Abstract
Recent epidemiological studies show a strong reduction in the incidence of Alzheimer's disease in patients treated with cholesterol-lowering statins. Moreover, elevated Aβ42 levels and the ɛ4 allele of the lipid-carrier apolipoprotein E are regarded as risk factors for sporadic and familial Alzheimer's disease. Here we demonstrate that the widely used cholesterol-lowering drugs simvastatin and lovastatin reduce intracellular and extracellular levels of Aβ42 and Aβ40 peptides in primary cultures of hippocampal neurons and mixed cortical neurons. Likewise, guinea pigs treated with high doses of simvastatin showed a strong and reversible reduction of cerebral Aβ42 and Aβ40 levels in the cerebrospinal fluid and brain homogenate. These results suggest that lipids are playing an important role in the development of Alzheimer's disease. Lowered levels of Aβ42 may provide the mechanism for the observed reduced incidence of dementia in statin-treated patients and may open up avenues for therapeutic interventions.
Apart from age, environmental factors have only slight influence on the incidence of Alzheimer's disease (AD). Very recently, two independent reports showed a strong decrease in the incidence of AD and dementia for patients that were treated with statins (1, 2). Both studies were retrospective, and statins were not given in any relation to dementia. Usually statins are prescribed for treatment of elevated serum cholesterol levels in patients. They reduce cholesterol levels by inhibiting the bottleneck enzyme of cholesterol synthesis, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. They are widely used drugs, well characterized and considered to be very safe for long-time treatment, and approved for use in elderly patients (3, 4).
The ɛ4 allele of the apolipoprotein E (apoE) is the major genetic risk factor for AD (5). Several lines of evidence indicate that apoE ɛ4 and statins have a related influence on AD. The normal cellular function of apoE is uptake and delivery of lipids. The isoform apoE ɛ4 correlates with an increased risk for atherosclerosis (6) and amyloid plaque formation (7). Moreover, elevated cholesterol uptake increases amyloid plaque formation or amyloid deposition (8, 9). This correlation may be extended to Aβ production, since cellular cholesterol levels affect neuronal Aβ production in vitro (10). Initially, Aβ has been a focus of AD research, because it was found to be the major constituent of the amyloid plaque. It is unknown whether the amyloid plaque is actively involved in the neurodegenerative process in AD or instead is a consequence of the disease process. More recently, however, Aβ has been a focus of AD research not because of it presence in the amyloid plaque, but because an overproduction of a minor Aβ isoform, Aβ42, is linked to all identified inherited forms of AD (11–13).
Aβ is produced during normal cellular processing of the Alzheimer amyloid precursor protein (APP) (14) by β-secretase and γ-secretase (15). While the majority of all Aβ isoforms produced is Aβ40, ≈10% of total Aβ consists of the two-amino-acid longer Aβ42 (11). Two neuronal Aβ42 and Aβ40 pools are known, secretory and intracellular Aβ (16–18). Familial AD mutations increase both Aβ pools (19), but the relative contribution to AD pathogeneses is not known (20).
Here we used simvastatin and lovastatin alone or in combination with methyl-β-cyclodextrin (CDX), which physically removes cholesterol from the plasma membrane, to investigate their potential to reduce intracellular and secretory Aβ levels, in particular Aβ42, and to address our hypothesis that statins in vivo may reduce the risk of developing AD by reducing Aβ42 production.
Our results demonstrate that cholesterol-lowering drugs and the cholesterol-extracting toxin CDX strongly reduce intracellular and secretory neuronal Aβ42 and Aβ40 levels in vitro, and that administration of simvastatin to guinea pigs strongly reduces cerebral Aβ levels, including the Aβ42 isoform.
Materials and Methods
Cell Culture.
Hippocampal neurons were prepared from 17-day-old fetal rats as described (21). Mixed cortical neurons were prepared and cultivated by using the same protocol, except that mixed cortical neurons from 14-day-old fetal rats were used (22). After 5 days in culture, 4 μM lovastatin (Calbiochem) or simvastatin (a gift from Merck Sharp and Dohme, Rahway, NJ) and 0.25 mM mevalonate (Sigma) were added for 48 h or 72 h. Recombinant Semliki Forest virus (SFV) encoding human APP695 was prepared as described previously (23). Neurons were infected for 60 or 90 min at 37°C under a 5% CO2/95% air atmosphere with recombinant SFV-APP695. The virus solution was replaced by maintenance medium and cells were incubated for 2 h. With the original protocol (10), we found that Aβ levels were too highly reduced in treated cells to properly quantify intracellular Aβ42 levels. Therefore, cells were exposed for 10 min instead of 20 min to 5 mM CDX (Sigma). This short extraction time removes less cholesterol and Aβ levels are less reduced, thus allowing us to quantify the effect of cholesterol depletion in regulating Aβ40 and Aβ42 levels with higher fidelity. After an additional 3.5–4.5 h of incubation in medium, cells were harvested. This treatment time is well within the nontoxic window for SFV infection. Control cells were processed in parallel in the absence of CDX and statin.
Protein Analysis.
Monoclonal antibodies G2–10 (2 μg/ml) and G2–11 (4 μg/ml) specific for Aβ40 and Aβ42, respectively, were used for immunoprecipitation. W0–2 monoclonal antibody directed against amino acids 4–10 of human Aβ was used for Western blot detection (24). W0–2 does not cross-react with rat Aβ but does cross-react with guinea pig Aβ, which is identical to human Aβ. Rabbit polyclonal antibodies used were B7/6 (1:500) against Aβ-1–16, B12/4 (1:1000) against the cytosolic domain of APP (10), and anti-FdAPP (1:750) directed against the ectodomain of APP (25). Cell culture media were collected and cell extracts were prepared in 2% Nonidet P-40/0.2% SDS/5 mM EDTA supplemented with complete protease inhibitor (Roche). In our experiments, relative intracellular Aβ levels in cell lysates are ≈4/5 Aβ40 and 1/5 Aβ42. These cell lysates were divided differently for Aβ42 (79%) and Aβ40 (19%) immunoprecipitation, and 20 μl (2%) was saved for direct loading of cell lysates for detection of APP and total Aβ. The conditioned media were divided 90% for Aβ42 and 10% for Aβ40 for immunoprecipitation. A 15-μl sample (1%) was saved for direct loading of conditioned media for detection of APP and total Aβ. This procedure results in approximately equal intensities for all Aβ forms analyzed. Quantitative immunoprecipitation was performed according to Schröder et al. (26), and precipitates were analyzed on Tris-Tricine 10–20% polyacrylamide gels (Novex). Quantitative Western blotting was performed as described (26, 27), except that 3% milk powder instead of BSA was used for blocking and W0–2 antibody (1 μg/ml) was used for detection of the G2–11 and G2–10 precipitates to facilitate a greater linear range and better detection levels and to avoid the detection of endogenous rat Aβ40, Aβ42, and APP. Quantification was performed by using MACBAS 2 software with either a PhosphorImager or densitometry scans from Western blots, in the latter case calibrated against control Aβ made of different volumes of an Aβ-rich conditioned cell culture medium. The calibration standard was applied to all Western blots.
Metabolic Labeling.
After treatment of the SFV-infected and lovastatin-pretreated cells for 10 min with 5 mM CDX, cells were labeled for 2.5 h with 150 μCi of [35S]methionine (Amersham Pharmacia; 1 Ci = 37 GBq) in methionine-free medium (MEM with 1/10 N2 supplement; GIBCO/BRL). For the determination of total protein biosynthesis rate, carefully washed cell lysates of treated and untreated cells were applied on SDS/polyacrylamide gels, and total radioactivity incorporated was determined by using a PhosphorImager and MACBAS 2 software.
Infection rate was determined by immunocytochemical analysis of treated vs. untreated acetone-fixed cells and stained for SFV proteins (anti-SFV 2–6, gift from Kai Simons, Max-Planck-Institute, Dresden, Germany) and myc-tagged APP (anti-myc 9E10, Upstate Biotechnology). As counterstaining, anti-calnexin (Transduction Laboratories) was used. Subsequently, neurons were microscopically counted (Zeiss LSM510 confocal microscope) and the numbers of infected neurons were determined.
All results are reported as mean ± SD. For nonparametric statistical analysis of changes compared with baseline values the Wilcoxon rank test was used.
Animals.
Adult male guinea pigs (Dunkin Hartley, 400 g) were obtained from Harlan-Winkelmann (Borchen, Germany), maintained in a 6 p.m.–6 a.m. dark and 6 a.m.–6 p.m. light alternating cycle and had free access to both water and food. Animals were fed with a standard laboratory chow diet supplemented with or without 0.5% simvastatin. Animals were weighted and food intake was controlled for all animals on a daily basis. The average weight of the animals over the course of the experiment was 485 g.
For collecting cerebrospinal fluid (CSF) samples the animals were anesthetized by an intraperitoneal injection of 60 mg/kg body weight ketamine (Hostaket; Hoechst) and 3 mg/kg body weight xylazine (Rompun 2%; Bayer) followed by further relaxation with ether. After shaving and disinfection of the neck, CSF puncture was performed by carefully insertion of a cannula (Microlance 3 REF300900, Becton Dickinson) with a 1-ml syringe between the spinous process of the first and second cervical vertebrae. After the cannula passed through the skin a small negative pressure was created in the syringe before the cannula was pushed deeper until CSF streamed into the syringe.
At the end of each experiment, trunk blood was collected from killed animals. One hemisphere of the guinea pig brain of each animal was used to determine the Aβ tissue levels. Aβ was precipitated with W02 antibody from detergent-lysed cortex samples, followed by W02 Western blotting.
Clinical Chemistry.
To estimate the range of cholesterol levels, total cholesterol was quantified by using the CHOD-PAP method (Roche Diagnostics) in the blood samples obtained at the end of each experiment. Alanine aminotransferase (ALT; EC 2.6.1.2), creatine kinase (CK; EC 2.7.3.2), creatine, cholesterol, and triglycerides in the serum of guinea pigs were measured on a fully automated Dade Behring Dimension RxL System analyzer with flex reagent cassette.
Brain lathosterol and cholesterol were extracted from homogenized guinea pig brain according to Folch et al. (28), incubated at 37°C for 60 min, reduced under N2 atmosphere, dissolved in isopropyl alcohol, and kept frozen at −20°C until analysis. Concentrations of cholesterol were estimated by the CHOD-PAP method and, for the more precise determinations, by GLC (28, 29).
As an internal standard, 50 μg of 5-α-cholestane was added to 500 μl of the lipid extract containing neutral sterols such as cholesterol and lathosterol. The samples were evaporated under nitrogen gas. After alkaline hydrolysis with 1 ml of 1 M NaOH in 90% ethanol for 120 min at 50°C, the solution was neutralized with phosphoric acid (50%, vol/vol) and the sterols were extracted twice with 4 ml of cyclohexane. Solvents were evaporated and sterols were trimethylsilylated. Neutral sterols were separated on an Ultra2 fused silica capillary column (Hewlett–Packard). GC flame ionization was performed in an HP5890 chromatograph. The initial temperature was 150°C for 3 min, and the temperature was increased at a rate of 30°C/min to 290°C. One microliter was injected by an automated liquid injector (Hewlett–Packard) in splitless mode at 280°C. Hydrogen was used as carrier gas with an inlet pressure of 10 psi (69 kPa), and flame ionization detection was done at 280°C.
Results
Effect of Cholesterol Depletion on Aβ Secretion.
We investigated whether cholesterol depletion would affect secretion of Aβ40 and Aβ42 in neurons. We expressed the human APP gene in primary rat hippocampal neurons by infection with SFV encoding the human APP (SFV-APP) (23, 30–32). Cholesterol was depleted from hippocampal neurons by two different strategies. First, de novo synthesis of cholesterol was inhibited by lovastatin or simvastatin. Biochemical pathways for nonsteroidal products were allowed to proceed by supplementing cells with mevalonate in the culture medium (33, 34). Second, plasma membrane cholesterol was extracted by CDX, which is very efficient in selectively and rapidly extracting cholesterol from the plasma membrane (35). Apart from cholesterol reduction, treatment resulted in reduced Aβ secretion. SFV-APP expression and total APP levels including endogenous APP and cell viability remained unaffected in both treated and control cells during the time course of our experiments as reported (10). Equally, no differences were found in total protein biosynthesis rate, as determined by [35S]methionine incorporation, or SFV infection rate, as assayed by the expression of SFV proteins and expression of myc-tagged APP, analyzed by quantitative immunocytochemistry (infection rates: 87% ± 8.4% untreated; 80% ± 7% treated; n > 85 for both groups, no significant difference between the two groups). SFV protein uptake by almost all neurons was found in both groups. These results corroborate previous findings (36) that cholesterol-dependent Aβ production does not depend on the use of SFV systems.
Aβ40 and Aβ42 isoforms were immunoprecipitated from conditioned media by using either Aβ40- or Aβ42-specific monoclonal antibodies, G2–10 and G2–11 (27), respectively. Because Aβ42 is present in lower amounts, higher sample volumes were used for Aβ42 precipitation to reliably quantify relative levels. To avoid detection of endogenous rat Aβ produced by rat hippocampal neurons, human Aβ was then detected with a human APP/Aβ-specific antibody (W0–2). Aβ levels were quantified against Aβ standards. Cholesterol depletion by lovastatin and CDX decreased Aβ40 levels to 50% ± 13% and Aβ42 levels dropped to 29% ± 10% in comparison to untreated control cells (n = 9, Fig. 1c). Similarly, intracellular Aβ40 levels decreased upon combined lovastatin and CDX treatment to 42 ± 18% and intracellular Aβ42 levels decreased to 33 ± 15% as compared with control cells (n = 7, Fig. 1c). Total human APP695 production was slightly reduced in treated cells (6% reduction) as compared with control cells (n = 4), this reduction, however, was not statistically significant.
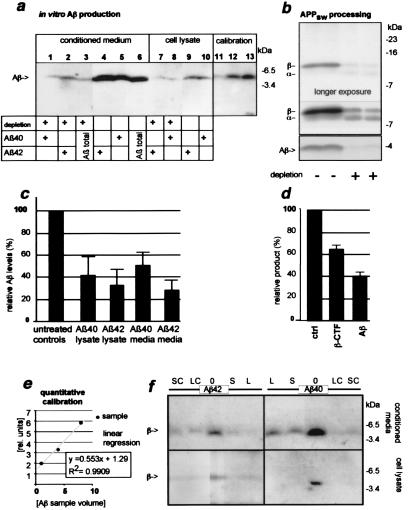
Figure 1.
Cholesterol depletion reduces secretory and intracellular Aβ levels in hippocampal neurons expressing SFV-APP695 or the “Swedish” mutant SFV-APP695sw. Hippocampal neurons were grown for 2 days in the presence of lovastatin/mevalonate. Next, cells were infected with SFV-APP695 or SFV-APP695sw and were treated after infection with 5 mM CDX for 10 min; control cells were not treated. After an additional 3.5 h, cells were lysed and whole-cell lysates were analyzed. (a) Reduced Aβ levels from APP695-expressing cells. Immunoprecipitation and Western blot analysis of Aβ from untreated cells and lovastatin/mevalonate- and CDX-treated cells (depletion). Lanes 1, 5, 8, and 10 show Aβ40 precipitates (antibody G2–10); lanes 2, 4, and 7 show Aβ42 precipitates (antibody G2–11); and lanes 3 and 6 show Aβ from directly loaded conditioned media. Intracellular Aβ was below detection limit without immunoprecipitation. Please note that different sample volumes were loaded for Aβ40 and Aβ42. Lanes 11–13 show calibration Aβ samples for quantification. Aβ was detected with human anti-Aβ-1–16 (W02). A strong reduction of secreted Aβ is observed in lanes 1–3 and 7 and 8 as compared with untreated cells in lanes 4–6 and 9 and 10. (b) Reduced Aβ levels and effect on C-terminal fragments from APP695sw. Cells were metabolically labeled for 2.5 h after infection. The lower gel shows reduced levels of total secretory Aβ from treated vs. untreated cells (immunoprecipitated with antibody B7/6). The upper gel shows immunoprecipitated APP C-terminal fragments from cell homogenates detected by antibody B12/4. Fragments presumably generated by α- and β-secretase cleavage are indicated. Unlike peripheral cells, hippocampal neurons prefer β-secretase-mediated cleavage of APP over α-secretase-mediated cleavage; this effect is enhanced by the use of the preferentially β-secretase-cleaved APP695sw (APPsw). Upon cholesterol depletion, levels of β-cleaved fragment were highly reduced, indicating an inhibition of β-secretase activity. (c) Quantification of intracellular and secretory Aβ40 and Aβ42 levels (n = 7, n = 9, respectively). (d) Quantification of APP695sw C-terminal fragments and secretory Aβ (n = 7, n = 9, respectively). (e) Quantitative calibration standard used for Western blot detection, lanes 11–13 in a. (f) Lovastatin and simvastatin without CDX are sufficient to reduce Aβ levels from primary mixed cortical neurons. Mixed cortical neurons were grown for 3 days in the presence of lovastatin or simvastatin (S or L) and mevalonate. After infection with SFV-APP695, cells were treated with 5 mM CDX for 10 min (SC or LC), not treated with CDX (S or L), or left untreated (0). After further 3.5 h cells were lysed. (Upper) Aβ40 or Aβ42 detected from conditioned media. (Lower) Aβ40 or Aβ42 detected from cell lysates (n = 6). A strong reduction of intracellular and secretory Aβ40 and Aβ42 was observed.
Familial AD Mutation.
In familial AD an overproduction of Aβ or Aβ42 has been observed. To analyze whether cholesterol reduction might reduce Aβ production from mutated APP we analyzed “Swedish” APP (APP695sw). The Swedish APP mutation results in increased β-cleavage of APP and subsequently leads to a strong overproduction of Aβ42 and Aβ40 and to AD (37). To test whether Swedish APP processing is sensitive to cellular cholesterol levels, we infected primary rat hippocampal neurons with SFV-APP695sw, and Aβ was immunoprecipitated from the cultured medium with antibody B7/6 directed against Aβ-1–16. Treatment resulted in a strong reduction in Aβ secretion in cholesterol-depleted neurons expressing human APP695sw as compared with control cells (39.8% ± 3.5%; n = 5, Fig. 1d). The β-fragment is the ultimate precursor of Aβ. In treated cells, a 12-kDa APP fragment, presumably the β-cleaved fragment, decreased while a 10-kDa fragment, presumably the α-secretory fragment, increased in amount (Fig. 1). These results suggest that cholesterol depletion affects β-cleavage of APP695sw while allowing α-cleavage to occur.
Statins Reduce Aβ Production in the Absence of CDX.
CDX and statins deplete cellular cholesterol by different mechanisms. The toxin CDX cannot be used for in vivo experiments. To investigate the in vivo Aβ-reducing potential of statins it was therefore important to determine whether lovastatin alone is sufficient to suppress Aβ production. Albeit hippocampal neurons are very important for brain function, they are a minority of total brain neurons. To avoid analyzing effects limited to hippocampal neurons, we used rat mixed cortical neurons rather than hippocampal neurons. Cells were treated for 3 days, instead of the 2 days used before, with lovastatin or simvastatin alone and were compared with control cells that were either untreated or treated together with statins and CDX. As in the previous experiments, the Aβ levels were analyzed in the cell lysate and in the culture medium. We found that Aβ production by mixed cortical neurons was lower than by that by hippocampal neurons. Lovastatin treatment with or without CDX reduced intracellular and extracellular Aβ42 and Aβ40 levels so strongly that it could not be quantified within our linear detection range (n = 6, Fig. 1f). When simvastatin was used instead of lovastatin similar results were obtained (n = 6, Fig. 1f). These data indicate that simvastatin and lovastatin may be used in vivo without CDX to lower Aβ levels, an important precondition for in vivo analysis.
Simvastatin Reduces Aβ Levels in Vivo.
To investigate the therapeutic potential of cholesterol reduction in vivo we studied the effect of simvastatin on Aβ levels in guinea pigs. Guinea pigs were used as the animal model in this study because they offer advantages over mice or rats. In guinea pigs higher CSF volumes are available and serial samples can be collected without sacrificing the animal. This availability enabled us to monitor Aβ levels during treatment instead of being limited to a single data point at the end of the experiment, and longitudinal studies in several single individual animals provide within-animal controls, which are more reliable than cross-sectional data. Furthermore, recovery of initial Aβ levels can be monitored easily after drug removal. Importantly, guinea pigs are well-established animals for lipid physiology studies as they offer several advantages over the use of mice because they are more closely related to the human situation (38–41). Unlike mice or rats, guinea pigs have the human Aβ sequence. In comparison to most transgenic animals, guinea pigs will reflect Aβ production by cell types that express endogenous APP levels. The major disadvantage of this model is that amyloid plaque formation cannot be monitored. We regarded this lack as minor compared with the other advantages of this model, because a reduced Aβ42 production rate results in reduced plaque formation in APP transgenic mice and the correlation of Aβ42 production and AD is very well documented for familial AD. Furthermore, the influence of cholesterol on plaque formation in animals is established, but the mechanism for this plaque formation in vivo is unclear (8, 9).
To achieve readily quantifiable changes in Aβ levels, animals were exposed to a high dosage simvastatin diet (0.5% of diet) for 3 weeks (42). Treated and control animals were pair fed. Animals removed similar amounts of food from the storage container (control animals, 38.3 ± 1.0 g/day; treated animals, 44.8 ± 6.5 g/day; 3-week animal data). More important, however, is weight gain, because guinea pigs do not eat all of the food they remove from the food container. We calculated that the animals ate ≈25 g/day, corresponding to a simvastatin uptake dose of ≈250 mg per kg. In the unlikely case that the animals used all of the food supplied a maximum uptake of 448 mg/kg could be achieved. For comparison, the maximum dose of simvastatin approved for human use is 80 mg/day or ≈1.1 mg/kg, equivalent to a 227–407 times lower dose than applied in our short-time animal experiments. All animals gained weight during the course of the experiment (control animals, 33.8% ± 3.5%; treated animals, 22.8% ± 1.9%; 3-week animal data). At the end of the experiments plasma cholesterol levels were determined. As expected, simvastatin treatment strongly reduced plasma cholesterol concentrations in guinea pigs (Table 1). Total brain cholesterol levels were not significantly reduced, which was expected because of its long half-life. However, the ratio of the cholesterol precursor lathosterol to cholesterol was significantly reduced (controls: cholesterol 12.0 ± 2.94 mg/g, lathosterol 108 ± 55 μg/g, ratio 9.27 ± 4.49; treated animals: cholesterol 13.1 ± 2.82 mg/g, lathosterol 56 ± 17 μg/g, ratio 4.2 ± 0.57; P = 0.003 for lathosterol and P = 0.001 for the ratio thereof).
Table 1.
Serum cholesterol, markers of hepatic and renal injury, and creatine kinase in guinea pigs treated with simvastatin or control diet
| Treatment | Cholesterol, mg/dl | Triglycerides, mg/dl | ALT, units/liter | Creatine kinase, units/liter | Creatinine, mg/dl |
|---|---|---|---|---|---|
| Control diet | 52.0 ± 8.7 | 100.0 ± 61.9 | 31.5 ± 9.6 | 159.0 ± 43.9 | 0.44 ± 0.02 |
| Simvastatin, 3 wk | 8.5 ± 2.2* | 63.0 ± 24.7 | 59.8 ± 14.7* | 414.8 ± 348.4 | 0.56 ± 0.0 |
| Simvastatin, 3 wk and washing-out for 3 wk | 43.5 ± 17.6 | 149.6 ± 94.6 | 21.5 ± 12.5 | 129.1 ± 43.4 | 0.57 ± 0.17 |
ALT, alanine aminotransferase; *, significant at α = 0.05 (after Bonferroni correction).
This finding strongly indicates that simvastatin treatment reduced de novo brain cholesterol synthesis (43). Markers indicative of muscle, renal, and hepatic toxicity showed alterations in the expected range. Creatine levels remained unchanged, whereas creatine kinase activity increased and alanine aminotransferase activity also increased transiently upon treatment (Table 1). Changes in animal behavior were not evident. At the end of the experiments all animals were subject to patho-anatomical examination and no macroscopic alterations were found.
The in vivo effects of simvastatin treatment on Aβ levels were analyzed by collecting two CSF samples per week. Samples from each week were pooled to gain higher volumes for quantification. A minimum volume (≈100 μl) of CSF was removed to avoid cerebral damage. Quantitative Western blotting was performed as in the previous experiments. Baseline CSF Aβ levels were determined from samples collected before treatment. We first examined the influence on total Aβ levels in animals that were treated for 3 weeks and then were placed back on simvastatin-free diet. Aβ levels declined continuously during treatment and then returned to normal values (Aβ, 60.1% ± 12.7% at the end of treatment, P = 0.004; Aβ, 87.9% ± 28.7% after recovery, no significant difference from control animals; n = 5) (Fig. 2a). In independent experiments Aβ40 and Aβ42 levels were determined (6 treated and 6 control animals). Animals were treated exactly as before for 3 weeks. Aβ levels decreased over the entire period of the experiment and were maximally decreased at the end of treatment (Aβ40, 46.6% ± 8.5%, P = 0.002; Aβ42, 62.32% ± 19.4%, P = 0.009) (Fig. 2b).
Figure 2.
In vivo treatment of guinea pigs with simvastatin reduced total Aβ, Aβ40, and Aβ42 levels in guinea pig CSF. Aβ levels recovered after treatment was stopped. CSF was collected at day 0 and 3 from guinea pigs. After this, animals were given a simvastatin-containing diet and CSF samples were drawn twice weekly. (a) After 3 weeks simvastatin-containing food was replaced by standard diet. Aβ levels declined for the first 3 weeks and recovered subsequently. In control animals Aβ levels remained constant (n = 5). (b) In a second experiment animals were treated as before for 3 weeks. A continuous decline in all Aβ levels was found. In control animals Aβ levels remained constant. For clarity, Aβ42 and Aβ40 levels of control animals are given in a and Aβ42 and Aβ40 level measurement was extended for control animals to 6 weeks as in the previous experiment (n = 6). (c) Western blot determination of CSF Aβ levels after 3 weeks of treatment. (d) Aβ tissue levels. Aβ was precipitated with W02 antibody from detergent-lysed cortex samples. Simvastatin-treated animals showed a strong reduction of brain tissue Aβ levels equivalent to the reduction observed in the CSF in a (n = 4). Mean level of control animals was set to 100% (±22% SD; n = 6). As expected from the CSF results, the recovery animals had normal Aβ levels (n = 6). The reduction in treated as compared with control animals was significant (P = 0.012).
To control for potential differences between CSF and brain-tissue Aβ levels at the end of treatments, one hemisphere of the guinea pig brain of each animal was used to determine brain-tissue Aβ levels. Simvastatin-treated animals showed a strong reduction of brain-tissue Aβ levels equivalent to the reduction observed in the CSF (Aβ, 54.6% ± 21.7%, P = 0.012; n = 4). Mean level of control animals was set to 100% (±22% SD; n = 6). As expected from the CSF results, the recovery animals had normal Aβ levels (n = 6), indicating that Aβ levels from CSF and brain tissue were equally affected (Fig. 2d).
Discussion
Low Aβ levels are considered protective for AD (Aβ42) and vascular dementia (Aβ40). Recent retrospective studies with human patients treated with statins for years show a drastically reduced risk for developing AD and dementia (1, 2). Here we have shown that statins may reduce the risk for AD by decreasing Aβ42 and Aβ40 levels. Aβ levels declined in vitro and in vivo upon statin treatment, and it is most likely that cholesterol influences APP-secretase activities. CDX alone has a marked effect on Aβ levels in hippocampal neurons (10). However, it is conceivable that cholesterol metabolites, such as cholesterol esters, or an imbalance in other lipids could be a critical factor in regulating APP-secretases. At present, we cannot exclude the possibility that statin side effects observed in humans (3, 4) might affect Aβ levels in our in vivo model. However, our data strongly indicate that cholesterol depletion or a change of lipid ratio is responsible for Aβ reduction in vitro. This is because Aβ levels were affected by two different cholesterol-reducing agents (statins and CDX) that achieve their effect through different and unrelated modes of action (cholesterol synthesis inhibition at the endoplasmic reticulum vs. physical removal of cholesterol from the plasma membrane). It, therefore, seems plausible that cholesterol depletion is also responsible for the reduced Aβ levels in vivo.
Cholesterol is important for protein trafficking (44), and the Aβ domain in APP has been identified to be important for axonal transport of APP (32). Furthermore, Aβ is an apical targeted peptide in cholesterol-depleted MDCK cells (45). It seems possible that Aβ production is sensitive to subtle changes in lipid-dependent proteolysis. An example of such a mechanism is the sterol concentration-sensitive mechanism of SCAP (SREBP cleavage activating factor), which translocates SREBP (sterol regulatory element binding protein) from the endoplasmic reticulum to the Golgi apparatus. The latter compartment contains a SREBP cleaving protease (46). However, the SREBP proteases are not identical to APP secretases (47). Moreover, Aβ generation has recently been shown to involve a specific lipid environment, as γ-secretase activity is sensitive to the detergent Triton X-100 but insensitive to CHAPS {3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate} (48).
Subtle increases in Aβ42 levels have been reported to be the likely cause for hereditary AD involving point mutations in the APP and presenilin genes (11, 12). This overproduction of Aβ42 is life-long and its effect presumably accumulates over time. Conversely, it can be speculated that even slightly decreased Aβ42 levels over many years are protective for AD and familial AD. The observed in vivo effects are very strong and were achieved by an experimental dosage that cannot be directly applied to humans. However, this was useful to achieve a readily measurable and significant effect over the time course of the study (weeks instead of years or decades as in AD and the human retrospective studies mentioned before). In accordance with the results from familial AD studies, the desired Aβ42 reduction in humans may very well be much smaller, allowing the use of a lower statin dose. Such long-term effects, however, preclude the direct analysis of Aβ42 production in animal models.
The results on the rare “Swedish” APP AD-causing mutation indicate in principle that cholesterol-lowering strategies might have therapeutic potential for “Swedish” familial AD, or familial AD in general, as well.
Our results show that Aβ levels can be regulated by treatment with cholesterol-lowering drugs, offer a potential mechanism for the protective effect of long-term statin treatment in AD, and raise the possibility that cholesterol-lowering strategies might be useful for the prevention of AD.
Acknowledgments
We thank I. Tomic and P. Samenfeld for excellent technical assistance and M. Hasan for editorial help. This work was supported in part by grants from the Alzheimer Forschung Initiative, the Deutsche Forschungsgemeinschaft through SFB 488, and the Bundesministerium für Bildung, Forschung, Wissenschaft und Technologie (01EC9402).
Abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- APP695sw
“Swedish” mutant of 695-aa APP
- CDX
methyl-β-cyclodextrin
- SFV
Semliki Forest virus
- CSF
cerebrospinal fluid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 5371.
References
- 1.Wolozin B, Kellman W, Ruosseau P, Celesia G G, Siegel G. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 2.Jick H, Zornberg G L, Jick S S, Seshadri S, Drachman D A. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen T R, Berg K, Cook T J, Faergeman O, Haghfelt T, Kjekshus J, Miettinen T, Musliner T A, Olsson A G, Pyorala K, et al. Arch Intern Med. 1996;156:2085–2092. [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group. Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 5.Corder E H, Saunders A M, Strittmatter W J, Schmechel D E, Gaskell P C, Small G W, Roses A D, Haines J L, Pericak-Vance M A. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 6.Hofman A, Ott A, Breteler M M, Bots M L, Slooter A J, van Harskamp F, van Duijn C N, Van Broeckhoven C, Grobbee D E. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 7.Bales K R, Verina T, Dodel R C, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone E M, Little S P, Cummins D J, et al. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 8.Sparks D L, Scheff S W, Hunsaker J C, 3rd, Liu H, Landers T, Gross D R. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 9.Refolo L M, Pappolla M A, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint G S, Sambamurti K, Duff K. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 10.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C G, Simons K. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younkin S G. Annu Neurol. 1995;37:287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- 12.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 13.St George-Hyslop P H. Biol Psychiatry. 2000;47:183–199. doi: 10.1016/s0006-3223(99)00301-7. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Lemaire H G, Unterbeck A, Salbaum J M, Masters C L, Grzeschik K H, Multhaup G, Beyreuther K, Muller Hill B. Nature (London) 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 15.Haass C, Schlossmacher M G, Hung A Y, Vigo Pelfrey C, Mellon A, Ostaszewski B L, Lieberburg I, Koo E H, Schenk D, Teplow D B, et al. Nature (London) 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 16.Wertkin A M, Turner R S, Pleasure S J, Golde T E, Younkin S G, Trojanowski J Q, Lee V M. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann T, Bieger S C, Bruhl B, Tienari P J, Ida N, Allsop D, Roberts G W, Masters C L, Dotti C G, Unsicker K, et al. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 18.Cook D G, Forman M S, Sung J C, Leight S, Kolson D L, Iwatsubo T, Lee V M, Doms R W. Nat Med. 1997;3:1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- 19.Wild Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C. J Biol Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann T. Eur Arch Psychiatry Clin Neurosci. 1999;249:291–298. doi: 10.1007/s004060050102. [DOI] [PubMed] [Google Scholar]
- 21.Tienari P J, Ida N, Ikonen E, Simons M, Weidemann A, Multhaup G, Masters C L, Dotti C G, Beyreuther K. Proc Natl Acad Sci USA. 1997;94:4125–4130. doi: 10.1073/pnas.94.8.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 23.de Hoop M J, Olkkonen V M, Ikonen E, Williamson E, von Poser C, Meyn L, Dotti C G. Gene Ther. 1994;1:S28–S31. [PubMed] [Google Scholar]
- 24.Jensen M, Hartmann T, Engvall B, Wang R, Uljon S N, Sennvik K, Naslund J, Muehlhauser F, Nordstedt C, Beyreuther K, et al. Mol Med. 2000;6:291–302. [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann T, Bergsdorf C, Sandbrink R, Tienari P J, Multhaup G, Ida N, Bieger S, Dyrks T, Weidemann A, Masters C L, et al. J Biol Chem. 1996;271:13208–13214. doi: 10.1074/jbc.271.22.13208. [DOI] [PubMed] [Google Scholar]
- 26.Schröder J, Pantel J, Ida N, Essig M, Hartmann T, Knopp M V, Schad L R, Sandbrink R, Sauer H, Masters C L, et al. Mol Psychiatry. 1997;2:505–507. doi: 10.1038/sj.mp.4000313. [DOI] [PubMed] [Google Scholar]
- 27.Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, Sandbrink R, Masters C L, Beyreuther K. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 28.Folch J, Lees M, Sloane-Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 29.Czubayko F, Beumers B, Lammsfuss S, Lütjohann D, von Bergmann K. J Lipid Res. 1991;32:1861–1867. [PubMed] [Google Scholar]
- 30.Turner R S, Suzuki N, Chyung A S C, Younkin S G, Lee V M Y. J Biol Chem. 1996;271:8966–8970. doi: 10.1074/jbc.271.15.8966. [DOI] [PubMed] [Google Scholar]
- 31.De Strooper B, Simons M, Multhaup G, Van Leuven F, Beyreuther K, Dotti C G. EMBO J. 1997;14:4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tienari P J, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters C L, et al. EMBO J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- 33.Rao S, Porter D C, Chen X, Herliczek T, Lowe M, Keyomarsi K. Proc Natl Acad Sci USA. 1999;96:7797–7802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langan T J, Volpe J J. J Neurochem. 1987;49:513–521. doi: 10.1111/j.1471-4159.1987.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld E B, Cooney A M, Pitha J, Dawidowicz E A, Dwyer N K, Pentchev P G, Blanchette-Mackie E J. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- 36.Frears E R, Stephens D J, Walters C E, Davies H, Austen B M. NeuroReport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- 37.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo Pelfrey C, Lieberburg I, Selkoe D J. Nature (London) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 38.Terpstra A H, Sanchez-Muniz F J, West C E, Woodward C J. Comp Biochem Physiol B. 1982;71:669–673. doi: 10.1016/0305-0491(82)90479-5. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez M, Merlos M, Adzet T, Laguna J C. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:311–316. doi: 10.1016/s0305-0491(97)00331-3. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen L B, Xu G, Shefer S, Tint G S, Batta A, Salen G. Metabolism. 1999;48:1542–1548. doi: 10.1016/s0026-0495(99)90243-3. [DOI] [PubMed] [Google Scholar]
- 41.Harden K K, Robinson J L. J Nutr. 1984;114:411–421. doi: 10.1093/jn/114.2.411. [DOI] [PubMed] [Google Scholar]
- 42.Horsmans Y, Desager J P, Harvengt C. Pharmacol Toxicol. 1990;67:336–339. doi: 10.1111/j.1600-0773.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 43.Miettinen T A, Tilvis R S, Kesaniemi Y A. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 44.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 45.Mizuno T, Haass C, Michikawa M, Yanagisawa K. Biochim Biophys Acta. 1998;1373:119–130. doi: 10.1016/s0005-2736(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 46.Brown M S, Goldstein J L. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita T, Chang T Y, Kodama T, Iwatsubo T. NeuroReport. 1998;9:911–913. doi: 10.1097/00001756-199803300-00027. [DOI] [PubMed] [Google Scholar]
- 48.Li Y M, Lai M T, Xu M, Huang Q, DiMuzio-Mower J, Sardana M K, Shi X P, Yin K C, Shafer J A, Gardell S J. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. . (First Published May 9, 2000, 10.1073/pnas.110126897) [DOI] [PMC free article] [PubMed] [Google Scholar]