Abstract
Investigations have outlined pancreatic secretory and synthetic responses to gastrointestinal hormones. However, there is little information concerning hormonal influences on pancreatic growth.
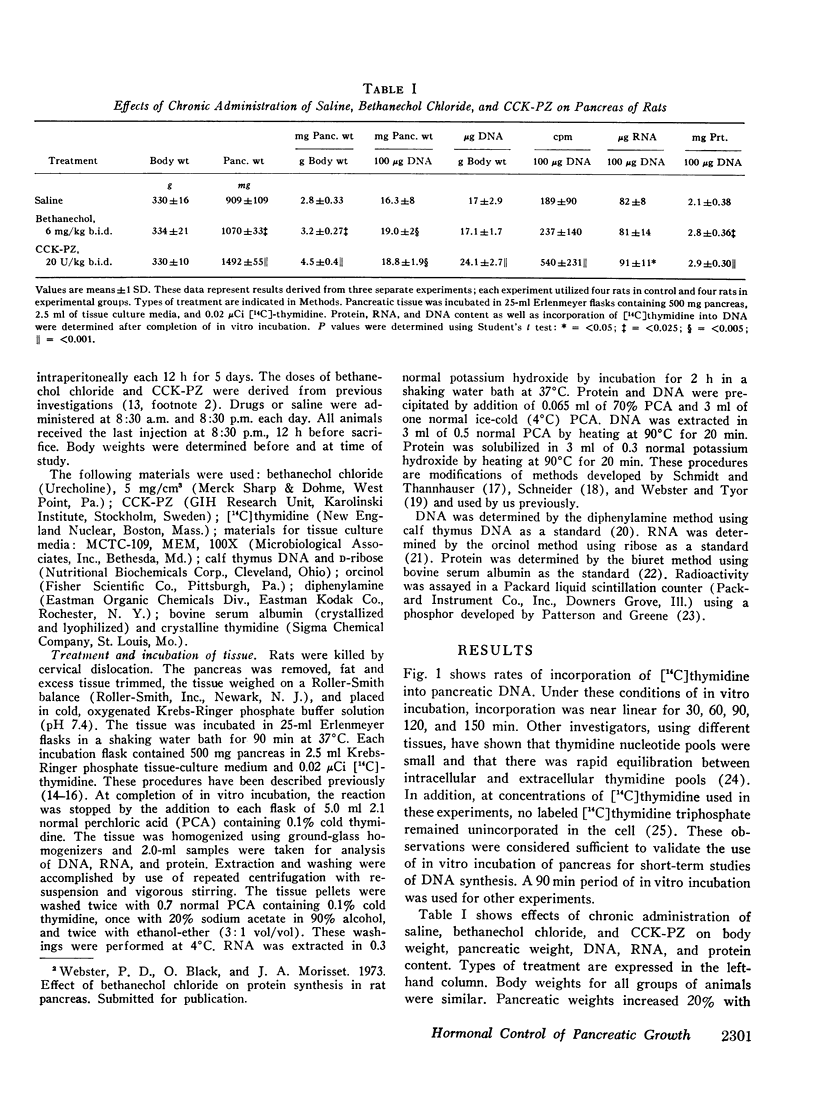
These studies were designed to examine effects of chronic administration of bethanechol and cholecystokinin-pancreozymin (CCK-PZ) on the pancreas. Male albino rats were given saline, bethanechol, 6 mg/kg, or CCK-PZ, 20 U/kg, intraperitoneally twice daily and killed after 5 days. The following changes were studied; pancreatic weight; RNA, DNA, and protein content; and [14C]thymidine incorporation into DNA. Bethanechol administration was associated with a 20% increase in pancreatic weight and a 33% increase in mg protein/100 μg DNA. In bethanechol-treated groups, amounts of DNA/gram body weight and incorporation of [14C]thymidine into DNA were similar to controls. CCK-PZ administration was associated with a 71% increase in pancreatic weight and a 38% increase in mg protein/100 μg DNA. In CCK-PZ-treated groups, amounts of DNA/gram body weight were increased by 42% and [14C]thymidine incorporation into DNA was increased by 185%.
These studies indicate that bethanechol administration was associated with increases in pancreatic cell mass (hypertrophy). CCK-PZ administration was associated with increases in cell mass and cell numbers (hypertrophy and hyperplasia). This information suggests the importance of CCK-PZ in maintaining pancreatic functional integrity. Although bethanechol and CCK-PZ elicit similar secretory responses, their mode of action on the cell, at least as far as growth influences are concerned, appears to be different.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler A. M., Johnson L. R. Pentagastrin-stimulated incorporation of 14 C-orotic acid into RNA of gastric and duodenal mucosa. Proc Soc Exp Biol Med. 1972 Oct;141(1):110–113. doi: 10.3181/00379727-141-36727. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. J., Herman L., Carol B., Roque A., Marsh W. H., Rosenstock L., Richards C., Perl D. Pancreatic acinar cell regeneration. Am J Pathol. 1968 May;52(5):983–1011. [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. J., Vinijchaikul K., Carol B., Rosenstock L. Pancreatic acinar cell regeneration. 3. DNA synthesis of pancreas nuclei as indicated by thymidine-H3 autoradiography. Am J Pathol. 1968 May;52(5):1039–1065. [PMC free article] [PubMed] [Google Scholar]
- GENTRY G. A., MORSE P. A., Jr, IVES D. H., GEBERT R., POTTER V. R. PYRIMIDINE METABOLISM IN TISSUE CULTURE CELLS DERIVED FROM RAT HEPATOMAS. II. THYMIDINE UPTAKE IN SUSPENSION CULTURES DERIVED FROM THE NOVIKOFF HEPATOMA. Cancer Res. 1965 May;25:509–516. [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Chymotrypsin inhibitor stimulation of pancreatic enzyme secretion in the rat. Proc Soc Exp Biol Med. 1971 Feb;136(2):649–654. doi: 10.3181/00379727-136-35332. [DOI] [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. 1972 May;140(1):6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Aures D., Yuen L. Pentagastrin-induced stimulation of protein synthesis in the gastrointestinal tract. Am J Physiol. 1969 Jul;217(1):251–254. doi: 10.1152/ajplegacy.1969.217.1.251. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., WALKER B. E. Renewal of cell populations. Physiol Rev. 1956 Apr;36(2):255–276. doi: 10.1152/physrev.1956.36.2.255. [DOI] [PubMed] [Google Scholar]
- Lehv M., Fitzgerald P. J. Pancreatic acinar cell regeneration. IV. Regeneration after resection. Am J Pathol. 1968 Oct;53(4):513–535. [PMC free article] [PubMed] [Google Scholar]
- Leroy J., Morisset J. A., Webster P. D. Dose-related response of pancreatic synthesis and secretion to cholecystokinin-pancreazymin. J Lab Clin Med. 1971 Jul;78(1):149–157. [PubMed] [Google Scholar]
- Mayston P. D., Barrowman J. A. The influence of chronic administration of pentagastrin on the rat pancreas. Q J Exp Physiol Cogn Med Sci. 1971 Apr;56(2):113–122. doi: 10.1113/expphysiol.1971.sp002105. [DOI] [PubMed] [Google Scholar]
- Morisset J. A., Webster P. D. Effects of fasting and feeding on protein synthesis by the rat pancreas. J Clin Invest. 1972 Jan;51(1):1–8. doi: 10.1172/JCI106779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset J. A., Webster P. D. In vitro and in vivo effects of pancreozymin, urecholine, and cyclic AMP on rat pancreas. Am J Physiol. 1971 Jan;220(1):202–208. doi: 10.1152/ajplegacy.1971.220.1.202. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Thymidine transport by cultured Novikoff hepatoma cells and uptake by simple diffusion and relationship to incorporation into deoxyribonucleic acid. J Cell Biol. 1972 Oct;55(1):161–178. doi: 10.1083/jcb.55.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. S., Wells H. Enhancement of pancreatic enzyme synthesis by pancreozymin. Am J Physiol. 1967 Jul;213(1):215–218. doi: 10.1152/ajplegacy.1967.213.1.215. [DOI] [PubMed] [Google Scholar]
- Stening G. F., Grossman M. I. Gastrin-related peptides as stimulants of pancreatic and gastric secretion. Am J Physiol. 1969 Jul;217(1):262–266. doi: 10.1152/ajplegacy.1969.217.1.262. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- Webster P. D., 3rd Effect of methacholine on pancreatic amylase synthesis. Gastroenterology. 1968 Sep;55(3):375–385. [PubMed] [Google Scholar]
- Webster P. D., 3rd Effect of stimulation on pancreatic amylase secretion and nuclear RNA synthesis. Proc Soc Exp Biol Med. 1969 Dec;132(3):1072–1076. doi: 10.3181/00379727-132-34369. [DOI] [PubMed] [Google Scholar]
- Webster P. D., Gunn L. D., Tyor M. P. Effect of in vivo pancreozymin and methacholine on pancreatic lipid metabolism. Am J Physiol. 1966 Sep;211(3):781–785. doi: 10.1152/ajplegacy.1966.211.3.781. [DOI] [PubMed] [Google Scholar]
- Webster P. D., Tyor M. P. Effect of intravenous pancreozymin on amino acid incorporation in vitro by pancreatic tissue. Am J Physiol. 1966 Jul;211(1):157–160. doi: 10.1152/ajplegacy.1966.211.1.157. [DOI] [PubMed] [Google Scholar]
- Webster P. D., Tyor M. P. Effects of fasting and feeding on uridine-3-H incorporation into RNA by pancreas slices. Am J Physiol. 1967 Jan;212(1):203–206. doi: 10.1152/ajplegacy.1967.212.1.203. [DOI] [PubMed] [Google Scholar]


