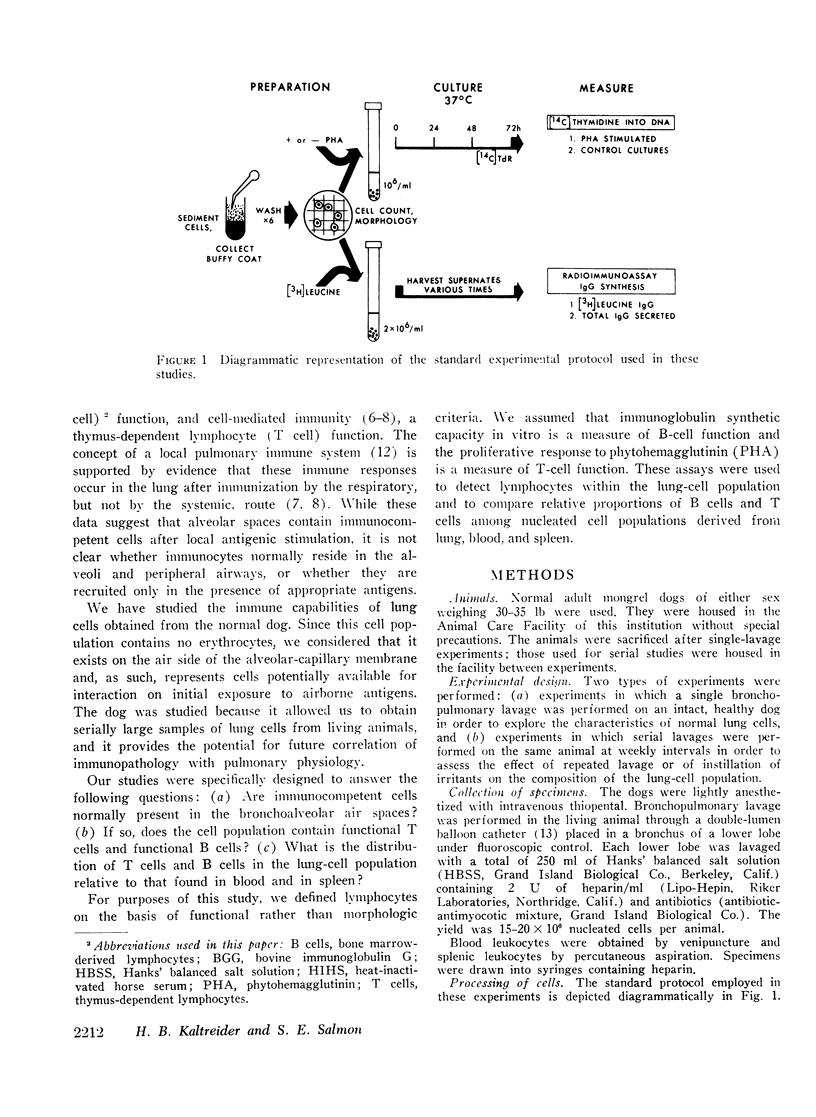
Abstract
While the alveolar macrophage has been studied extensively, little attention has been directed toward the immune functions of the bronchoalveolar lymphocyte. These cells, obtained by bronchopulmonary lavage of the normal canine lung, are derived from the air side of the alveolar-capillary membrane of the lower respiratory tract. The distribution of lymphocyte types within the bronchoalveolar cell population was determined and compared with that of leukocytes from blood and spleen.
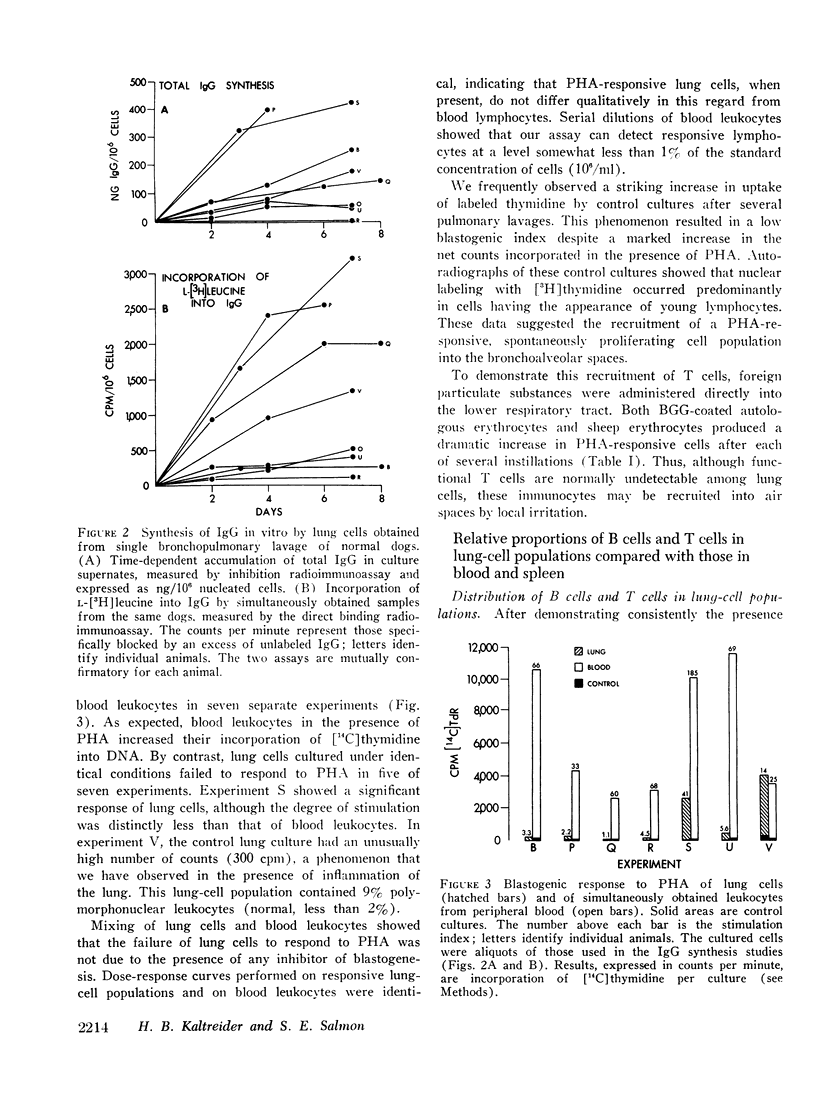
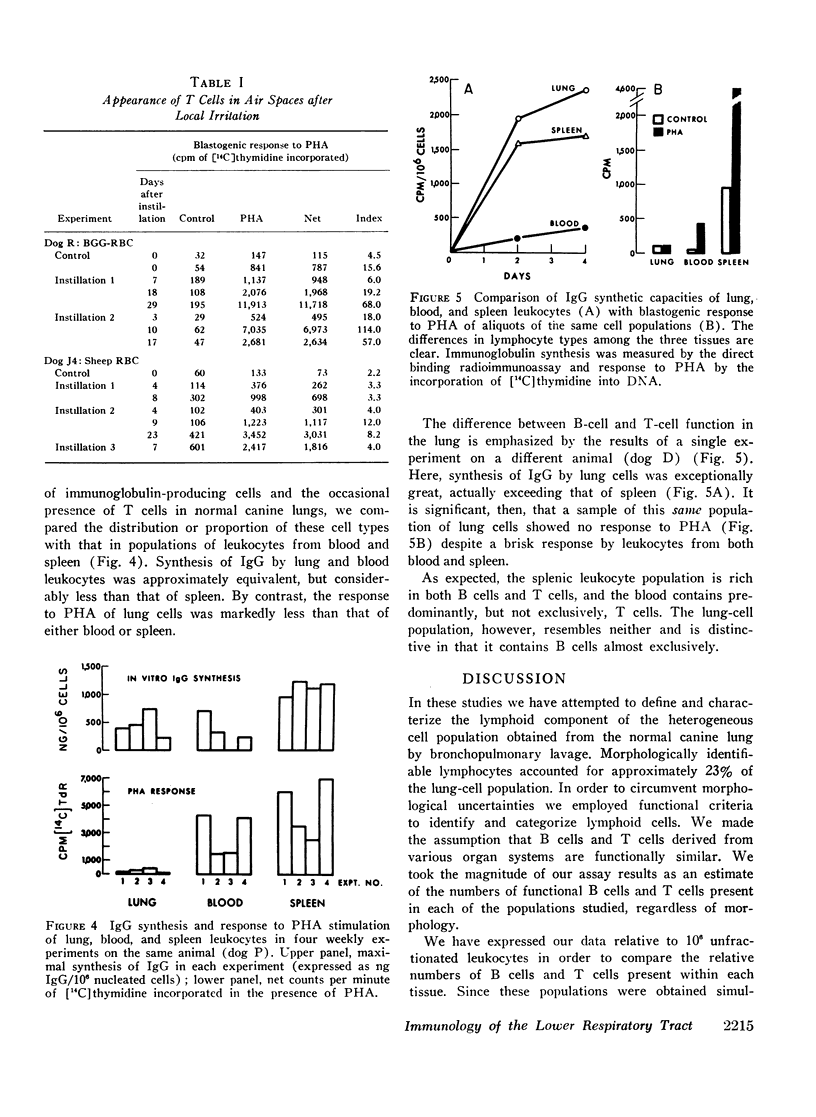
IgG synthesis in vitro was used as a measure of bone marrow-derived lymphocyte (B cell) function, and the blastogenic response of cell cultures to phytohemagglutinin was used as a measure of the presence or absence of thymus-dependent lymphocytes (T cells). De novo synthesis of IgG by bronchoalveolar cells was demonstrated consistently by two independent radio-immunoassays. Therefore, B cells are present in the air spaces of normal canine lungs. T cells were generally not detectable in aliquots of the same cell populations but could be recruited into alveolar spaces after local irritation.
The distribution of lymphocyte types within the bronchoalveolar cell population is unique and distinctly different from that of blood and spleen. The spleen is rich in both T cells and B cells, blood is rich in T cells but poor in B cells, whereas the lung lacks T cells and contains substantial numbers of immunoglobulin-producing B cells. The findings indicate that bronchoalveolar lymphocytes do not reflect simply the lymphoid composition of peripheral blood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Finley T. N., Swenson E. W., Curran W. S., Huber G. L., Ladman A. J. Bronchopulmonary lavage in normal subjects and patients with obstructive lung disease. Ann Intern Med. 1967 Apr;66(4):651–658. doi: 10.7326/0003-4819-66-4-651. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Green G. M. Pulmonary clearance of infectious agents. Annu Rev Med. 1968;19:315–336. doi: 10.1146/annurev.me.19.020168.001531. [DOI] [PubMed] [Google Scholar]
- Green G. M. The J. Burns Amberson Lecture--in defense of the lung. Am Rev Respir Dis. 1970 Nov;102(5):691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Holub M., Hauser R. E. Lung alveolar histiocytes engaged in antibody production. Immunology. 1969 Aug;17(2):207–226. [PMC free article] [PubMed] [Google Scholar]
- Leikola J., Fudenberg H. H., Vyas G. N., Perkins H. A. Isoantibodies to human IgM: serologic and immunochemical investigations. J Immunol. 1971 May;106(5):1147–1153. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- McCombs R. P. Diseases due to immunologic reactions in the lungs (first of two parts). N Engl J Med. 1972 Jun 1;286(22):1186–1194. doi: 10.1056/NEJM197206012862205. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Mackey G., Fudenberg H. H. "Sandwich" solid phase radioimmunoassay for the quantitative deterimination of human immunoglobulins. J Immunol. 1969 Jul;103(1):129–137. [PubMed] [Google Scholar]
- Salmon S. E., Smith B. A. Sandwich solid phase radioimmunoassays for the characterization of human immunoglobulins synthesized in vitro. J Immunol. 1970 Mar;104(3):665–672. [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasi T. B., Jr Secretory immunoglobulins. N Engl J Med. 1972 Sep 7;287(10):500–506. doi: 10.1056/NEJM197209072871008. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]


